Abstract
A full-length drought-responsive gene Ccrboh, encoding the respiratory burst oxidase homologue (rboh), was cloned in Citrullus colocynthis, a very drought-tolerant cucurbit species. The robh protein, also named NADPH oxidase, is conserved in plants and animals, and functions in the production of reactive oxygen species (ROS). The Ccrboh gene accumulated in a tissue-specific pattern when C. colocynthis was treated with PEG, abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), or NaCl, while the homologous rboh gene did not show any change in C. lanatus var. lanatus, cultivated watermelon, during drought. Grafting experiments were conducted using C. colocynthis or C. lanatus as the rootstock or scion. Results showed that the rootstock significantly affects gene expression in the scion, and some signals might be transported from the root to the shoot. Ccrboh in C. colocynthis was found to function early during plant development, reaching high mRNA transcript levels 3 d after germination. The subcellular location of Ccrboh was investigated by transient expression of the 35S::Ccrboh::GFP fusion construct in protoplasts. The result confirmed that Ccrboh is a transmembrane protein. Our data suggest that Ccrboh might be functionally important during the acclimation of plants to stress and also in plant development. It holds great promise for improving drought tolerance of other cucurbit species.
Keywords: Citrullus colocynthis, C. lanatus, drought stress, grafting, NAPDH oxidase, RBOH, watermelon
Introduction
Water deficit is considered to be the main environmental stress of plants and a major constraint of plant growth and productivity. Tolerance to drought stress is a complex phenomenon, comprising a number of physio-biochemical processes which are activated during different stages of plant development (Ramanjulu and Bartels, 2002; Wang et al., 2003). Production of ROS at the cell surface is one of the earliest events detected in the plant defence response. ROS can function as signalling molecules that mediate responses to various processes in plant cells such as development, pathogen defence, programmed cell death, and stomatal behaviour. Plants have evolved mechanisms of ROS generation, signalling, and removal during development, and biotic and abiotic stress (Apel and Hirt, 2004; Miller et al., 2009).
The respiratory burst oxidase homologue (rboh), also named NADPH oxidase, in mammalian neutrophils has two components located in the plasma membrane (gp91–phox and p22–phox), which interact with several cytosolic proteins (p47–phox, p67–phox, and the small G protein Rac) to become active (Wientjes and Segal, 1995). RBO homologues in the plant and animal kingdoms contain cytosolic FAD- and NADPH-binding domains and six conserved transmembrane helices. In addition, some include calcium-binding elongation factor (EF) hands (Sagi and Fluhr, 2006; Torres et al., 1998). The Arabidopsis genome contains ten members (Atrboh A–J) with two EF hands at the N terminus (Sagi and Fluhr, 2006). Function overlap between different rboh proteins has been observed (Torres et al., 2002; Kwak et al., 2003).
The gp91–phox homologues AtrbohD and AtrbohF from Arabidopsis, NtrbohD from Nicotiana tabacum, and tomato rboh were shown to be required for ROS accumulation in plant defence responses (Simon-Plas et al., 2002; Torres et al., 2002; Sagi et al., 2004; Miller et al., 2009; Pogany et al., 2009). ABA signal transduction is located upstream and downstream of ROS production. ROS is synthesized in response to exogenous ABA, and ROS mediates, at least in part, ABA responses including stomatal closure and gene expression (Pei et al., 2000; Desikan et al., 2001). The Arabidopsis genes (AtrbohD and AtrbohF) function in ROS-dependent ABA signalling for stomatal closure (Kwak et al., 2003). Ethylene also functions in regulating stomatal aperture, inducing stomatal closure dependent on H2O2 production in guard cells generated by AtrbohF (Desikan et al., 2006). These data suggest a complex signalling network with interaction between rbohs and other signalling molecules. In maize, a cross-talk between Ca2+ and ROS generated by NADPH oxidase is involved in the ABA signalling pathway leading to the induction of antioxidant enzyme activity and antioxidant metabolism (Jiang and Zhang, 2002, 2003). Water stress-induced ABA accumulation triggered the generation of ROS by NADPH oxidase, resulting in the induction of the antioxidant defence system against oxidative damage. Ca2+ functions upstream and downstream of ROS production in signal transduction in plants. Ogasawara et al. (2008) showed that Ca2+ binding and phosphorylation synergistically activate the ROS-producing enzyme activity of AtrbohD.
The family Cucurbitaceae includes several economically important cultivated species such as watermelon (Citrullus lanatus var. lanatus), melon (Cucumis melo L.), cucumber (C. sativus L.), squashes, pumpkins, and gourds (Cucurbita species). Watermelons are often grafted onto Cucurbita moschata, C. maxima, Benincasa hispida, or Lagenaria siceraria to impart levels of resistance to soil-borne pathogens (such as Fusarium oxysporum), low soil temperatures, salinity, and water stress (Chouka and Jebari, 1999; Lee, 1994; Yetisir et al., 2003, 2006; Yetisir and Sari, 2003). One species, C. colocynthis, in the genus Citrullus is widely distributed in the Sahara–Arabian desert areas and well adapted to drought stress (Dane et al., 2006). It is a potential rootstock to improve the drought tolerance of watermelon.
Since rboh proteins are important components of signalling pathways, the aim of this study was to isolate and identify the rboh gene from drought-tolerant C. colocynthis, and to gain information on its functions under stress conditions and during plant development. To our knowledge, this is the first report on the cloning of a full-length rboh gene and the analysis of transcriptional profiles of this gene in Citrullus species.
Materials and methods
Plant material and treatments
C. colocynthis seeds (No. 34 256) from Israel and C. lanatus var. lanatus seeds (‘AU Producer’) were sown in turface or soil in the greenhouse with a 14 h photoperiod at temperatures ranging from about 22 °C to 30 °C and ambient relative humidity. A half-strength Hoagland's nutrient solution (PhytoTechnology Laboratories, Shawnee Mission, KS) was used to irrigate plants daily after germination.
The seedlings with at least one true leaf were grafted using one cotyledon or the slant graft method (Davis et al., 2008). To facilitate rootstock and scion union, seedlings were placed in a shaded plastic tunnel with a humidifier (Fedders, Sanford, NC) to maintain 100% humidity and temperatures around 28 °C for a period of 7–10 d, followed by acclimation for 7 d to the natural conditions of the greenhouse.
Seedlings at the 5–6 leaf stage were placed in 20% PEG 8000 solution to induce drought. Leaf and root samples were collected at 0, 4, 8, 12, 24, and 48 h and immediately stored at –80 °C. Relative water content (RWC) was measured during PEG treatment according to the method by Smart and Bingham (1974). For other treatments, seedlings at the 5–6 leaf stage were treated with 100 μM ABA, 1 mM SA, 50 μM JA or 150 mM NaCl by spraying and/or irrigation. Shoots and roots were harvested at 8 h for ABA, 4 h for SA, 4 h for JA, and 24 h for NaCl treatment. Shoots and roots from untreated seedlings were harvested as controls. For gene expression during vegetative growth, samples were collected at 1, 3, 7, 14, 21, 30, and 60 d after germination.
RNA isolation and cDNA synthesis
RNA was extracted from roots or shoots according to the RiboPure kit protocol (Ambion, Austin, TX). To eliminate the remaining genomic DNA, RNA was treated with DNase I (Ambion) according to the manufacturer's instruction. cDNA was synthesized using RETROscript™ (Ambion).
Cloning of full-length Ccrboh cDNA using rapid amplification of cDNA ends (RACE)
The primers CcrbohFW1 and CcrbohRV1 (Table 1) used for the cloning of Ccrboh core cDNA fragment were designed and synthesized according to the conserved regions of the rboh gene sequences of Arabidopsis thaliana, Oryza sativa, Triticum sativum, Lycopersicon esculentum, and Nicotiana tabacum, deposited in GenBank. PCR analysis was initiated with the hot start method using a single-strand cDNA template and Taq DNA polymerase (New England BioLabs, Ipswich, MA). The PCR product was subcloned into pGEM-T Easy vector (Promega, Madison, WI) and sequenced.
Table 1.
Oligonucleotide primer sequences for Ccrboh cDNA cloning and relative quantitative real-time RT PCR
| Primer name | Sequence (5′–3′) |
| CcrbohFW1 | CCTGTTTGTCGAAACACCATCACT |
| CcrbohRV1 | GAATGATCCTTGTTCCCTAGTCAC |
| CcrbohRV2 | AATGGGCGATTGCGTGTAATCCC |
| CcrbohRV3 | AGGAACGATGACGCCTAATT |
| CcrbohFW2 | GGAGGAGCTCCTAATCCTAAGT |
| CcrbohFW3 | ATGAGACCTCACGAACCTTATTCTG |
| CcrbohRV4 | AGTGCGGTATGTGTCAACCTTCACC |
| CcrbohFW4 | AATTAGGCGTCATCGTTCCT |
| CcrbohRV2 | AATGGGCGATTGCGTGTAATCCC |
| CcrbohFW3 | ATGAGACCTCACGAACCTTATTCTG |
| CcrbohRV5 | AGTGGATGTTTTACGAGAGAAAT |
| ACTFW | CAACATACATAGCAGGCACA |
| ACTRV | TGACTGAGGCTCCACTCAAC |
RACE was performed according to the manual of the 5′-RACE System Version 2.0 and 3′-RACE System (Invitrogen, Carlsbad, CA). Gene-specific primers CcrbohRV1 and CcrbohRV2 for 5′-RACE, and CcrbohFW2 for 3′-RACE (Table 1) were generated based on the cloned conserved core cDNA sequences.
Sequences analysis
Amino acid sequences encoding rboh genes from Arabidopsis were chosen from the NCBI database. Multiple sequence alignment was carried out with CLUSTAL W at the default setting. Treeview software was used for displaying the phylogenetic trees, and pSORT was used to predict protein localization.
Relative quantitative (RQ) real-time RT-PCR
RQ real-time RT-PCR was carried out using an ABI 7500 RealTime PCR System and 7500 System software version 1.2.3 (Applied Biosystems or ABI, Foster City, CA). The C. colocynthis-specific actin gene (GH626171), used as the reference gene, was amplified in parallel with the target gene allowing normalization of gene expression and providing quantification. Primers were designed based on conserved regions, and primer sequences of the Ccrboh (CcrbohFW4 and CcrbohRV2) and actin (ACTFW and ACTRV) are listed in Table 1. Detection of RQ real-time RT-PCR products was done using the SYBR® Green PCR Master mix kit (Applied Biosystems) following the manufacturer's recommendations. Quantification of the relative transcript levels was performed using the comparative CT method. The induction ratio (IR) was calculated as recommended by the manufacturer and corresponds to 2−ΔΔCT, where
Relative quantification relies on the comparison between expression of a target gene versus a reference gene and the expression of same gene in the target sample versus the reference sample (Pfaffl, 2001).
Southern blot analysis
Genomic DNA isolated from C. colocynthis seeds (20 μg per sample) was digested with different restriction enzymes (HindIII, EcoRV, or XbaI 20 unit g−1 DNA) for 16 h at 37 °C, followed by separation on a 0.8% agarose gel. After electrophoresis, gels were washed with water and 10× SSC and blotted with Hybond N+ (Amersham Pharmacia Biotech, Piscataway, NJ) prewetted with 10× SSC. Hybridization was performed at 65 °C with Church buffer (1% BSA, 200 μM EDTA, 0.5 M sodium phosphate, 7% SDS) containing a 32P-labelled probe. Full-length cDNA of the Ccrboh gene as a probe was obtained by PCR using the following gene-specific primers: CcrbohFW3 and CcrbohRV4 (Table 1).
Green fluorescent protein (GFP) conjugated plasmid construction
The plasmid for protoplast transformation was generated using the Invitrogen Gateway system according to the manufacturer's instructions. Ccrboh DNA lacking a stop codon was amplified by PCR using CcrbohFW3 and CcrbohRV5 (Table 1), and subcloned into a TOPO vector (Invitrogen, Carlsbad, CA). The TOPO vector with the gene and pENTR 1A dual selection vector were cut by KpnI and NotI, and the cut pENTR vector and gene were ligated using T4 DNA ligase (Invitrogen). Entry clones containing the Ccrboh gene lacking a stop codon were transferred from the entry clone vector to the destination clone vector pEarleyGate 103 with GFP on C-terminal using the LR reaction (Earley et al., 2006).
Protoplast isolation and transformation
C. colocynthis cotyledons from soil-grown plants were excised, cut into 1 mm strips and immediately placed into an enzyme solution for overnight digestion in the dark. The enzyme solution which contained 2% cellulose R10, 0.5% macerozyme R10, 0.5% driselase, 2.5% KCl, 0.2% CaCl2, pH 5.7, was filter sterilized. After overnight incubation, leaf tissue was gently shaken for 30 min at 40 rpm to release leaf mesophyll protoplasts, followed by filtration through a 40 μm cell sifter to remove debris and centrifugation at 150 g to pellet the protoplasts. Protoplasts were washed twice with a washing solution (0.5 M mannitol, 4 mM MES pH 5.7, and 20 mM KCl) and re-centrifuged at 150 g. The protoplasts were suspended in washing solution on ice for electroporation.
Protoplasts were transformed in a manner essentially as described previously (Sheen, 1991; Rashotte et al., 2006). Electroporation was typically carried out with 1–2×105 protoplasts in 300 μl of wash solution and about 40–50 μg of plasmid DNA, and treated for electroporation at 300 V in a 0.1 mm electroporation cuvette using an Eppendorf Electroporator 2510. After overnight incubation in the dark, protoplasts were examined under Nikon Eclipse 80i epifluorescence microscope with a UV source. A standard UV filter was used in addition to 1 ng ml−1 of Hoechst 33342 dye initially to observe cells and to identify nuclei in intact cells as a measure of the cells’ viability. A GFP filter that blocks both chlorophyll fluorescence and Hoechst 33342 fluorescence was used to examine the localization of GFP fusion proteins. All photographs were taken with a Qimaging Fast 1394 digital camera and composed as a composite image in Adobe Photoshop CS3.
Results
Cloning and sequence analysis of the Ccrboh gene
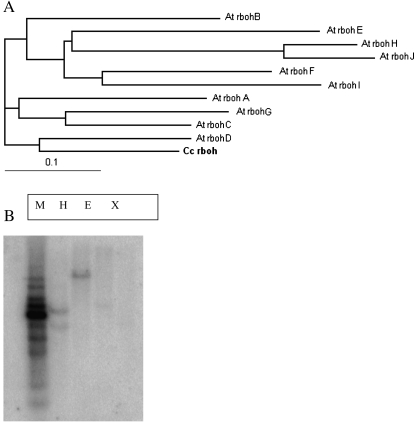
The cDNAs Ccrboh (EU580727) encoding respiratory burst oxidase protein was cloned from C. colocynthis and sequenced. Sequence analysis indicated that the full-length cDNA contains the 5′-UTR, the complete open reading frame (ORF), 3′-UTR and the Poly (A) tail. Ccrboh has a 2781 bp ORF encoding a protein of 926 amino acids. The BLASTp search utility identified the ATG translation start in Ccrboh as well as a Ca2+-binding motif of the EF-hand loop type occurring at the N-terminal. Six tentative transmembrane segments were predicted by pSORT. In Arabidopsis, the EF-hands are present in the third small exon of rbo homologues (Torres et al., 1998). The presence of the highly conserved motif in rboh proteins suggests a possible direct effect of Ca2+ on the function of rbohs in plants. It has been shown that the EF-hand motif in plant rbohs bind 45Ca2+ (Keller et al., 1998), and Ca2+ stimulates rboh to produce ROS in plasma membranes (Sagi and Fluhr, 2001; Heyno et al., 2008). The C-terminal of the Ccrboh protein contains other functional motifs such as the ferric reductase-like transmembrane component domain, the FAD-binding domain, and the NAD-binding domain which have greater overall sequence similarity to gp91phox in animals (Torres et al., 1998; Torres and Dangl, 2005). Phylogenetic analysis of Ccrboh and 10 rboh proteins from Arabidopsis showed that Ccrboh has high homology to the AtrbohD (Fig. 1A). Plant rbohs are structurally related to human NOX and are part of a large superfamily that arose early in evolution before the animal/plant divergence (Fluhr, 2009).
Fig. 1.
(A) Phylogenetic tree of Ccrboh and 10 Arabidopsis rbohs. Ccrboh (EACF05505), AtrbohJ (Q9LZU9), AtrbohI (Q9SUT8), AtrbohC (O81210), AtrbohD (Q9FIJ0), AtrbohA (O81209), AtrbohG (Q9SW17), AtrbohF (O48538), AtrbohE (O81211), AtrbohB (Q9SBI0), and AtrbohH (Q9FJD6). (B) Southern blot analysis of Ccrboh. Genomic DNA digested with HindIII (H), EcoRV (E), or XbaI (X), respectively, followed by hybridization using full-length gene as probe. M=1 kb DNA marker.
To investigate the genomic organization of the Ccrboh gene in C. colocynthis, genomic DNA was digested with HindIII, EcoRV, or XbaI, respectively, and hybridized with a probe, which was a full-length cDNA of the Ccrboh gene generated by PCR. The result showed that at least two hybridizing bands ranging from 2 kb to 10 kb were present in the first lane (digested with HindIII), and one band in the second (digested with EcoRV) and third lane (digested with XbaI) under high stringency conditions (Fig. 1B), indicating that Ccrboh potentially exists as one or two copies in the genome.
Expression profiles of the Ccrboh gene in C. colocynthis
Leaf RWC of C. colocynthis was measured to define the induction of the drought condition following PEG treatment. The leaf RWC in control C. colocynthis and watermelon was 90% and 95%, respectively. Upon treatment with PEG, the leaf RWC of both species decreased rapidly to 75% within 12 h. However, C. colocynthis gradually adjusted its leaf RWC to maintain an 80% level during treatment (Si et al., 2009), while the RWC of watermelon continually decreased to 68% resulting in the wilting of leaves. This indicates that C. colocynthis has some mechanism to cope with drought stress.
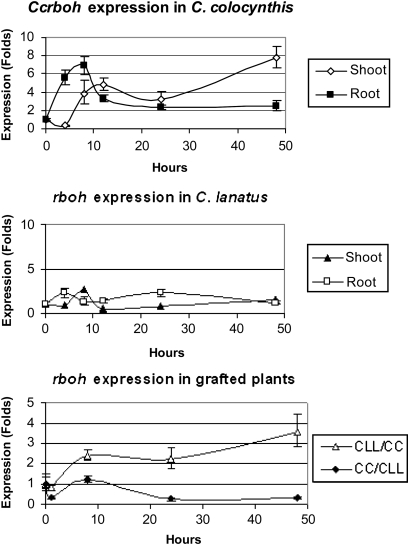
Ccrboh was induced in both roots and shoots (Fig. 2) following drought (PEG) treatment, but the induction in roots was 4 h earlier than in shoots. The highest induction level in roots was between 4 h and 8 h and decreased to control levels after 12 h, while it was highly induced in shoots after 12 h of treatment and continually increased up to 48 h. Although roots and shoots contain different sets of specialized cells, it is not known to what extent the stress response programmes differ between these tissues. The tissue-specific division of transcript distribution falls into three basic classes in Arabidopsis: expression throughout the plant (AtrbohD and F), in the roots (Atrboh A–G, I), or in a pollen-specific manner (Sagi and Fluhr, 2006; Fluhr, 2009).
Fig. 2.
Comparison of expression profiles of the rboh gene in the root and shoot of C. colocynthis and C. lanatus var. lanatus during drought (PEG) treatments and grafting. CLL/CC: C. lanatus var. lanatus grafted onto the C. colocynthis rootstock; CC/CLL: C. colocynthis grafted onto the C. lanatus var. lanatus rootstock. Gene expression was normalized by comparing ΔΔCt to control (0 h) (n=3).
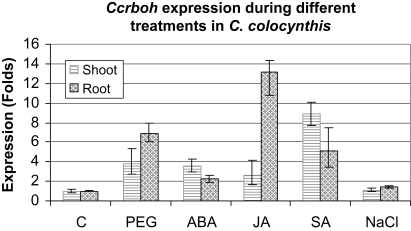
Ccrboh was also induced by ABA, JA, SA, but not NaCl in a tissue-specific pattern (Fig. 3). The results are similar to other reports, showing that application of ABA, IAA, or BA leads to the accumulation of rboh transcript (Kwak et al., 2003; Sagi et al., 2004). It is likely that rboh could function as a signal transponder for hormone action (Sagi et al., 2004). SA and JA act as local and systemic signal molecules in plant defence against pathogen attack and a complex interplay between SA and rboh occurs in the regulation of cell death (Torres et al., 2005; Pogany et al., 2009). Tissue-dependent variations in gene expression patterns under different abiotic stress conditions have been documented in many plant species (Kawasaki et al., 2001; Kreps et al., 2002; Liu and Baird, 2003; Baisakh et al., 2006). Regulation of the temporal and spatial expression patterns is an important part of the plant stress response.
Fig. 3.
Comparison of expression profiles of the Ccrboh gene in the root and shoot during different treatments in C. colocynthis. Gene expression was normalized by comparing ΔΔCt to control (0 h) (n=3).
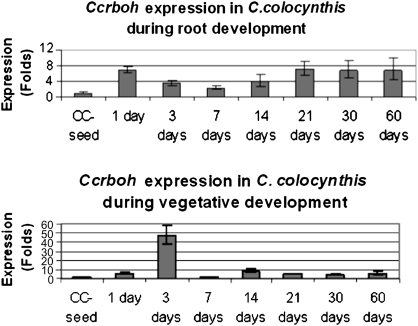
To understand how Ccrboh functions in plant development, the expression of the Ccrboh gene was analysed during root and vegetative growth (Fig. 4) using C. colocynthis seeds as a control. In roots, Ccrboh expression levels showed a 7-fold increase in 1-d-old roots, followed by a 3–4 fold increase in 3–14-d-old roots, and 7-fold induction in older seedlings. In shoots, Ccrboh expression levels increased 1 d after germination, and then dramatically increased up to 48-fold in the 3-d-old seedlings, followed by a reduction to 5–10 fold in 7–60-d-old seedlings. The results indicate that the Ccrboh gene also functions during root growth and leaf morphogenesis.
Fig. 4.
Comparison of expression profiles of the Ccrboh gene in the roots and shoots following days after germination in C. colocynthis (CC). Gene expression was normalized by comparing ΔΔCt to control (CC seeds) (n=3).
Expression analysis of Ccrboh in C. lanatus var. lanatus (watermelon) and grafted plants during drought
Watermelon plants, treated with 20% PEG 8000 to induce drought stress, were examined at 0, 4, 8, 12, and 48 h. Several pairs of primers were designed based on the conserved regions of rbohD. After testing, primers CcrbohFW4 and CcrbohRV2 (Table 1) were used to analyse rboh gene expression in two species. The rhoh mRNA transcript levels in watermelon did not change in roots and shoots during PEG treatment (Fig. 2). Interestingly, when watermelon was grafted onto a C. colocynthis rootstock, rboh was induced following 8 h of treatment and continually increased up to 48 h in watermelon scions (Fig. 2). The expression pattern in grafted plants [watermelon scion/C. colocynthis rootstock (CLL/CC)] was the same as that in non-grafted C. colocynthis, but the induction level was lower (Fig. 2). The Ccrboh gene expression level did not change in C. colocynthis grafted onto watermelon rootstock (CC/CLL) during drought (Fig. 2). Miller et al. (2009) reported that plants can mediate rapid cell to cell communication over a long distance which is dependent on the rbohD gene. This grafting experiment indicates that the rootstock is important for the regulation of genes in the scion as a result of long-distance signalling of ROS (Miller et al., 2009), ABA (Thompson et al., 2007), microRNA (Ruiz-Medrano et al., 1999), or some small proteins (Corbesier et al., 2007).
Subcellular localization of the Ccrboh protein
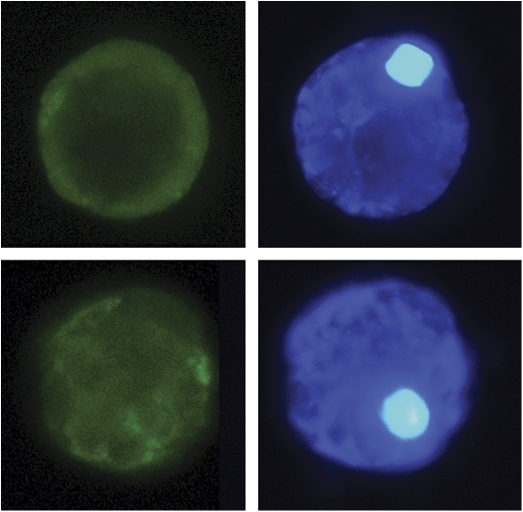
To address the subcellular localization of Ccrboh in living cells, a construct containing Ccrboh fused in-frame with GFP driven by the CaMV 35S promoter (35S::Ccrboh::GFP) was transiently expressed in leaf protoplasts. Hoechst 33342 dye was used to observe if protoplasts were intact and viable initially (Fig. 5, right panels). As a control the unconjugated GFP vector was transformed into protoplasts as shown in Fig. 5 (bottom), revealing cells that exhibit a diffused distribution of green fluorescence throughout the cell. By contrast, when GFP was fused with Ccrboh, the GFP signal was confined to the outermost region of the cell (Fig. 5, top), which could be followed under high magnification by focusing through the z-axis of the cell. This result is consistent with the localization of the Ccrboh protein to the plasma membrane, in agreement with results from previous studies using the cellular fractionation of plant tissues in tobacco (Sagi and Fluhr, 2001; Simon-Plas et al., 2002), and localization of NtrbohD on chemically distinct membrane microdomains called lipid rafts (as reviewed by Fluhr, 2009). It also confirmed the prediction by pSORT that the Ccrboh protein has six transmembrane segments, and is localized to plasma membrane with a probability of 52.2%.
Fig. 5.
The subcellular localization of the Ccrboh protein in C. colocynthis protoplasts. A representative example of 35S::Ccrboh::GFP fusion in a leaf mesophyll protoplast of C. colocynthis (top) compared with a protoplast transformed with the control vector pEarleyGate 103 (bottom). Protoplasts were visualized under UV light in the presence of Hoechst 33342 dye showing the subcellular position of the nucleus (right). Visualization using a GFP wavelength filter (left) blocks general background and Hoechst dye fluorescence thus revealing the location of Ccrboh to the outermost region of the cell (top) in contrast to the control with diffusely distributed fluorescence around the cell (bottom). (This figure is available in colour at JXB online.)
Discussion
Arabidopsis rboh genes are differentiated by their expression sensitivity to environmental inputs (Sagi and Fluhr, 2006). The most common abiotic inducers are nitrogen stress and conditions of anoxia/hypoxia, and AtrbohC–F are also induced by various biotic stresses. AtrbohD is identified as the major constitutively active form (Torres et al., 2002). Ccrboh was induced by hormones such as ABA, JA, and SA (Fig. 3). The results correspond with other reports, showing that application of ABA, IAA, or BA leads to the accumulation of rboh transcript (Kwak et al., 2003; Sagi et al., 2004). It is likely that rboh could function as a signal transponder for hormone action (Sagi et al., 2004; as reviewed by Mori et al., 2009). Hormones might regulate rboh in two ways. Hormones might affect rboh by generating ROS burst that may be mediated by functions in N-terminal regulatory regions. A more lasting and long-term effect of hormones may be achieved by the up-regulation of rboh levels. The diverse transcription patterns of rboh genes suggest that rboh proteins function in a broad range of growth, biotic, and abiotic stress responses (Torres and Dangl, 2005). It was recently discovered that the initiation and propagation of a systemic signal in Arabidopsis requires the function of the rbohD gene. Surprisingly, this systemic signal is independent of JA, SA or ethylene, hormones known to be involved in stress and wounding responses and ROS production (Miller et al., 2009). It is possible that ROS act downstream of these hormones.
A role for AtrbohC in root hair growth and in mediating the tip-focused Ca2+ gradient was discovered in Arabidopsis root hair cells (Foreman et al., 2003). AtrbohC transcript is present in the epidermis in the proximal regions of the meristem, in the elongation zone, in the differentiation zone, and in elongating root hairs. The AtrbohC mutant RHD2 (ROOT HAIR DEFECTIVE2) has short root hairs and stunted roots. Lowered rboh levels in the antisense lines of tomato were shown to have a profound influence on plant growth. Curled and inverted leaves, and abnormal flowers and fruits were observed in the mutants. Rboh induction responded to application of ABA, IAA, BA, and ACC (Sagi et al., 2004). All these observations indicate that rboh proteins function in a plethora of developmental effects in many plant organs, and imply the involvement of a range of hormones (Harir and Mittler, 2009; Mori et al., 2009). This is not surprising, because ROS are required for cell expansion during the morphogenesis of organs such as roots, leaves, and pollen (as reviewed by Carol and Dolan, 2006). A number of histone 3.3 variants were modulated in tomato rboh antisense mutants, implying that attenuation of rboh activity may influence chromosome structure, and eventually impinge on fundamental cellular processes (Sagi et al., 2004).
Domesticated watermelons have been selected for their productivity and quality. Domestication and breeding have contributed to an increased susceptibility to environmental stresses, diseases, and pests. For this reason, watermelons are often grafted onto different rootstocks. Root characteristics are of primary importance in determining stress tolerance in plant species (Fernández-García et al., 2002; Jensen et al., 2003; Ruiz et al., 2006). The identity and relative contribution of signals from the root during water deficit conditions remains controversial (Schachtman and Goodger, 2008), but rboh appears to be a highly regulated, sensitive, and versatile mediator of developmental and environmental signals (Miller et al., 2009). Since rboh gene induction was observed in watermelon grafted onto a C. colocynthis rootstock during drought, C. colocynthis can be considered as a potential rootstock for watermelon production or as a source for improving drought tolerance via gene manipulation. However, how the rootstock affects the fruit quality and how the grafted plants perform under stress conditions in the field environment needs to be investigated further.
Conclusions
In summary, a full-length cDNA clone, Ccrboh, encoding respiratory burst oxidase has been identified in C. colocynthis. Sequence analysis showed that Ccrboh is highly homologous to AtrbohD. Ccrboh transcript was expressed in a tissue-specific pattern following drought stress, ABA, JA, and SA treatment in C. colocynthis seedlings. Although no change in rboh transcript level was observed in watermelon under drought stress, the rboh gene was induced in the watermelon scion grafted onto a C. colocynthis rootstock. Ccrboh also functions in leaf morphogenesis because of changes in expression that were detected during vegetative growth. Transient expression of Ccrboh::GFP fusion protein in protoplasts confirmed that the Ccrboh protein is localized on the plasma membrane. Depending on the incoming signals from the plant, pathogen, or environment, the redox state might be altered such that it governs a transcriptional response aimed at maximizing plant fitness in a changing environment. All these results provide very useful information for the functional analysis of Ccrboh and its implications in plant genetic improvement.
Further studies, including characterizing the regulation of the signal transduction network that controls Ccrboh production and activity, as well as primary downstream targets modulated by ROS bursts, will extend our understanding of the biological role and function of rboh in plant development and growth, as well as the responses to various biotic and abiotic stresses.
References
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Baisakh N, Subudhi KP, Parami PN. cDNA-AFLP analysis reveals differential gene expression in response to salt stress in a halophyte Spartina alterniflora Loisel. Plant Science. 2006;170:1141–1149. [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. Journal of Experimental Botany. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Chouka AS, Jebari H. Effect of grafting of watermelon on vegetative and root development, production and fruit quality. Acta Horticulturae. 1999;492:85–93. [Google Scholar]
- Corbesier L, Vincent C, Jang S, et al. FT protein movement contributes to long-distance signalling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Dane F, Liu J, Zhang C. Phylogeography of the bitter apple, Citrullus colocynthis. Genetic Resources and Crop Evolution. 2006;54:327–336. [Google Scholar]
- Davis AR, Perkins-Veazie P, Sakata Y, et al. Cucurbit grafting. Critical Reviews in Plant Sciences. 2008;27:50–74. [Google Scholar]
- Desikan R, Mackerness S, Hancock JT, Neill S. Regulation of the Arabidopsis transcriptome by oxidase stress. Plant Physiology. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill S. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. The Plant Journal. 2006;47:907–916. doi: 10.1111/j.1365-313X.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- Earley K, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. Gateway compatible vectors for plant functional genomics and proteomics. The Plant Journal. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- Fernández-García N, Martínez V, Cerdá A, Carvajal M. Water and nutrient uptake of grafted tomato plants grown under saline conditions. Journal of Plant Physiology. 2002;159:899–905. [Google Scholar]
- Fluhr R. Reactive oxygen-generating NAPDH oxidases in plants. In: del Rio LA, Puppo A, editors. Reactive oxygen species in plant signalling. Berlin: Springer-Verlag; 2009. pp. 1–23. [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Harir Y, Mittler R. The ROS signalling network of cells. In: del Rio LA, Puppo A, editors. Reactive oxygen species in plant signalling. Berlin: Springer-Verlag; 2009. pp. 165–174. [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytologist. 2008;179:687–699. doi: 10.1111/j.1469-8137.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Rytter J, Detwiler EA, Travis JW, McNellis TW. Rootstock effects on gene expression patterns in apple tree scions. Plant Molecular Biology. 2003;493:493–511. doi: 10.1023/B:PLAN.0000019122.90956.3b. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defence in leaves of maize seedlings. Planta. 2002;215:1022–1030. doi: 10.1007/s00425-002-0829-y. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedings. Plant, Cell and Environment. 2003;26:929–939. doi: 10.1046/j.1365-3040.2003.01025.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S, Kawai K, Galbraith D, Bohnert JH. Gene expression profiles during the initial phase of salt stress in rice. The Plant Cell. 2001;13:889–905. doi: 10.1105/tpc.13.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner D, Dixon RA, Lamb C. A plant homologue of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. The Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps AJ, Wu Y, Chang H, Shu T, Wang X, Harper FJ. Transcriptome changes for Arabidopsis in response to salt, osmotic and cold stress. Plant Physiology. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS dependent ABA signalling in. Arabidopsis. European Molecular Biology Organization Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM. Cultivation of grafted vegetables. Part I. Current status, grafting methods and benefits. HortScience. 1994;29:235–239. [Google Scholar]
- Liu X, Baird WV. The ribosomal small-subunit protein S28 gene from Helianthus annuus (Asteraceae) is down-regulated in response to drought, high salinity, and abscisic acid. American Journal of Botany. 2003;90:526–531. doi: 10.3732/ajb.90.4.526. [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. The plant NADPH oxidase mediates rapid systemic signalling in response to diverse stimuli. Science Signalling. 2009;2 doi: 10.1126/scisignal.2000448. ra45. [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Uraji M. Integration of ROS and hormone signalling. In: del Rio LA, Puppo A, editors. Reactive oxygen species in plant signalling. Berlin: Springer-Verlag; 2009. pp. 25–42. [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, et al. Synerigistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. Journal of Biological Chemistry. 2008;14:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany M, von Rad U, Grun S, Dongo A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis– Alternaria pathosystem. Plant Physiology. 2009;151:1459–1475. doi: 10.1104/pp.109.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanjulu S, Bartels D. Drought- and desiccation-induced modulation of gene expression in plants. Plant, Cell and Environment. 2002;25:141–151. doi: 10.1046/j.0016-8025.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proceedings of the National Academy of Sciences, USA. 2006;103:11081–11085. doi: 10.1073/pnas.0602038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JM, Ríos JJ, Rosales MA, Rivero RM, Romero L. Grafting between tobacco plants to enhance salinity tolerance. Journal of Plant Physiology. 2006;163:1229–1237. doi: 10.1016/j.jplph.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 1999;126:4405–4419. doi: 10.1242/dev.126.20.4405. [DOI] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologues impinge on wound responsiveness and development in Lycopersicon esculentum. The Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Goodger JQD. Chemical root to shoot signalling under drought. Trends in Plant Science. 2008;13:281–287. doi: 10.1016/j.tplants.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Sheen J. Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. The Plant Cell. 1991;3:225–245. doi: 10.1105/tpc.3.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Zhang C, Meng S, Dane F. Gene expression changes in response to drought stress in Citrullus colocynthis. Plant Cell Reports. 2009;28:997–1009. doi: 10.1007/s00299-009-0703-5. [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein J. The plasma membrane oxidase Ntrboh is responsible for AOS production in elicited tobacco cells. The Plant Journal. 2002;31:137–147. doi: 10.1046/j.1365-313x.2002.01342.x. [DOI] [PubMed] [Google Scholar]
- Smart RE, Bingham GE. Rapid estimates of relative water content. Plant Physiology. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Mulholland BJ, Jackson AC, Mckee JMT, Hilton HW, Symond RC, Sonneveld T, Burbidge A, Stevenson P, Taylor LB. Regulation and manipulation of ABA biosynthesis in roots. Plant, Cell and Environment. 2007;30:67–78. doi: 10.1111/j.1365-3040.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) The Plant Journal. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defence response. Proceedings of the National Academy of Sciences, USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wientjes FB, Segal AW. NADPH oxidase and the respiratory burst. Seminars in Cell Biology. 1995;6:357–365. doi: 10.1016/s1043-4682(05)80006-6. [DOI] [PubMed] [Google Scholar]
- Yetisir H, Sari N, Yucel S. Rootstock resistance to Fusarium wilt and effect on watermelon fruit yield and quality. Phytoparasitica. 2003;31:163–169. [Google Scholar]
- Yetisir H, Sari N. Effects of different rootstock on plant growth, yield and quality of watermelon. Australian Journal of Experimental Agriculture. 2003;43:1269–1274. [Google Scholar]
- Yetisir H, Caliskan ME, Soylu S, Sakar M. Some physiological and growth responses of watermelon [ Citrullus lanatus (Thunb.) Matsum. And Nakai] grafted onto Lagenaria siceraria to flooding. Environmental and Experimental Botany. 2006;58:1–8. [Google Scholar]