Abstract
Spartina densiflora is a C4 halophytic species that has proved to have a high invasive potential which derives from its clonal growth and its physiological plasticity to environmental factors, such as salinity. A greenhouse experiment was designed to investigate the synergic effect of 380 and 700 ppm CO2 at 0, 171, and 510 mM NaCl on the growth and the photosynthetic apparatus of S. densiflora by measuring chlorophyll fluorescence parameters, gas exchange and photosynthetic pigment concentrations. PEPC activity and total ash, sodium, potassium, calcium, magnesium, and zinc concentrations were determined, as well as the C/N ratio. Elevated CO2 stimulated growth of S. densiflora at 0 and 171 mM NaCl external salinity after 90 d of treatment. This growth enhancement was associated with a greater leaf area and improved leaf water relations rather than with variations in net photosynthetic rate (A). Despite the fact that stomatal conductance decreased in response to 700 ppm CO2 after 30 d of treatment, A was not affected. This response of A to elevated CO2 concentration might be explained by an enhanced PEPC carboxylation capacity. On the whole, plant nutrient concentrations declined under elevated CO2, which can be ascribed to the dilution effect caused by an increase in biomass and the higher water content found at 700 ppm CO2. Finally, CO2 and salinity had a marked overall effect on the photochemical (PSII) apparatus and the synthesis of photosynthetic pigments.
Keywords: Chlorophyll fluorescence, CO2 enrichment, cordgrass, gas exchange, growth rate, PEPC activity, photosynthetic pigments, salinity
Introduction
Global environmental changes such as climatic change and biological invasions are common conservation problems affecting ecosystems worldwide (Occhipinti-Ambrogi, 2007). Climate change is likely to alter patterns of alien plant invasions through its effect on three general aspects: the invasibility of the ecosystem, climate impacts on indigenous species, and the invasive potential of the alien species (Dukes and Mooney, 1999).
Spartina densiflora is a C4 halophytic species with a South American origin that is invading salt marshes as far apart as southern Europe (Tutin, 1980; Mateos-Naranjo et al., 2007), North Africa (Fennane and Mathez, 1988) and North America (Kittelson and Boyd, 1997). In Spain, S. densiflora has proved to have a high invasive potential which derives from its prolific seed production and from its clonal growth (Figueroa and Castellanos, 1988; Nieva et al., 2001). In addition, its physiological and morphological versatility apparently allows S. densiflora to tolerate a wide range of salinity, tidal submergence, and drainage (Castillo et al., 2005; Mateos-Naranjo et al., 2007). This is consistent with S. densiflora having colonized high, middle, and low marshes, with their different characteristic assemblages of native species (Nieva et al., 2001). Many ecological and physiological aspects of S. densiflora have hitherto been analysed (Castillo et al., 2005; Mateos-Naranjo et al., 2007, 2008a, b). Nevertheless, so far no studies have assessed the influence of climatic change and of rising atmospheric CO2 concentrations on the invasive potential of S. densiflora.
Predictions regarding climate change indicate that the CO2 concentration in the atmosphere is expected to have undergone a 2-fold increase by the end of the century with a value of c. 760 ppm by 2100 (IPCC, 2001). There is a general consensus on the direct physiological impact of increasing CO2 concentration on plant photosynthesis and metabolism, stimulating growth and development in hundreds of plants species (Ghannoum et al., 2000). However, recent evidence from free-air CO2 enrichment experiments suggested that elevated CO2 concentration did not directly stimulate C4 photosynthesis. Nonetheless, drought stress can be ameliorated at elevated CO2 concentration as a result of lower stomatal conductance and greater intercellular CO2 concentration (Leakey, 2009). Furthermore, the effects of CO2 enrichment on plants can be modified by other environmental factors, such as salinity (Lenssen et al., 1993, 1995; Rozema, 1993) and temperature.
The aims of this study were to investigate (i) whether CO2 enrichment stimulates the growth of the invasive species S. densiflora, and whether this stimulation is mediated by an improvement of photosynthetic activity, and/or (ii) whether salinity stress can be ameliorated at high CO2 concentration. The specific objectives were to: (i) to analyse the growth of plants in experimental salinity concentrations from 0 to 510 mM NaCl at ambient and elevated CO2 concentrations (380 and 700 ppm, respectively); (ii) determine the extent of the effects on the photosynthetic apparatus (PSII chemistry), gas exchange characteristics, phosphoenolpyruvate carboxylase activity (PEPC), and photosynthetic pigments; and (iii) examine the possible role of concentrations of mineral matter (ash), calcium, potassium, sodium, and zinc accumulated and C/N ratio in response to increasing external salinity at both CO2 levels.
Materials and methods
Plant material
Seeds of S. densiflora were collected in December 2006 from Odiel Marshes (37°15’ N, 6°58’ W; SW Spain), and subsequently stored at 4 °C (in darkness) for three months. After the storage period, seeds were placed in a germinator (ASL Aparatos Científicos M-92004, Madrid, Spain), and subjected to an alternating diurnal regime of 16 h of light (photon flux rate, 400–700 nm, 35 μmol m−2 s−1) at 25 °C and 8 h of darkness at 12 °C, for a month. Seedlings were planted in individual plastic pots (9 cm and 11 cm of height and diameter, respectively) filled with perlite and placed in a greenhouse (during spring 2007) with minimum-maximum temperatures of 21–25 °C, 40–60% relative humidity and natural daylight (minimum and maximum light flux: 200 and 1000 μmol m−2 s−1, respectively). Pots were carefully irrigated with 20% Hoagland's solution (Hoagland and Arnon, 1938) as necessary.
Growth conditions
In April 2007, after a month of seedling cultures, the pots were allocated to three NaCl treatments in shallow trays: 0, 171, and 510 mM in Hoagland's solution. Afterwards, they were exposed to ambient (380 ppm) or elevated CO2 concentration (700 ppm), in a controlled-environment chamber, in the same greenhouse and supplied with Hoagland's solution with or without NaCl (ten pots per tray, with one tray per NaCl and CO2 treatments) for a further three months. The CO2 concentration was within 10% of the target concentration for 85% of the time, on the basis of 1 min averages. NaCl concentrations were chosen to cover variations recorded by Mateos-Naranjo et al. (2008a) in the salt marshes of the Odiel River where S. densiflora occurs.
The CO2 concentration in the greenhouse with ambient CO2 was not controlled, but it was measured with a CO2 analyser (Testo 535, Germany). The controlled-environment chamber consists of transparent chamber tops, 3.3×1.4×1.1 m (length×width×height) made with 0.005 thick acrylic glass, and aluminium angular frame elements. The CO2 level in the enriched chamber was maintained by supplying pure CO2 from a compressed gas cylinder (Air liquide, B50 35K) into the chamber. The CO2 concentration in the chamber was continuously recorded by a CO2 sensor (Vaisala CARBOCAP GMT220, Finland), the signal being received by a computer (ASCON M3, Italy) that activated, if necessary, CO2 injection into the enriched chamber so as to reach the desired 700 ppm.
At the beginning of the experiment, 3.0 l of the appropriate solution were placed in each of the trays down to a depth of 1 cm. During the experiment, the levels in the trays were monitored and they were topped up to the marked level with 20% Hoagland's solution as a way to limit the change of NaCl concentration due to water evaporation of the nutritive solution.
Growth analysis
At the beginning and at the end of the experiment, three and seven entire plants (roots and leaves) from each treatment, respectively, were dried at 80 °C for 48 h and then weighed. Dried, ground samples were ignited in lidded, ceramic crucibles and ash weights were recorded; the furnace temperature was raised slowly over 6 h to 550 °C and this temperature was maintained for a further 8 h. Also, the number of tillers was measured.
A classical growth analysis (Evans, 1972) was carried out with ash-free dry masses. The relative growth rate in whole plant dry mass (RGR) was calculated and partitioned into its three components, unit leaf rate (ULR), specific leaf area (SLA), and leaf mass fraction (LMF), using the software tool of Hunt et al. (2002):
where t is time, W is total dry mass per plant, LA is total leaf area per plant, and LW is total leaf dry mass per plant. Leaf area was calculated by superimposing the surface of each leaf over a mm-square paper.
Leaf elongation rate (LER) was measured in random leaves (n=14, per treatment; two measurements per plant) at 90 d of treatment by placing a marker of inert sealant at the base of the youngest accessible leaf. The distance between the marker and the leaf base was measured after 24 h (Mateos-Naranjo et al., 2008b).
Gas exchange
Gas exchange measurements were taken on random, fully expanded penultimate leaves (Fig. 1; n=10, one measurement per plant and three extra taken randomly) using an infrared gas analyser in an open system (Li-6400, Li-Cor Inc., Nebraska, USA) after 7, 30, and 90 d of treatment. Net photosynthetic rate (A), intercellular CO2 concentration (Ci), and stomatal conductance to CO2 (Gs) were determined at ambient CO2 concentration of 380 and 700 ppm CO2, temperature of 20 °C, 50±5% relative humidity, and a photon flux density of 1000 μmol m−2 s−1. A, Ci, and Gs were calculated using standard formulae of Von Caemmerer and Farquhar (1981). Photosynthetic area was approximated as the area of a trapezium. The water use efficiency (WUE) was calculated as the ratio between A and transpiration rate (mmol CO2 assimilated mol−1 H2O transpired).
Fig. 1.
Pot of Spartina densiflora with fully expanded penultimate leaves marked in red. (This figure is available in colour at JXB online.)
Leaf water content
Leaf water content (WC) was calculated after 90 d of treatment as:
where FW is the fresh mass of the leaves, and DW is the dry mass after oven-drying at 80 °C for 48 h.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured in random, fully developed penultimate leaves (n=10, one measurement per plant and three extra taken randomly) using a portable modulated fluorimeter (FMS-2, Hansatech Instrument Ltd., England) after 7, 30, and 90 d of treatment. Light- and dark-adapted fluorescence parameters were measured at dawn (stable, 50 μmol m−2 s−1 ambient light) and at midday (1600 μmol m−2 s−1) to investigate whether NaCl and CO2 concentration affected the sensitivity of plants to photoinhibition.
Plants were dark-adapted for 30 min, using leaf-clips exclusively designed for this purpose. The minimal fluorescence level in the dark-adapted state (F0) was measured using a modulated pulse (<0.05 μmol m−2 s−1 for 1.8 μs) which was too small to induce significant physiological changes in the plant. The data stored were an average taken over a 1.6 s period. Maximal fluorescence in this state (Fm) was measured after applying a saturating actinic light pulse of 15 000 μmol m−2 s−1 for 0.7 s. The value of Fm was recorded as the highest average of two consecutive points. Values of the variable fluorescence (Fv=Fm–F0) and maximum quantum efficiency of PSII photochemistry (Fv/Fm) were calculated from F0 and Fm. This ratio of variable to maximal fluorescence correlates with the number of functional PSII reaction centres, and dark-adapted values of Fv/Fm can be used to quantify photoinhibition (Krivosheeva et al., 1996).
The same leaf section of each plant was used to measure light-adapted parameters. Steady-state fluorescence yield (Fs) was recorded after adapting plants to ambient light conditions for 30 min. A saturating actinic light pulse of 15 000 μmol m−2 s−1 for 0.7 s was then used to produce the maximum fluorescence yield () by temporarily inhibiting PSII photochemistry.
Using fluorescence parameters determined in both light- and dark-adapted states, the following were calculated: quantum efficiency of PSII 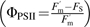 and non-photochemical quenching
and non-photochemical quenching 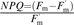 ; Redondo-Gómez et al., 2006).
; Redondo-Gómez et al., 2006).
Photosynthetic pigments
At the end of the experiment period, photosynthetic pigments in fully expanded penultimate leaves (n=5) were extracted using 0.05 g of fresh material in 10 ml of 80% aqueous acetone. After filtering, 1 ml of the suspension was diluted with a further 2 ml of acetone and chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid (Cx+c) contents were determined with a spectrophotometer (Hitachi U-2001, Hitachi Ltd, Japan), using three wavelengths (663.2, 646.8, and 470.0 nm). Concentrations of pigments (μg g−1 FW) were obtained by calculation, using the method of Lichtenthaler (1987).
Determination of sodium, potassium, calcium, magnesium, zinc, and nitrogen
In accordance with protocols of Redondo-Gómez et al. (2007), at the end of the experiment leaf and root samples were dried at 80 °C for 48 h and ground. Leaves and roots were carefully washed with distilled water before any further analysis. Then 0.5 g samples, taken from a mixture of the leaves or the roots belonging to the seven plants used for each treatment, were tripicately digested with 6 ml HNO3, 0.5 ml HF, and 1 ml H2O2. Ca2+, K+, Mg2+, Na+, P, and Zn were measured by inductively coupled plasma (ICP) spectroscopy (ARL-Fison 3410, USA). Total N and C concentrations were determined for undigested dry samples with an elemental analyser (Leco CHNS-932, Spain).
Preparation of desalted protein extracts
Plants were exposed to 2 h of direct sunlight at midday. To extract PEPC (n=5), 0.2 g of leaf tissue were taken and immediately ground in a chilled mortar with 1 ml of extraction buffer A containing 100 mM TRIS-HCl pH 7.5, 20% (v/v) glycerol, 1 mM EDTA, 10 mM MgCl2, 14 mM β-mercaptoethanol, 1 mM phenylmethysulphonyfluoride (PMSF), 10 μg ml−1 chymostatin, 10 μg ml−1 leupeptin, and 10 mM potassium fluoride. The homogenate was centrifuged at 15 000 g for 2 min and the supernatant was filtered through Sephadex G-25 equilibrated with buffer A without β-mercaptoethanol. The desalted extract was used rapidly to determine the activity and sensitivity of PEPC to L-malate, as described below.
Assay of PEPC activity and its inhibition by L-malate
PEPC activity was measured spectrophotometrically at the optimal and suboptimal pH values of 8 and 7.3, respectively, using the NAD-malate dehydrogenase-coupled assay at 2.5 mM phosphoenolpyruvate (PEP) described by Echevarria et al. (1994). Assays were initiated by the addition of an aliquot of crude extract (n=5). An enzyme unit is defined as the amount of PEPC that catalyses the carboxylation of 1 μmol of phosphoenolpyruvate min−1 at pH 8 and 30 °C. Malate sensitivity was determined at suboptimal pH 7.3 in the presence or absence of various concentrations of L-malate (IC50, 50% inhibition of initial PEPC activity by L-malate; Echevarría et al., 1994). A high IC50 is related to a high degree of PEPC phosphorylation.
Protein quantification
Protein amounts were determined by the method of Bradford (1976), using bovine serum albumin (BSA) as standard.
Statistical analysis
Statistical analysis was carried out using Statistica v. 6.0 (Statsoft Inc.). Pearson coefficients were calculated to assess correlation between different variables. Data were analysed using one-, two-, and three-way analysis of variance (F-test). Data were first tested for normality with the Kolmogorov–Smirnov test and for homogeneity of variance with the Brown–Forsythe test. Significant test results were followed by Tukey tests for identification of important contrasts. The comparison between measurements of fluorescence at dawn and midday and between the means of ambient and elevated CO2 concentration treatments in all parameters were made by using the Student test (t test).
Results
Growth analysis
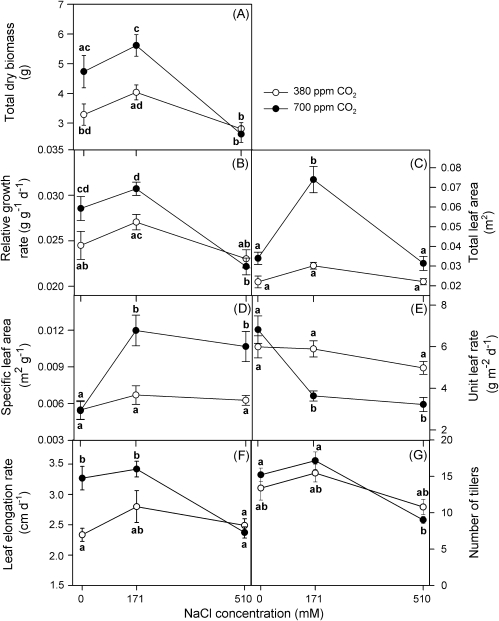
Total dry mass showed a broad optimum at 171 mM NaCl external salinity for both 380 and 700 ppm CO2 (Fig. 2A); dry mass was substantially reduced at the highest salinity (510 mM NaCl) for both CO2 concentrations. On the other hand, total dry mass was higher at 700 ppm CO2 and 0 and 171 mM NaCl than at 380 ppm CO2 for the same salinity treatments (two-way ANOVA: CO2×salinity, F2,32=4.1, P <0.05; Fig. 2A). The same trends were evident in mean relative growth rate (RGR; Fig. 2B), and the effects of salinity and CO2 concentration on RGR were highly significant after 90 d of treatment (two-way ANOVA: CO2×salinity, F2,34=3.6, P <0.05). The peak at 171 mM NaCl was associated with higher values of total leaf area (Fig. 2C) and specific leaf area (Fig. 2D), whereas the lower values of RGR at 510 mM were linked to lower values of unit leaf rate (Fig. 2E). Leaf mass fraction did not show any relationship either with salinity or CO2 treatments, showing values c. 0.7 g g−1 in all cases (data not presented).
Fig. 2.
Growth analysis of Spartina densiflora in response to treatment with a range of salinity concentrations at ambient and elevated CO2 concentration over 90 d. Total dry mass (A), relative growth rate (B), total leaf area (C), specific leaf area (D), unit leaf rate (E), leaf elongation rate (F), and number of tillers (G). Values represent mean ±SE, n=7 (n=14 for total leaf area). The analysis was carried out on an ash-free dry mass basis. Different letters indicate means that are significantly different from each other (two-way ANOVA, CO2×salinity; Tukey test, P <0.05).
Finally, RGR was directly correlated with leaf elongation rate (LER; r=0.84, P <0.0001) and number of tillers (r=0.81, P <0.0001) at 700 ppm CO2. There were not significant correlations between these parameters at 380 ppm CO2, although they showed similar trends (Fig. 2F, G).
Gas exchange
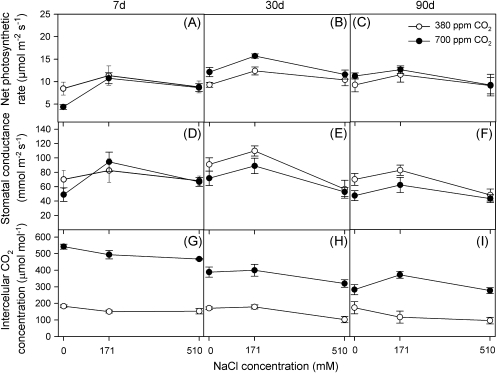
Overall net photosynthetic rate (A) data were similar for all salinity and CO2 treatments at each of the three measurement times; except at the 30 d treatment, where plants grown at 700 ppm CO2 with 0 and 171 mM NaCl showed higher A values than those at ambient CO2 (t test, P <0.01; Fig. 3A–C). Stomatal conductance (Gs) showed a trend that was extremely similar to that of A (Fig. 3D–F). Nonetheless, Gs values were lower at 700 ppm CO2 after 30 d and 90 d of treatment (t test, P <0.05). Intercellular CO2 concentration (Ci) at 700 ppm CO2 responded differently to salinity at the earlier stages of the experiment than at the later stage: salinity had no effect on Ci after 7 d and 30 d but Ci reached a peak at 171 mM NaCl after 90 d (Fig. 3G–I). Ci values were higher at 700 ppm CO2 at each of the three measurement times for all salinity treatments (t test, P <0.05).
Fig. 3.
Net photosynthetic rate, A (A–C), stomatal conductance, Gs (D–F), and intercellular CO2 concentration, Ci (G–I) in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment with a range of NaCl concentrations at ambient and elevated CO2 concentration after 7 d (A, D, G), 30 d (B, E, H), and 90 d (C, F, I). Values represent mean ±SE, n=10.
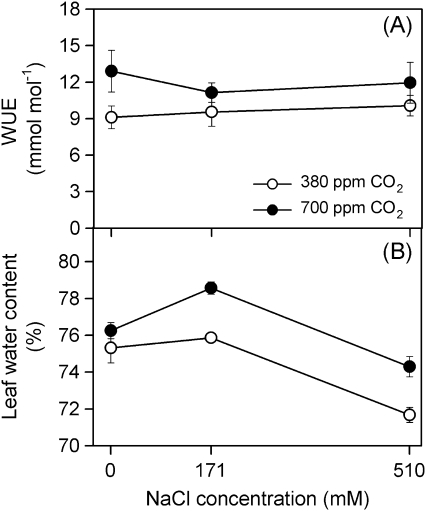
Plants grown at 700 ppm CO2 showed consistently higher water use efficiency (WUE), although significant differences were only recorded at 0 mM NaCl (t test, P >0.05). Leaf water content (WC) was higher at 700 ppm CO2 for all salinity treatments after 90 d of treatment (t test, P >0.0001; Fig. 4). Furthermore, WC was higher at 171 mM NaCl at 700 ppm CO2 concentration (one-way ANOVA: F2,17=23.4, P <0.0001).
Fig. 4.
Water use efficiency, WUE (A) and leaf water content (B) in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment with a range of NaCl concentrations at ambient and elevated CO2 concentration over 90 d. Values represent mean ±SE, n=10 and n=7 for WUE and water content, respectively.
Chlorophyll fluorescence
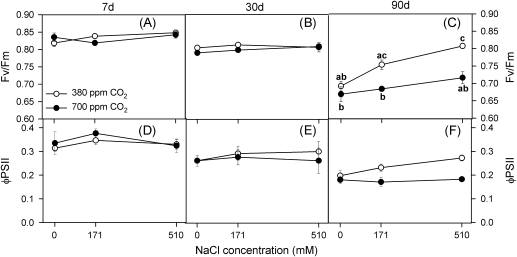
Values of Fv/Fm and quantum efficiency of PSII (ΦPSII) at dawn were high at both 380 ppm and 700 ppm CO2 at all external NaCl concentrations after 7, 30, and 90 d of treatment, varying between 0.81 and 0.84 for Fv/Fm, and between 0.80 and 0.83 for ΦPSII. Fv/Fm and ΦPSII, respectively, were always lower at midday and the reductions resulted mainly from lower values of Fm and qP (data not presented), respectively, at midday than at dawn (t test, P <0.05). On the other hand, the midday Fv/Fm values at both CO2 concentrations decreased during the course of the experiment, especially in plants grown with 700 ppm CO2; again as a consequence of lower values of Fm (Fig. 5A–C). The same trends were evident in ΦPSII at midday (Fig. 5D–F).
Fig. 5.
Maximum quantum efficiency of PSII photochemistry, Fv/Fm (A–C) and quantum efficiency of PSII, ΦPSII (C–E) at midday in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment with a range of salinity concentrations at ambient and elevated CO2 concentration after 7 d (A, C), 30 d (B, D), and 90 d (C, E). Values represent mean ±SE, n=10. Different letters indicate means that are significantly different from each other (two-way ANOVA, CO2×salinity; Tukey test, P <0.05).
The effects of salinity and of CO2 concentration on Fv/Fm at midday were highly significant after 90 d of treatment (two-way ANOVA: CO2×salinity, F2,41=5.0, P <0.05; Fig. 5C). Moreover, ΦPSII values at midday increased with external salinity in plants grown at 380 ppm CO2 after 90 d of treatment (one-way ANOVA; F2,46=4.0, P <0.05; Fig. 5F); and ΦPSII values were lower at 700 ppm CO2 in the presence of NaCl than at 380 ppm CO2 (t-test, P <0.05).
Finally, plants treated with both CO2 concentrations maintained nearly constant NPQ at each of the three measurement times, irrespective of the salinity treatment (c. 1.0; P >0.05).
Photosynthetic pigments
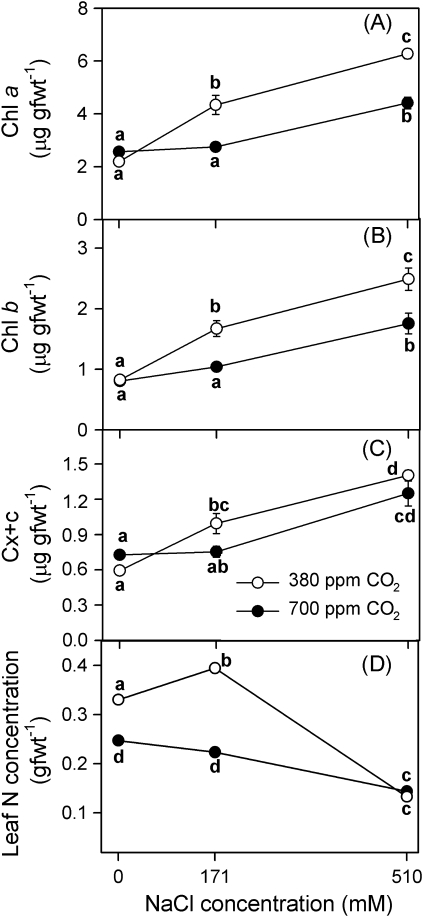
The effects of salinity and CO2 concentration on pigment concentrations (Chl a, Chl b, and Cx+c, all in μg g−1 FW; Fig. 6A–C) were highly significant after 90 d of treatment (two-way ANOVA, CO2×salinity: Chl a, F2,23=17.7, P <0.0001; Chl b, F2,22=4.6, P <0.05; Cx+c, F2,23=5.0, P <0.05). Pigment concentrations increased with increasing salinity treatment at both CO2 concentrations (one-way ANOVA, P <0.05). Contrary to that, leaf nitrogen content diminished with increasing salinity (Fig. 6D). Furthermore, plants treated with NaCl showed higher Chl a and b concentrations at 380 ppm CO2 than under 700 ppm CO2 concentration (t test, P <0.0001). In the case of carotenoids, there were not any differences between CO2 treatments (P >0.05).
Fig. 6.
Chlorophyll a, Chl a (A), chlorophyll b, Chl b (B), carotenoid, Cx+c (C), and leaf nitrogen (D) concentrations in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment with a range of salinity concentrations at ambient and elevated CO2 concentration over 90 d. Values represent mean ±SE, n=5. Different letters indicate means that are significantly different from each other (two-way ANOVA, CO2×salinity; Tukey test, P <0.05).
Determination of sodium, potassium, calcium, magnesium, zinc, and nitrogen
Overall, the mineral (ash) contents of both leaves and roots were higher at 380 ppm CO2, and increased with increasing external NaCl concentration. Likewise, ash content was greater in roots than in leaves (three-way ANOVA, CO2×salinity×tissue: F2,23=3.7, P <0.05; Table 1).
Table 1.
Ash, total sodium, potassium, calcium, magnesium and zinc, and C/N ratio for leaves and roots of Spartina densiflora in response to a treatment with a range of salinity concentrations at ambient and elevated [CO2] over 90 d
| [CO2] (ppm) | 380 | |||||
| Tissue | Leaves | Roots | ||||
| Salinity (mM) | 0 | 171 | 510 | 0 | 171 | 510 |
| Ash (%) | 13.4 (0.03) a | 15.8 (0.13) b | 15.4 (0.02) b | 19.0 (0.22) c | 19.4 (0.39) c | 23.8 (0.43) d |
| Na (mg g−1) | 8.5 (0.10) a | 31.9 (0.10) b | 47.4 (0.27) c | 5.5 (0.04) de | 23.8 (0.05) f | 38.2 (0.22) g |
| K (mg g−1) | 53.8 (1.24) a | 24.8 (0.62) b | 14.7 (0.12) c | 41.6 (0.17) e | 37.7 (0.30) ef | 50.1 (0.58) a |
| Ca (mg g−1) | 7.9 (0.05) a | 6.2 (0.02) b | 3.8 (0.00) c | 3.8 (0.02) c | 2.1 (0.00) d | 1.7 (0.00) e |
| Mg (mg g−1) | 3.6 (0.02) a | 4.1 (0.01) b | 2.0 (0.01) c | 6.1 (0.03) d | 5.2 (0.01) e | 2.2 (0.00) f |
| Zn (mg kg−1) | 40.7 (0.93) a | 30.9 (0.56) b | 41.8 (0.23) ac | 44.2 (0.56) c | 40.0 (0.26) a | 87.7 (1.24) d |
| C/N | 15.3 (0.05) a | 17.6 (0.00) b | 17.1 (0.10) b | 10.0 (0.02) c | 14.1 (0.23) d | 16.4 (0.30) e |
| [CO2] (ppm) | 700 | |||||
| Tissue | Leaves | Roots | ||||
| Salinity (mM) | 0 | 171 | 510 | 0 | 171 | 510 |
| Ash (%) | 11.3 (0.05) e | 15.4 (0.15) b | 16.9 (0.27) b | 11.3 (0.36) e | 12.9 (0.42) f | 21.2 (0.48) g |
| Na (mg g−1) | 6.3 (0.02) d | 36.3 (0.29) h | 47.8 (0.64) c | 5.4 (0.05) de | 23.6 (0.42) f | 44.5 (0.40) i |
| K (mg g−1) | 32.8 (0.62) fg | 17.4 (0.16) cd | 10.6 (0.15) c | 30.3 (0.87) g | 19.6 (0.36) d | 25.4 (0.23) b |
| Ca (mg g−1) | 6.7 (0.00) f | 5.0 (0.00) g | 4.2 (0.00) h | 2.6 (0.00) i | 1.5 (0.00) j | 1.2 (0.00) k |
| Mg (mg g−1) | 2.7 (0.03) g | 3.7 (0.04) a | 2.5 (0.02) h | 3.7 (0.05) a | 3.9 (0.03) b | 2.9 (0.02) i |
| Zn (mg kg−1) | 20.2 (0.04) e | 21.6 (0.07) e | 28.4 (0.14) bf | 16.9 (0.23) g | 17.0 (0.07) g | 28.9 (0.16) bf |
| C/N | 16.0 (0.05) e | 16.3 (0.12) e | 16.4 (0.05) e | 12.9 (0.08) f | 16.2 (0.00) e | 14.2 (0.11) d |
Values represent mean ±SE, n=3. Different letters indicate means that are significantly different from each other (three-way ANOVA, CO2×salinity×tissue; Tukey test, P <0.05).
By the end of the experiment, tissue Na concentrations were greater in leaves than in roots, and increased markedly with external NaCl concentration (t test, P <0.001). By contrast, leaf K, Ca, and Mg concentrations decreased with increasing salinity at both CO2 treatments. In addition, leaf and root K, Ca, and Mg concentrations were higher at 380 ppm CO2 (t test, P <0.05).
On the other hand, tissue zinc concentration was greater at 380 ppm CO2 (t test, P <0.0001) and Zn content was higher in the roots than in the leaves at ambient CO2 concentration (Table 1).
Finally, C/N ratio was considerably higher in leaves than in roots for all salinity and CO2 treatments (three-way ANOVA, P <0.0001). Tissue C/N ratio increased with external NaCl concentration at 380 ppm CO2. Furthermore, C/N ratio was greater at 380 ppm CO2 for leaves and at 700 ppm CO2 for roots.
PEPC activity
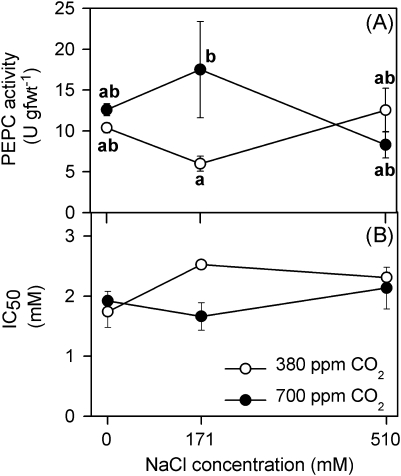
The effects of salinity and of CO2 concentration on PEPC activity were significant after 90 d of treatment (two-way ANOVA: CO2×salinity, F2,18=3.8, P <0.05). The lowest value of PEPC activity was recorded at 380 ppm CO2 and 171 mM NaCl, and the highest value at the same salinity and elevated CO2 concentration (Fig. 7A). Contrary to that, IC50 for malate was higher at 380 ppm CO2 and 171 mM NaCl, and lower at 700 ppm CO2 and the same salinity (Fig. 7B).
Fig. 7.
PEPC activity (A) and IC50 values for L-malate (B) in crude extracts of illuminated leaves of Spartina densiflora in response to treatment with a range of salinity concentrations at ambient and elevated CO2 concentration over 90 d. Values represent mean ±SE, n=5. Different letters indicate means that are significantly different from each other (two-way ANOVA, CO2×salinity; Tukey test, P <0.05).
Discussion
Significant long-term (i.e. months) effects of CO2 concentration on the growth of the C4 Spartina densiflora were observed, with plants grown at elevated CO2 concentration producing 35% and 20% more biomass, at 0 and 171 mM NaCl, respectively, than their ambient CO2-grown counterparts; although this effect was counterbalanced by high salinity (510 mM NaCl), recording similar total dry mass and RGR at ambient and at elevated CO2 concentrations. This response was apparent as the RGR of ash-free dry mass, total leaf area, leaf elongation rate and, by inference, the number of tillers produced. Castillo et al. (2005) found the highest rate of leaf elongation of S. densiflora in distilled water. Nevertheless, in our experiment, the growth at 171 mM NaCl was slightly higher than in the absence of salt under both CO2 concentrations. In this and other studies, the absence of salt has been proved to affect neither the photosynthetic function of S. densiflora nor its growth (Mateos-Naranjo et al., 2008b).
Enhanced growth at elevated CO2 concentration disagrees with results reported by Lenssen et al. (1993), who found that elevated CO2 concentration reduced plant weight of Spartina anglica by 20%, this reduction being associated with decreased SLA. In the current study, the stimulation of growth in S. densiflora at elevated CO2 concentration and 171 mM NaCl was linked to higher SLA, while leaf mass fraction was not affected by either salinity or CO2 concentration. The increase of SLA with CO2 concentration and salinity could be mediated by an improvement in water relations and/or an induction of cell expansion. Rozema et al. (1991) found that a higher turgor pressure might stimulate leaf expansion. In this way, higher leaf water content was observed in S. densiflora at elevated CO2 concentration in the presence of salt. On the other hand, De Souza et al. (2008) found that elevated CO2 concentration in sugarcane induced cell expansion through the action of XTH (xyloglucan endotransglycosylase/hydrolase) on leaf cell walls. A similar effect was observed by Ferris et al. (2001) for Populus species.
Enhanced growth of S. densiflora was higher than that reported by Rogers et al. (2008) for C4 invasive plants of Cyperus rotundus and C. esculentus (10–15%), even higher than the growth stimulation described in the literature for other C4 plants in response to a doubling of the current ambient CO2 concentration under non-saline conditions (22–33%; Ghannoum et al., 2000). On the other hand, Rozema et al. (1991) noted that Spartina patens showed higher RGR values at elevated CO2 concentration when plants were treated with low salinity (10 mM NaCl), while RGR of those treated with 250 mM NaCl decreased at 580 ppm CO2 (–48.3%, under aerated conditions in the culture solution). In the case of S. densiflora, the decrease of RGR at an elevated CO2 concentration was recorded in plants treated with 510 mM NaCl. Reduced RGR at high salinity can be attributed to lower unit leaf rate. This component of RGR was the most sensitive to salinity and CO2 concentration, underlining the primary importance of the rate of assimilation per unit leaf area.
Little effect of salinity and of CO2 concentration was detected on the net photosynthetic rate of S. densiflora. In this regard, starch accumulation has been described as a possible cause of the reduction of a CO2 stimulation of photosynthesis during long-term elevated CO2 concentration levels (DeLucia et al., 1985). However, our results showed lower values of foliar C/N ratio at 700 ppm CO2. Yelle et al. (1989) found in Lycopersicon esculentum that acclimation to elevated CO2 concentration was not a result of starch accumulation but was instead related to decreased activity of ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco).
Contrastingly, De Souza et al. (2008) found that A of sugarcane decreased because of root growth limitation after 22 weeks at 720 ppm CO2, since it is known that root growth limitation can result in lower photosynthetic rates (Arp, 1991). Thus, roots of S. densiflora occupied 5% less of the volume of the pot for all treatments after three months, so pot size did not limit root growth.
On the other hand, different works have shown that one of the most consistent responses of C4 plant species to elevated atmospheric CO2 concentration is a decrease in Gs (Ainsworth and Rogers, 2007). In the case of S. densiflora this decrease was only recorded after 30 d and 90 d of treatment. Robredo et al. (2007) explained that increased Ci originated by the elevated CO2 concentration could promote partial stomatal closure, although the mechanism whereby stomata respond to the CO2 signal remains unknown. Nevertheless, higher Ci values of S. densiflora at elevated CO2 were also recorded after 7 d of treatment without there being a lower Gs, which could be explained by the lower A values.
By contrast, the levels of PEPC protein of S. densiflora leaves were high at elevated CO2 concentration. Intermediate salinity and CO2 concentration enhanced PEPC activity, which could also contribute to the acclimation of A to CO2 enrichment. On the other hand, the lower PEPC activity recorded at 171 mM NaCl and ambient CO2 concentration was counterbalanced by a higher activation of PEPC (see IC50 for malate). This response has previously been reported by Echevarría et al. (2001) and García-Mauriño et al. (2003), who found that PEPC-kinase activity of Sorghum increased in salt-treated plants. Contrary to this, Sankhla and Huber (1974) found that high concentrations of Na within the plants might limit the activity of PEPC. Nevertheless, PEPC activity of S. densiflora was not limited by the progressive accumulation of Na+ in root and shoots, with increasing external salinity, at either CO2 concentration treatment.
Accumulation of Na+ with increasing external salinity concentration was accompanied by the decrease of leaf K, Ca, and Mg levels. Similar results have been recorded for other halophytes (Khan et al., 2000; Redondo-Gómez et al., 2007). The higher mineral (ash) contents reported at ambient CO2 concentration could be accounted for by the higher leaf and root K, Ca, Mg, and Zn concentrations. Rogers et al. (1999) reported that plant nutrient concentrations often decline under elevated CO2 concentration, and this decline can be ascribed to the dilution effect caused by increases in biomass. In the case of S. densiflora, this decline could be also ascribed, to a great extent, to the dilution effect caused by the higher water content found at elevated CO2. Ainsworth and Rogers (2007) explained this decline by a reduction of leaf transpiration rate under elevated CO2 concentration, which may cause a lower flux of nutrient through the soil to the root surface, thereby reducing nutrient uptake. All these possibilities could explain the lower nutrient content recorded in S. densiflora under elevated CO2 concentration.
On the other hand, Randall and Bouma (1973) found that carbonic anhydrase (involved in photosynthesis, facilitating the diffusion of CO2 through the liquid phase of the cell to the chloroplast) demonstrated a lower efficiency at concentrations approaching CO2 saturation with Zn deficiency, suggesting that zinc deficiency impairs the biochemical capacity of the plant to fix CO2. Nonetheless, net photosynthesis of S. densiflora was not affected, instead a lower leaf Zn concentration was recorded at 700 ppm CO2.
There was evidence that elevated salinity and CO2 concentration affected the integrity or function of the photochemical apparatus in the long term, and there was an impact on chlorophyll concentrations in the leaves. Chlorophyll content was enhanced by salinity; although at an elevated CO2 concentration this enhancement was only recorded at 510 mM NaCl. According to Geissler et al. (2009), plants seemed to use the additional energy supply under an elevated CO2 concentration for increasing the investment in salinity tolerance mechanisms; for example, for reducing oxidative stress and water loss. The NaCl-induced increase in the chlorophyll level has been previously reported in S. densiflora (Castillo et al., 2005). Furthermore, as in our experiment, Chen et al. (1999) found that the impairment of the photosynthetic pigments was greater in crop plants at an elevated CO2 concentration. These findings seem to point to CO2 enrichment as an accelerator of pigment degradation and of leaf senescence (Munns, 1993). However, leaf N concentration was not lower at elevated CO2, so there was no link between leaf nitrogen and pigment concentration, as would be expected due to the fact that N is mostly contained in chlorophyll molecules (Torres Netto et al., 2005). This may be explained by the previously referred dilution effect, caused by the higher water content found at elevated CO2. In fact, the response of leaf N concentration and of water content to salinity and CO2 concentration were quite similar.
On the other hand, the response of A to salinity and to CO2 concentration did not track that of ΦPSII, since the quantum efficiency of PSII data suggest a long-term negative effect of high CO2 in the presence of salt. This disparity could have been caused by changes in the relative rates of CO2 fixation, photorespiration, nitrogen metabolism, and electron donation to oxygen (the Mehler reaction; Fryer et al., 1998). Redondo-Gómez et al. (2010) suggested that photorespiration and cyclic electron transport could be mechanisms to protect Arthrocnemum macrostachyum against an excess of radiation under high salinities. Both pathways can lead to the additional consumption of reducing equivalents and can thus function as sinks for excessive excitation energy (Asada, 1996). These two physiological processes could be relevant mechanisms to protect S. densiflora against an excess of radiation under high salinities, since, as in the case of A. macrostachyum, the relatively stable NPQ across the salinity range could indicate that salt does not cause an increase in thermal dissipation in the PSII antennae. By contrast, Chen et al. (1999) reported enhanced ΦPSII yield at 700 ppm CO2 without additional NaCl in the nutrient solution.
The maximum quantum efficiency of PSII photochemistry (Fv/Fm) did show a significant reduction at midday compared to dawn values. This midday depression of Fv/Fm was greater in the long term. It was also dependent on salinity treatment, in the same way as ΦPSII. The decreased Fv/Fm values at midday with the duration of treatment and the NaCl and CO2 concentration could have been caused by a lower proportion of open reaction centres (lower values of Fm), which could be attributed to a decrease in chlorophyll a content. The fact that photoinhibition was not more severe in salt-adapted plants, even when exposed to high light, suggests that they have mechanisms by which excess energy is dissipated safely (Qiu et al., 2003).
In summary, the comparison of growth and photosynthetic responses of S. densiflora has provided a new insight into the response to climatic change and rising atmospheric CO2 concentration in a competitive coastal invader, which experiences salinity levels as high as those present in seawater. Differences in growth rate over this range of salinity can be accounted for by the ability to develop and maintain an assimilatory surface area combined with an improvement of water relations at elevated CO2 concentration, rather than by variations in net photosynthetic rate. Salinity and CO2 concentration have a marked effect on the photochemical (PSII) apparatus in the long term, but not on photosynthesis. However, the absence of a CO2-enrichment effect on net photosynthesis might be explained by enhanced PEPC carboxylation capacity. By contrast, photosynthetic pigments seem to be adversely affected by elevated CO2 concentration in the presence of NaCl. Finally, our results suggest that the productivity of this invader might increase in a scenario of future increase in atmospheric CO2 concentration for plants growing at salinities ranging between 0 mM and 171 mM NaCl. Nevertheless, the salt stress that may be experienced by plants growing at 510 mM NaCl would offset this fertilizer effect.
Acknowledgments
We are grateful to Mr F Fernández-Muñoz for technical assistance. We also thank the Spanish Science and Technology and Environmental Ministries for their support (projects PCI2006-A7-0641 and 042/2007 Organismo Autónomo Parques Nacionales, respectively), and Seville University Greenhouse General Service for collaboration.
References
- Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell and Environment. 2007;30:258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Arp WJ. Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell and Environment. 1991;14:869–875. [Google Scholar]
- Asada K. Radical production and scavenging in the chloroplasts. In: Baker NR, editor. Photosynthesis and the environment. Dordrecht, Netherlands: Kluwer Academic Publishers; 1996. pp. 123–150. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castillo JM, Rubio-Casal E, Redondo S, Álvarez-López AA, Luque T, Luque C, Nieva FJ, Castellanos EM, Figueroa ME. Short-term responses to salinity of an invasive cordgrass. Biological Invasions. 2005;7:29–35. [Google Scholar]
- Chen K, Hu G, Keutgen N, Janssen MJJ, Lenz F. Effects of NaCl and CO2 enrichment on pepino (Solanum muricatum Ait). II. Leaf photosynthetic properties and gas exchange. Scientia Horticulturae. 1999;81:43–56. [Google Scholar]
- DeLucia EH, Sasek TW, Strain BR. Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynthesis Research. 1985;7:175–184. doi: 10.1007/BF00037008. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Gaspar M, Alvez da Silva E, Ulián EC, Waclawovsky AJ, Nishiyama MY, Dos Santos RV, Teixeira MM, Souza GM, Buckeridge MS. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant, Cell and Environment. 2008;31:1116–1127. doi: 10.1111/j.1365-3040.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Dukes JS, Mooney HA. Does global change increase the success of biological invaders? Trends in Ecology & Evolution. 1999;14:135–196. doi: 10.1016/s0169-5347(98)01554-7. [DOI] [PubMed] [Google Scholar]
- Echevarría C, García-Mauriño S, Alvarez R, Soler A, Vidal J. Salt stress increases the Ca2+-independent phosphoenolpyruvate carboxylase kinase activity in Sorghum leaves. Planta. 2001;214:283–287. doi: 10.1007/s004250100616. [DOI] [PubMed] [Google Scholar]
- Echevarría C, Pacquit V, Bakrim N, Osuna L, Delgado B, Arriodupont M, Vidal J. The effect of pH on the covalent and metabolic control of C4 phospho enolpyruvate carboxylase from Sorghum leaf. Archives of Biochemistry and Biophysic. 1994;315:425–430. doi: 10.1006/abbi.1994.1520. [DOI] [PubMed] [Google Scholar]
- Evans GC. The quantitative analysis of plant growth. Oxford, UK: Blackwell Scientific Publications; 1972. [Google Scholar]
- Fennane M, Mathez J. Nouveaux materiaux pour la flore de Maroc. Fascicule 3. Naturalia Montspieliensia. 1988;52:135–141. [Google Scholar]
- Ferris R, Sabatti M, Miglietta F, Mills RF, Taylor G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant, Cell and Environment. 2001;24:305–315. [Google Scholar]
- Figueroa ME, Castellanos EM. Vertical structure of Spartina maritimaand Spartina densiflorain Mediterranean marshes. In: Werger MJA, van der Aart PJM, During HJ, Verhoeven JTA, editors. Plant form and vegetation structure. The Hague, Netherlands: SPB Academic Publishing; 1988. pp. 105–108. [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology. 1998;116:571–580. doi: 10.1104/pp.116.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mauriño S, Monreal JA, Alvarez R, Vidal J, Echevarría C. Characterization of salt stress-enhanced phosphoenolpyrivate carboxylase kinase activity in leaves of Sorghum vulgare: independence from osmotic stress, involvement of ion toxicity and significance of dark phosphorylation. Planta. 2003;216:648–655. doi: 10.1007/s00425-002-0893-3. [DOI] [PubMed] [Google Scholar]
- Geissler N, Hussin S, Koyro HW. Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. Journal of Experimental Botany. 2009;60:137–151. doi: 10.1093/jxb/ern271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, von Caemmerer S, Ziska LH, Conroy JP. The growth response of C4 partial pressure: a reassessment. Plant, Cell and Environment. 2000;23:931–942. [Google Scholar]
- Hoagland D, Arnon DI. The water culture method for growing plants without soil. California Agricultural Experiment Station Bulletin. 1938;347:1–39. [Google Scholar]
- Hunt R, Causton DR, Shipley B, Askew AP. A modern tool for classical plant growth analysis. Annals of Botany. 2002;90:485–488. doi: 10.1093/aob/mcf214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Climatic change 2001: the scientific bases. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Khan MA, Ungar IA, Showalter AM. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. Stocksii. Annals of Botany. 2000;85:225–232. [Google Scholar]
- Kittelson PM, Boyd MJ. Mechanisms of expansion for an introduced species of cordgrass, Spartina densiflora, in Humboldt Bay, California. Estuaries. 1997;20:770–778. [Google Scholar]
- Krivosheeva A, Tao DL, Ottander C, Wingsle G, Dube SL, Öquist G. Cold acclimated and photoinhibition in Scots pine. Planta. 1996;200:296–305. [Google Scholar]
- Leakey ADB. Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proceedings of the Royal Society B-Biological Sciences. 2009;276:2333–2343. doi: 10.1098/rspb.2008.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen GM, Lamers J, Stroetenga M, Rozema J. Interactive effects of atmospheric CO2 enrichment, salinity and flooding on growth of C3 (Elymus athericus) and C4 (Spartina anglica) salt species. Vegetatio. 1993;104/105:379–388. [Google Scholar]
- Lenssen GM, van Duin WE, Jak P, Rozema J. The response of Aster tripolium and Puccinellia maritima to atmospheic carbon dioxide enrichment and their interactions with flooding and salinity. Aquatic Botany. 1995;50:181–192. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Cambrollé J, Luque T, Figueroa ME. Growth and photosynthetic responses to zinc stress of an invasive cordgrass. Spartina densiflora. Plant Biology. 2008b;10:754–762. doi: 10.1111/j.1438-8677.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Luque CJ, Castellanos EM, Davy AJ, Figueroa ME. Environmental limitations on recruitment from seed in invasive Spartina densiflora on a southern European salt marsh. Estuarine, Coastal and Shelf Science. 2008a;79:727–732. [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Silva J, Santos R, Figueroa ME. Effect of prolonged flooding on the invader Spartina densiflora Brong. Journal of Aquatic Plant Management. 2007;45:121–123. [Google Scholar]
- Munns R. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell and Environment. 1993;16:15–24. [Google Scholar]
- Nieva FJ, Diaz-Espejo A, Castellanos EM, Figueroa ME. Field variability of invading populations of Spartina densiflora Brongn. grown in different habitats of the Odiel marshes (SW Spain) Estuarine, Coastal and Shelf Science. 2001;52:515–527. [Google Scholar]
- Occhipinti-Ambrogi A. Global change and marine communities: alien species and climatic change. Marine Pollution Bulletin. 2007;55:342–352. doi: 10.1016/j.marpolbul.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Qiu N, Lu Q, Lu C. Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica. New Phytologist. 2003;159:479–486. doi: 10.1046/j.1469-8137.2003.00825.x. [DOI] [PubMed] [Google Scholar]
- Randall PJ, Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiology. 1973;52:229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Gómez S, Mateos-Naranjo E, Davy AJ, Fernández-Muñoz F, Castellanos E, Luque T, Figueroa ME. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Annals of Botany. 2007;100:555–563. doi: 10.1093/aob/mcm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Gómez S, Mateos-Naranjo E, Figueroa ME, Davy AJ. Salt stimulation of growth and photosynthesis in an extreme halophyte, Arthrocnemum macrostachyum. Plant Biology. 2010;12:79–87. doi: 10.1111/j.1438-8677.2009.00207.x. [DOI] [PubMed] [Google Scholar]
- Redondo-Gómez S, Wharmby C, Castillo JM, Mateos-Naranjo E, Luque CJ, de Cires A, Luque T, Davy AJ, Figueroa ME. Growth and photosynthetic responses to salinity in an extreme halophyte, Sarcocornia fruticosa. Physiologia Plantarum. 2006;128:116–124. [Google Scholar]
- Robredo A, Pérez-López U, Sainz de la Maza H, González-Moro B, Lacuesta M, Mena-Petite A, Muñoz-Rueda A. Elevated CO2 alleviated the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environmental and Experimental Botany. 2007;59:252–263. [Google Scholar]
- Rogers HH, Runion GB, Prior SA, Price AJ, Torbert HA. Effects of elevated atmospheric CO2 on invasive plants: comparison of Purple and Yellow Nutsedge (Cyperus rotundus L. and C. esculentus L. Journal of Environmental Quality. 2008;37:395–400. doi: 10.2134/jeq2007.0155. [DOI] [PubMed] [Google Scholar]
- Rogers HH, Runion GB, Prior SA, Torbert HA. Response of plants to elevated atmospheric CO2: root growth, mineral nutrition, and soil carbon. In: Luo Y, Mooney HA, editors. Carbon dioxide and environmental stress. San Diego, USA: Academic Press; 1999. pp. 215–244. [Google Scholar]
- Rozema J. Plant responses to atmospheric carbon dioxide enrichment: interactions with some soil and atmospheric condictions. Vegetatio. 1993;104/105:173–190. [Google Scholar]
- Rozema J, Dorel F, Janissen R, Lenssen G, Broekman R, Arp W, Drake BG. Effect of elevated atmospheric CO2 on growth, photosynthesis and water relations of salt marsh grass species. Aquatic Botany. 1991;39:45–55. [Google Scholar]
- Sankhla N, Huber W. Ecophysiological studies on Indian arid zone plants. IV. Effect of salinity and gibberellin on the activities of photosynthetic enzymes and 14CO2 fixation products in leaves of Pennisetum typhoides seedlings. Biochemie und Physiologie der Pflanzen. 1974;16:181–187. [Google Scholar]
- Torres Netto A, Campostrini E, Gonçalves de Oliveira J, Bressan-Smith RE. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Scientia Horticulturae. 2005;104:199–209. [Google Scholar]
- Tutin TG. Spartina Schreber. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea. Vol. 5. Cambridge, UK: Cambridge University Press; 1980. pp. 259–260. [Google Scholar]
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:377–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Yelle S, Beeson RC, Trudel MJ, Gosselin A. Acclimation of two tomato species to high atmospheric CO2. 2. Ribulose-1,5-biphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase. Plant Physiology. 1989;90:1473–1477. doi: 10.1104/pp.90.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]