Abstract
Leaves of many evergreen angiosperm species turn red under high light during winter due to the production of anthocyanin pigments, while leaves of other species remain green. There is currently no explanation for why some evergreen species exhibit winter reddening while others do not. Conditions associated with low leaf water potentials (Ψ) have been shown to induce reddening in many plant species. Because evergreen species differ in susceptibility to water stress during winter, it is hypothesized that species which undergo winter colour change correspond with those that experience/tolerate the most severe daily declines in leaf Ψ during winter. Six angiosperm evergreen species which synthesize anthocyanin in leaves under high light during winter and five species which do not were studied. Field Ψ, pressure/volume curves, and gas exchange measurements were derived in summer (before leaf colour change had occurred) and winter. Consistent with the hypothesis, red-leafed species as a group had significantly lower midday Ψ in winter than green-leafed species, but not during the summer when all the leaves were green. However, some red-leafed species showed midday declines similar to those of green-leafed species, suggesting that low Ψ alone may not induce reddening. Pressure–volume curves also provided some evidence of acclimation to more negative water potentials by red-leafed species during winter (e.g. greater osmotic adjustment and cell wall hardening on average). However, much overlap in these physiological parameters was observed as well between red and green-leafed species, and some of the least drought-acclimated species were red-leafed. No difference was observed in transpiration (E) during winter between red and green-leaved species. When data were combined, only three of the six red-leafed species examined appeared physiologically acclimated to prolonged drought stress, compared to one of the five green-leafed species. This suggests that drought stress alone is not sufficient to explain winter reddening in evergreen angiosperms.
Keywords: Anthocyanin, carbohydrates, drought, evergreen, osmolarity, photosynthesis, sugars, winter, water relations
Introduction
The question of a functional significance of anthocyanin pigments in leaves has received substantial attention in the recent literature (see reviews by Chalker-Scott, 1999; Manetas, 2006; Archetti et al., 2009). Comparatively little attention has been given to the question of why only certain species change leaf colour from green to red during certain ontogenetic stages or seasons while others do not. During winter, the leaves of many evergreen angiosperms turn a range of red to purple colours in response to high sunlight exposure, due to the synthesis of anthocyanin pigments (Oberbauer and Starr, 2002; Hughes and Smith, 2007; Kytridis et al., 2008). In some species, leaf colour change may be winter-transient, with leaves metabolizing anthocyanins to become green again with the return of springtime warming. Leaves of other winter-red species senesce while still red at winter's end, concomitant with a flush of new, green leaves. By contrast, other evergreen angiosperms maintain leaves that are entirely green throughout the winter. Many of these winter-green species do synthesize anthocyanins in other tissues or during different ontogenetic stages, such as in juvenile leaves, flowers, stems, roots, senescing leaves, and/or in response to pathogen infection. Their lack of anthocyanin in winter leaves suggests that anthocyanins are not beneficial for these species during the winter season. However, this assumption remains untested, and why some evergreen species synthesize anthocyanin in winter leaves, while others do not, is currently unknown (Hughes and Smith, 2007).
An explanation for redness versus greenness during winter is complicated by a lack of consensus among plant physiologists regarding the physiological function of anthocyanins in leaf tissues (see reviews by Manetas, 2006; Archetti et al., 2009). Most research seeking to determine a functional role of anthocyanins in evergreen leaves has focused on their putative roles in photoprotection (Hughes et al., 2005; Hughes and Smith, 2007; Kytridis et al., 2008). Winter leaves are especially vulnerable to high light stress, as low temperatures reduce the rate at which leaves may process sunlight for photosynthesis, thereby resulting in an imbalance of energy capture versus processing. This imbalance may lead to an increase in light energy that is transferred from chlorophyll to oxygen, resulting in the production of reactive oxygen species (ROS) and tissue damage (Powles, 1984; Hüner et al., 1998; Adams et al., 2004). Anthocyanins are thought to minimize photo-oxidative damage by either absorbing green light, thereby reducing the amount of light absorbed by photopigments (Feild et al., 2001; Lee and Gould, 2002; Hughes et al., 2005), and/or through neutralizing ROS directly as antioxidants (Gould et al., 2002; Nagata et al., 2003; Kytridis and Manetas, 2006). The idea that winter redness reflects an increased need for photoprotection has been supported in some studies (Kytridis et al., 2008), but not others (Hughes and Smith, 2007). Much evidence also exists counter to a photoprotective function in senescing (Lee et al., 2003), young (Dodd et al., 1998; Manetas et al., 2003; Karageorgou and Manetas, 2006), and mature (Kyparissis et al., 2007; Kytridis et al., 2008) leaves, rendering additional explanations for winter colour change timely and necessary.
In addition to the well-described relationship between anthocyanins and high light stress, there also exists some correlative evidence for a relationship between anthocyanins and osmotic stress (Chalker-Scottt, 1999, 2002). Specifically, anthocyanin synthesis is known to be inducible under high salinity (Dutt et al., 1991; Ramanjulu et al., 1993; Kaliamoorthy and Rao, 1994; Eryilmaz, 2006), drought (Spyropoulos and Mavrommatis, 1978; Balakumar et al., 1993; Sherwin and Farrant, 1998; Yang et al., 2000), and sugar treatments (Sakamoto et al., 1994; Suzuki, 1995; Tholakalabavi et al., 1997). Furthermore, species with high levels of foliar anthocyanin seem to be common in environments characterized by low soil moisture (Spyropoulos and Mavormmatis, 1978), and are more tolerant of drought conditions (Diamantoglou et al., 1989; Knox, 1989; Beeson, 1992; Paine et al., 1992). Because winter conditions are often accompanied by environmental and physiological factors that promote drought stress [e.g. low vapour pressure deficit (VPD) of air, low soil moisture, freezing of apoplastic water in leaves, and reduced hydraulic conductivity of xylem due to freeze–thaw embolisms], it is possible that anthocyanin synthesis during winter may correspond with relative differences in water stress in some species compared to others. Indeed, evergreen species are known to differ significantly in vulnerability to water stress during the winter months, due to differences in xylem cavitation, solute accumulation, cell wall hardening, freezing damage, and transpirational and cuticular water loss (Davis et al., 1999; Uemera and Steponkus, 1999; Taneda and Tateno, 2005). Because anthocyanin synthesis is known to be inducible by lower leaf water potentials, perhaps those species experiencing the greatest leaf water deficits during the day (due to any combination of the above) would correspond with those that synthesize anthocyanin. Within a functional context, a light-attenuating or antioxidant function of anthocyanin would be suitable under such conditions, as low leaf water potentials have been linked with the degradation of chlorophyll (Eryilmaz, 2006), increased photorespiration, and an increase in free radicals (Halliwell and Gutteridge, 1986; Xiong and Zhu, 2002), resulting in damage to structural and functional proteins, membrane lipids, and nucleic acids (Fridovich, 1986; Smirnoff, 1998; Xiong and Zhu, 2002).
The objective of this study was to test the hypothesis that species which synthesize anthocyanin during winter are those which experience (i.e. tolerate) the most extreme declines in daily water potentials. First, this hypothesis was tested directly by measuring seasonal predawn and midday water potentials (Ψ) in the field, and, secondly, by examining cell characteristics indicative of acclimation to prolonged water stress (e.g. osmotic adjustment and cell wall hardening).
Materials and methods
Six winter-red evergreen angiosperm species and five winter-green species were measured for field leaf Ψ to determine whether winter-red species had the greatest declines in leaf Ψ during the day. Pressure–volume methodology was also carried out on these species in the laboratory to assess relative physiological acclimation to water stress (e.g. osmotic adjustment and cell wall hardening), to determine whether red-leafed species exhibited physiological adjustments indicative of acclimation to prolonged drought stress.
Sites and species
Winter red-leafed species included: Galax urceolata (Poir.) Brummitt, Lonicera japonica (Thunb.), Gaultheria procumbens (L.), Leucothoe fontanesiana (Steud.) Sleumer, Hedera helix (L.), Rhododendron spp. (a horticultural azalea); winter green-leafed species included: Vinca minor (L.), Rhododendron catawbiense (Michx.), Kalmia latifolia (L.), Rhododendron maximum (L.), and Rhododendron spp. (a horticultural azalea). Study plants were mature individuals growing in sun-exposed embankments 5–20 m from roadsides in Jonas Ridge, NC, USA (35°57′20′′ N, 81°53′55′′ W; altitude: c. 1200 m) on south- or south-east-facing sites receiving >6 h full sunlight (i.e. >1350 μmol m−2 s−1 on a horizontal surface at solar noon) per day during both summer and winter months. Measurements were taken on clear sunny days, on sun-exposed, south-facing, first-year leaves. Detailed descriptions of most of these species are given in Hughes and Smith (2007). Most growth forms used were vines and herbs, in which case leaves were randomly sampled. In the case of shrubs, first-year leaves (i.e. apical leaves for all Rhododendron spp. and K. latifolia) were sampled at roughly 1–1.5 m above the ground. Field temperatures were derived from a local field station, approximately 8 km from the study site, archived online at http://www.wunderground.com/weatherstation/WXDailyHistory.asp?ID = KNCCROSS1.
Field Ψ measurements
Leaf water potentials (Ψ) were measured on four winter days: 14 December 2007 (max: 13 °C; min: 6 °C), 17 January 2008 (max: 3 °C; min: –3 °C), 15 February 2008 (max: 14 °C; min: 0 °C), and 2 March 2008 (max: 13 °C; min: –4 °C), and one autumn day before leaves had changed colour, 30 September 2008 (max: 21 °C; min: 6 °C). Since V. minor and L. japonica (green and red-leafed species, respectively) lacked petioles, stem Ψ with five attached leaves were substituted. First-year, sun-exposed leaves or stems from at least five separate individuals of each species were excised in the field at predawn (05.00–07.00 h) and midday (11.00–13.00 h, kept in plastic bags from which air had been removed, and stored on ice until measurement within 4 h. Ψ values were derived using a Scholander-type pressure chamber (Model 1000, PMS Instrument Company, Corvallis, OR) with nitrogen gas (Turner, 1981). Field measurements were compared with measurements after 4 h storage on ice, and no significant changes in Ψ were observed. It should be noted that measurements on leaves that still appeared to be frozen (i.e. initial Ψ values <–6 MPa, with Ψ becoming substantially less negative after 2–5 min of warming) were not used in the analyses. However, these potentially frozen leaves were only observed during the January predawn measurement of H. helix and G. urceolata. Because freezing could not be empirically verified for these leaves, statistical tests were run both with and without ‘frozen’ leaf values. Statistics were also run with and without the January measurement day entirely, as, even when frozen data were excluded, predawn Ψ of all species were suspiciously low (with Ψ for half of the species being more negative than the subsequent midday measurements). Unfortunately, because no Ψ measurements were made during the previous day, it was not possible to determine whether these low predawn values were due to an inability to recharge (which can be caused by a variety of factors known to limit water uptake under freezing or near-freezing temperatures), further declines in Ψ during the night, or measurement error.
Pressure–volume curves
Pressure–volume curves were plotted for each species using leaf or stem material excised one month before colour change (October) and one month after (December). First-year leaves were used in all cases. Three to five leaves were excised from separate individuals in the field, stored in sealed plastic bags, and transported on ice. Petioles were recut underwater, and leaves were hydrated overnight. The following day, pressure–volume curves were derived using methodology described in Turner (1981). Briefly, leaf Ψ was measured periodically as leaves transpired freely; leaf mass was determined immediately following each measurement. At least five points on the curve were derived for each leaf, and at least five points on the line following turgor loss. Dry mass was determined following completion of the curve by drying leaves in an oven at 80 °C until a constant mass was achieved. Water relations parameters derived from graphical and linear regression analyses included: osmotic potential at full turgor (Ψπ,100), osmotic potential at the turgor loss point (Ψπ,0), relative water content at the turgor loss point (%RWC0), symplastic water fraction (SWF), and the bulk modulus of elasticity (ε) between 95% and 98% RWC (Turner, 1981).
Leaf gas exchange
Maximum photosynthesis, Amax; stomatal conductance, gs; and transpiration, E, were measured from 4 December 2005 until 4 March 2006, and between 15–17 December 2007. Only first-year leaves under full ambient sunlight (>1350 μmol m−2 s−1) were measured at midday (11.00 h until 15.00 h) on warm days (daily high temperature >18 °C), in order to obtain maximum winter gas exchange values. The on-board CO2 mixer was set at 400 ppm, and VPD was adjusted to ambient conditions (c. 2.3). Plants were sampled via a standard random-walk procedure. A Li-Cor model Li-6400 (Li-Cor Inc., Lincoln, NE, USA) equipped with an LED chamber (model Li-6400-02B) was used to measure leaf gas exchange, with the LED light source set to 1500 μmol m−2 s−1 (simulating high light ambient conditions). Chamber temperature and relative humidity were matched to ambient conditions.
Sugars
Sugar concentrations in leaves were determined by High Pressure Liquid Chromatography (HPLC), using a Waters Alliance 2695 system. Two hundred milligrams of freeze-dried leaf tissue was weighed into a 10 ml disposable borosilicate test tube. Four millilitres of deionized water was added and the test tube was shaken at 350 rpm on an orbital shaker for 30 min. Standard reagents of sucrose, glucose, and fructose were obtained from Sigma Aldrich and were dissolved in distilled, deionized water to a concentration of 3.0 mg ml−1. Subsequent dilutions of the stock were prepared to 1.5, 0.9, and 0.3 mg ml−1 for 4-point quadratic calibration curves. Separations were carried out on a 7×53 mm Altech Prevail Carbohydrate ES Rocket column maintained at 50 °C, using an isocratic flow of 2.0 ml min−1, an injection volume of 2 μl, and an analysis time of 6 min.
The mobile phase consisted of 75% acetonitrile and 25% water. Sugars were detected with a Waters 2420 evaporative light scattering detector (ELSD) with a drift tube temperature of 50 °C; N2 as the nebulizer gas at 50 psi; and the nebulizer heater set to 40%.
Statistics
All data except sugar analyses were transformed by log10 for normality (determined as P >0.05 by the Shapiro–Wilks test). The association between leaf colour and predawn and midday Ψ were assessed for each measurement month separately using a random-effects, nested MANOVA with identity contrast (with species nested within colour, and species being the random effect). The change in winter Ψ between predawn and midday was calculated for each species as (average winter predawn Ψ – average winter mid-dawn Ψ); red and green species values were pooled and compared using a one-tailed Student's t test with unequal variance. The effects of leaf colour on Ψπ,100, Ψπ,0, RWC0, SWF, and ε were analysed using a nested, random-effects MANOVA with identity contrast. The effects of leaf colour on sucrose, glucose, fructose, and total soluble sugars were analysed using a nested standard least squares test for each sugar type separately. Significance was determined as P <0.05 for all tests. Seasonal comparisons (i.e. summer versus winter) for soluble sugars and pressure–volume curve measurements for individual species were compared using a one-tailed Student's t test with equal variance. Winter gas exchange parameters (photosynthesis, stomatal conductance, and transpiration) for red and green-leafed species were compared by random-effects, nested MANOVA.
Results
Seasonal Ψ
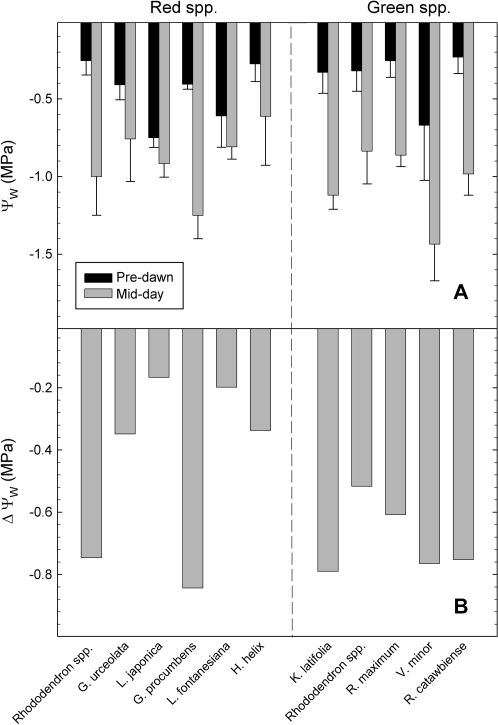
During September (before colour change had occurred), summer green leaves of winter-red species had significantly lower predawn Ψ compared with those of perennially green-leafed species (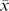 =–0.45 MPa for red, –0.38 MPa for green; P=0.04); during midday the reverse was observed—leaves of green-leafed species had significantly lower Ψ than those of red-leafed species (
=–0.45 MPa for red, –0.38 MPa for green; P=0.04); during midday the reverse was observed—leaves of green-leafed species had significantly lower Ψ than those of red-leafed species (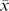 =–0.88 MPa for red, –1.03 MPa for green; P <0.01) (Fig. 1A). There was no significant difference in mean change in Ψ between predawn and midday in September between the two groups (P >0.05) (Fig. 1B).
=–0.88 MPa for red, –1.03 MPa for green; P <0.01) (Fig. 1A). There was no significant difference in mean change in Ψ between predawn and midday in September between the two groups (P >0.05) (Fig. 1B).
Fig. 1.
(A) Mean predawn and midday summer water potentials for species that either turn red (left half) or remain green (right half) during winter. (B) Delta water potential between predawn and midday. Bars represent means of 5–10 replicates; error bars represent standard deviation. Measurements were derived on 30September, 2008 (High: 21 °C, Low 5 °C).
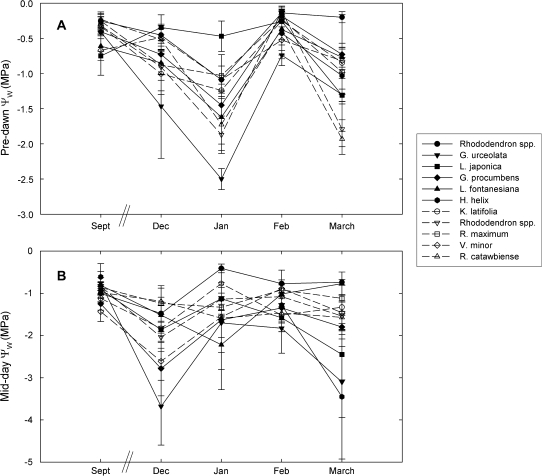
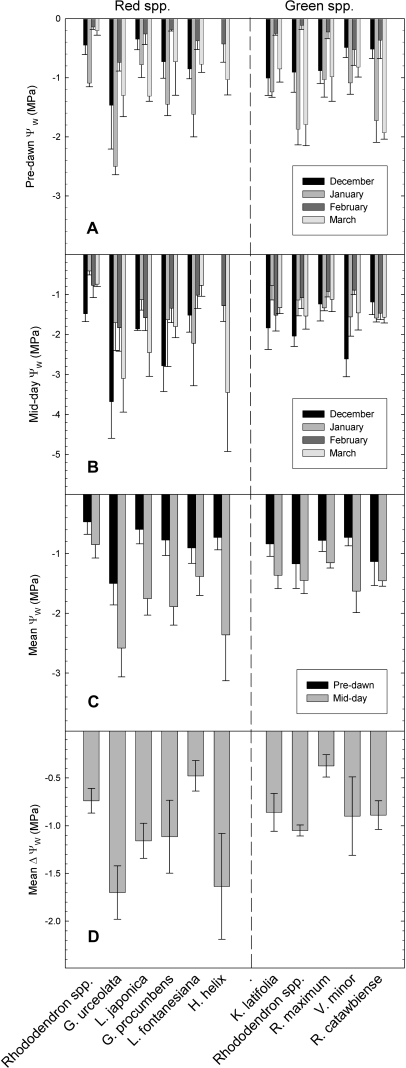
Winter predawn Ψ in December, January, and February showed no difference between red and green-leafed species (P=0.56, 0.25, and 0.52, respectively); during March, green-leafed species had significantly lower predawn Ψ compared to red (P <0.0001) (Figs 2, 3). When data for all winter months were pooled, red and green-leafed species did not significantly differ with regards to predawn Ψ (P=0.24). During midday, red-leafed species had significantly lower Ψ values compared to green-leafed species during December, January, and March (P <0.0001 for all) but not during February (P=0.37). When all data for the winter months were pooled, red-leafed species had significantly lower midday Ψ values compared to green-leafed species (P <0.0001). There was no significant change in daily Ψ between red and green-leafed species in December, January, or February (P=0.15, 0.48, 0.35). In March, red-leafed species had a significantly greater mean decline in Ψ compared to green-leafed species (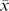 =1.2 MPa for red, 0.13 MPa for green; P=0.01). When all winter months were pooled, red-leafed species showed marginally greater decline in mean Ψ (P=0.09) (Fig. 3D).
=1.2 MPa for red, 0.13 MPa for green; P=0.01). When all winter months were pooled, red-leafed species showed marginally greater decline in mean Ψ (P=0.09) (Fig. 3D).
Fig. 2.
Mean predawn (A) and midday (B) water potential values of red-leafed species (solid lines, black symbols) and green-leafed species (dashed lines, white symbols) from September through March. Note that during September, leaves of all species were green. Points represent means of 5–10 replicates; error bars represent standard deviation. For dates and temperature details, refer to ‘Field water potential measurements’ in the Materials and methods.
Fig. 3.
Winter water potential values for red (left half of graphs) and green-leafed species (right half). Monthly mean predawn (A) and midday (B) water potentials; (C) average winter predawn and midday water potential values; (D) average delta water potentials between predawn and midday. Bars represent means of 5–10 replicates; error bars depict standard deviation (A, B, D) and standard error (C). For dates and temperature details, refer to ‘Field water potential measurements’ in the Materials and methods.
Most species had significantly lower predawn and midday Ψ during winter compared to summer (P < 0.05) with exceptions including the winter green-leafed V. minor, which had similar predawn and midday Ψ values during summer and winter (P=0.45 for predawn; 0.16 for midday); L. japonica, which had significantly less negative predawn Ψ during winter compared to summer (P=0.04); and the red-leafed Rhododendron sp., which had similar midday Ψ values between summer and winter (P=0.14) (Fig. 2).
Including potentially ‘frozen’ leaf Ψ measurements for H. helix and G. urceolata in January resulted in red-leafed species having significantly more negative predawn values compared to green-leafed species (with ‘frozen’ data, P=0.0001; without, P=0.25). However, when all winter measurements were pooled, inclusion or exclusion of the ‘frozen’ tissue did not affect the overall significance of predawn comparisons (without ‘frozen’ tissue: P=0.24; with P=0.77). Also, the inclusion or exclusion of these data did not affect the statistical significance of the mean change in Ψ between predawn and midday during January (P=0.48 both with and without ‘frozen’ data), or when all winter months were pooled (P=0.09 both with and without). Inclusion or exclusion of the January data for all species (as measurement error might have been responsible for very low predawn values observed in general) also did not affect the overall results (red versus green-leaf predawn Ψ with January data: P=0.83; without January data: P = 0.21; midday Ψ with January data: P <0.0001; without: P=0.0012).
Pressure–volume curves
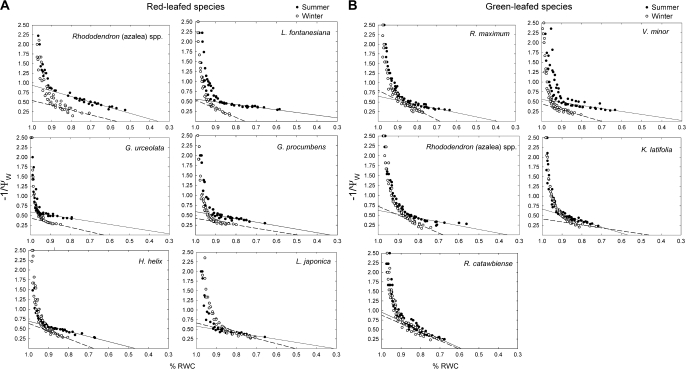
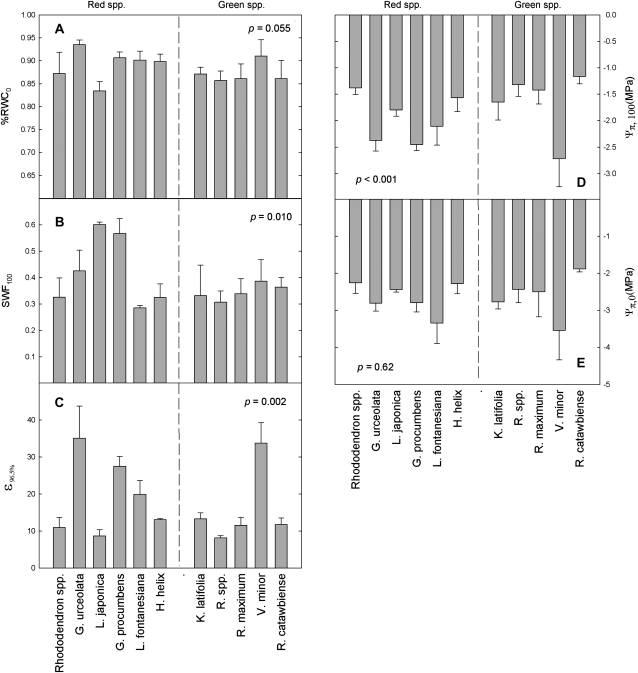
Pressure–volume curves revealed no significant difference in Ψπ,100 of summer leaves of green versus red species (P=0.29), but during winter, Ψπ,100 of red-leafed species were more negative (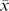 =–1.6 MPa for green and –2.0 MPa for red; P <0.01); Figs 4, 5D; Table 1. SWF at full turgor, and bulk modulus of elasticity at 96.5% RWC were significantly higher for red-leafed species compared to green during both summer (SWF=0.57 MPa for green and 0.66 for red; ε=12.9 MPa for green and 16.5 for red; P <0.05 in both cases) and winter (SWF=0.34 and 0.42 for green and red; ε=15.3 MPa and 19.1 MPa for red and green, respectively; P <0.01 for both).
=–1.6 MPa for green and –2.0 MPa for red; P <0.01); Figs 4, 5D; Table 1. SWF at full turgor, and bulk modulus of elasticity at 96.5% RWC were significantly higher for red-leafed species compared to green during both summer (SWF=0.57 MPa for green and 0.66 for red; ε=12.9 MPa for green and 16.5 for red; P <0.05 in both cases) and winter (SWF=0.34 and 0.42 for green and red; ε=15.3 MPa and 19.1 MPa for red and green, respectively; P <0.01 for both).
Fig. 4.
(A, B) Seasonal pressure–volume curves for winter red-leafed (A) and green-leafed (B) angiosperm evergreen species. Open circles with dashed lines represent winter measurements (after leaf colour change had occurred), solid circles represent summer measurements (prior to leaf colour change). Curves were derived from leaves of 3–5 separate individuals.
Fig. 5.
Data derived from winter pressure–volume curves. Left column, from top to bottom: (A) per cent relative water content at turgor loss point, (B) symplastic water fraction at full turgor, and (C) bulk modulus of elasticity between 95% and 98% RWC. Right column, from top to bottom: (D) osmotic potential at full turgor, (E) osmotic potential at turgor loss point. Bars represent means of 3–5 replicates, error bars are standard deviation.
Table 1.
Data summary for measurements derived from pressure–volume curves and sugar analyses.
| Ψπ,100 (MPa) | Ψπ,0 (MPa) | % RWC0 | SWF | ε96.5% (MPa) | Glucose (mg g−1) | Fructose (mg g−1) | Sucrose (mg g−1) | Total sugars (mg g−1) | ||
| *Rhododendron sp. | Summer | –1.0 (0.1) a | –1.3 (0.1) a | 0.90 (0.01) a | 0.63 (0.07) a | 10.7 (1.5) a | 28.9 (9.6) a | 34.5 (7.4) a | 26.5 (6.7) a | 90.0 (20) a |
| Winter | –1.4 (0.1) b | –2.3 (0.3) b | 0.87 (0.05) a | 0.33 (0.07) b | 10.9 (2.8) a | 35.4 (9.1) b | 40.6 (8.9) a | 42.3 (13) b | 118 (15) b | |
| *G. urceolata | Summer | –1.7 (0.1) a | –1.8 (0.0) a | 0.95 (0.01) a | 0.74 (0.05) a | 24.8 (4.1) a | 26.7 (6.5) a | 21.8 (5.9) a | 3.04 (2.8) a | 51.5 (15) a |
| Winter | –2.4 (0.2) b | –2.8 (0.2) b | 0.93 (0.01) a | 0.43 (0.08) b | 35.0 (8.7) a | 49.5 (9.6) b | 37.3 (8.8) b | 6.08 (4.2) a | 92.3 (18) b | |
| *L. japonica | Summer | –1.5 (0.1) a | –1.9 (0.2) a | 0.88 (0.04) a | 0.67 (0.1) a | 13.5 (3.5) a | 4.75 (4.4) a | 2.45 (3.4) a | 47.5 (11) a | 54.7 (17) a |
| Winter | –1.8 (0.1) a | –2.4 (0.1) b | 0.83 (0.02) a | 0.60 (0.01) a | 8.67 (1.7) a | 17.5 (4.7) b | 18.2 (2.5) b | 78.0 (14) b | 114 (20) b | |
| *G. procumbens | Summer | –1.7 (0.1) a | –2.0 (0.1) a | 0.91 (0.03) a | 0.62 (0.19) a | 18.5 (5.3) a | 30.9 (3.3) a | 41.8 (8.2) a | 54.3 (3.7) a | 127 (8.7) a |
| Winter | –2.5 (0.2) b | –2.8 (0.2) b | 0.91 (0.01) a | 0.57 (0.06) a | 27.5 (2.6) a | 46.8 (3.9) b | 58.6 (5.7) b | 29.7 (4.2) b | 135 (11) a | |
| *L. fontanesiana | Summer | –1.8 (0.1) a | –2.1 (0.1) a | 0.89 (0.02) a | 0.80 (0.16) a | 16.0 (5.4) a | 33.7 (3.0) a | 38.4 (2.6) a | 0.00 (0.0) a | 72.1 (5.2) a |
| Winter | –2.1 (0.3) a | –3.3 (0.6) b | 0.90 (0.02) a | 0.28 (0.01) b | 19.9 (3.8) a | 60.7 (20) b | 69.9 (25) b | 23.3 (11) b | 154 (48) b | |
| *H. helix | Summer | –1.5 (0.1) a | –1.7 (0.1) a | 0.92 (0.01) a | 0.56 (0.10) a | 18.1 (2.9) a | 28.9 (9.6) a | 34.5 (7.4) a | 26.5 (6.7) a | 90.0 (20) a |
| Winter | –1.6 (0.3) a | –2.3 (0.3) b | 0.90 (0.02) b | 0.32 (0.05) b | 13.1 (0.3) b | 35.4 (9.1) a | 40.6 (8.9) a | 42.3 (13) b | 118 (15) b | |
| K. latifolia | Summer | –1.6 (0.2) a | –2.0 (0.2) a | 0.90 (0.03) a | 0.49 (0.05) a | 15.7 (4.5) a | 34.2 (0.2) a | 33.7 (5.7) a | 7.29 (2.2) a | 75.2 (16) a |
| Winter | –1.6 (0.3) a | –2.8 (0.2) b | 0.87 (0.01) a | 0.33 (0.1) b | 13.3 (1.7) a | 29.6 (9.3) a | 47.7 (8.4) b | 60.9 (13.6) b | 138 (23) b | |
| Rhododendron sp. | Summer | –1.4 (0.1) a | –2.0 (0.1) a | 0.87 (0.01) a | 0.47 (0.08) a | 9.6 (0.75) a | 30.6 (1.4) a | 41.9 (6.5) a | 30.6 (1.4) a | 72.9 (8.0) a |
| Winter | –1.3 (0.2) a | –2.5 (0.4) a | 0.86 (0.02) a | 0.31 (0.04) b | 8.16 (0.5) a | 32.6 (3.5) b | 42.1 (2.6) a | 27.9 (2.3) a | 103 (4.4) b | |
| R. maximum | Summer | –1.5 (0.2) a | –2.0 (0.3) a | 0.86 (0.02) a | 0.62 (0.1) a | 10.9 (0.9) a | 22.3 (10) a | 35.9 (12) a | 8.65 (5.4) a | 66.8 (12) a |
| Winter | –1.4 (0.3) a | –2.5 (0.7) a | 0.86 (0.03) a | 0.34 (0.06) b | 11.5 (2.1) a | 33.6 (3.1) b | 44.8 (5.6) a | 5.48 (1.1) a | 83.8 (9.4) b | |
| V. minor | Summer | –2.0 (0.1) a | –2.3 (0.0) a | 0.90 (0.2) a | 0.79 (0.10) a | 18.3 (6.4) a | 30.8 (1.2) a | 23.2 (3.1) a | 22.6 (5.5) a | 76.7 (3.0) a |
| Winter | –2.7 (0.5) a | –3.5 (0.8) a | 0.91 (0.04) a | 0.39 (0.08) b | 33.7 (5.5) b | 24.5 (2.4) b | 31.5 (5.4) b | 116 (16) b | 172 (9.5) b | |
| R. catawbiense | Summer | –1.3 (0.1) a | –2.1 (0.2) a | 0.83 (0.01) a | 0.47 (0.03) a | 9.69 (1.6) a | ||||
| Winter | –1.2 (0.1) a | –1.9 (0.1) a | 0.86 (0.04) a | 0.36 (0.04) b | 11.7 (1.8) a |
Asterisks denote species which exhibit winter reddening.
During summer, Ψπ,0 was more negative and RWC0 lower in leaves of species that remain green during winter than in leaves that turned red (Ψπ,0 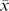 =–2.1 MPa for green, –1.8 MPa for red; P <0.0001; %RWC0
=–2.1 MPa for green, –1.8 MPa for red; P <0.0001; %RWC0 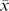 =0.87 for green, 0.91 for red P <0.0001). However, these two groups did not differ during winter after colour change had occurred (Ψπ,0
=0.87 for green, 0.91 for red P <0.0001). However, these two groups did not differ during winter after colour change had occurred (Ψπ,0 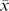 =–2.6 MPa and –2.7 MPa for green and red, respectively, P=0.23; %RWC0=0.87 for green, 0.89 for red; P=0.1).
=–2.6 MPa and –2.7 MPa for green and red, respectively, P=0.23; %RWC0=0.87 for green, 0.89 for red; P=0.1).
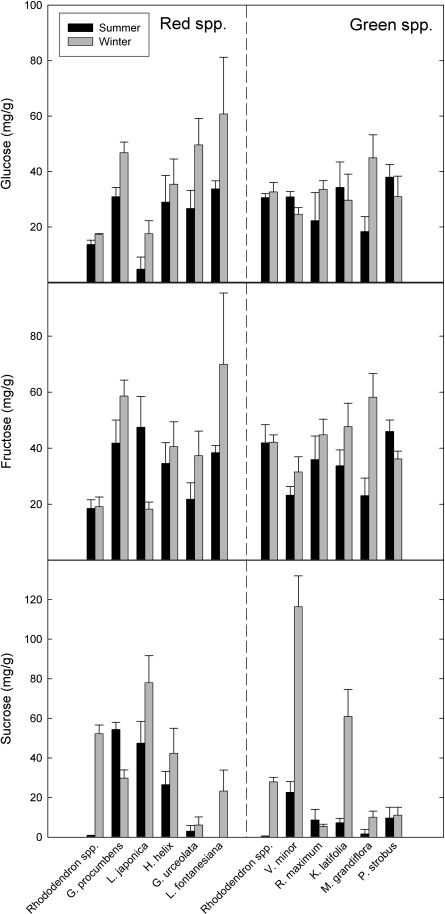
Sugars
All red and green-leafed species showed significant increases in the combined amounts of glucose+fructose+sucrose during winter, with the exception of the red-leafed G. procumbens (Table 1; Fig. 6). Seasonal levels of total sugars (glucose+fructose+sucrose) were not significantly different between red and green-leafed species during summer (P=0.66) or winter (P=0.23). All red-leafed species increased the glucose content during winter (significant at P <0.05 for all but H. helix), and most increased the fructose and sucrose contents as well (Table 1). Half of the green-leafed species measured did not show significant increases in fructose or sucrose contents during winter, although most significantly increased glucose (the only exception being K. latifolia). Red-leaved species had significantly higher sucrose contents during the summer than green-leafed species (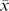 =9.7 mg g−1 for green, 22 mg g−1 for red; P <0.0001), but during winter, green-leafed species had significantly higher sucrose content (
=9.7 mg g−1 for green, 22 mg g−1 for red; P <0.0001), but during winter, green-leafed species had significantly higher sucrose content (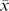 =53 mg g−1 for green, 39 mg g−1 for red; P <0.0001) (Fig. 6; Table 1). Green-leaved species had significantly higher glucose and fructose contents during summer than red leaves (P <0.0005 for both), but red-leaved species had significantly higher glucose during winter (P <0.01); red and green-leafed species did not differ in fructose content during winter (P=0.78).
=53 mg g−1 for green, 39 mg g−1 for red; P <0.0001) (Fig. 6; Table 1). Green-leaved species had significantly higher glucose and fructose contents during summer than red leaves (P <0.0005 for both), but red-leaved species had significantly higher glucose during winter (P <0.01); red and green-leafed species did not differ in fructose content during winter (P=0.78).
Fig. 6.
Seasonal sugar content of winter-red (left column) and winter-green (right column) angiosperm evergreens. Bars represent means of five replicates ±SD.
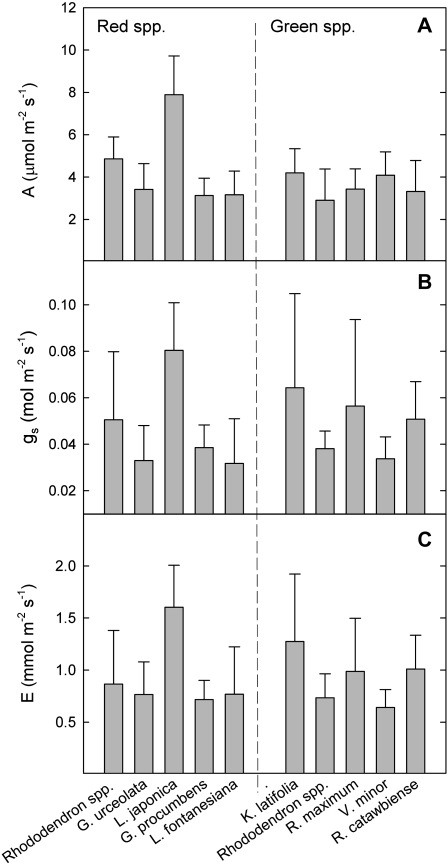
Leaf gas exchange
Red and green-leaved species did not significantly differ in any gas exchange parameters during winter (Fig. 7). Mean winter photosynthesis was 4.2 μmol CO2 m−2 s−1 for red and 3.6 μmol CO2 m−2 s−1 for green (P=0.14); mean winter stomatal conductance (gs) was 0.043 mol m−2 s−1 for red and 0.049 mol m−2 s−1 for green (P=0.12); and mean winter transpiration (E) was 79 mmol m−2 s−1 for red and 93 mmol m−2 s−1 for green (P=0.16).
Fig. 7.
Winter gas exchange of winter-red (left column) and winter-green (right column) leafed angiosperm evergreens. Photosynthesis is illustrated in (A), stomatal conductance (B), and transpiration (C). Bars represent means of 10–20 replicates ±SD.
Discussion
Consistent with our hypothesis, red-leafed species had significantly more negative midday Ψ during winter compared to green-leafed species during three of the four winter measurement days (Figs 2, 3), as well as when all winter days were pooled (Fig. 3C). Furthermore, pressure–volume curves showed that red-leafed species, as a group, were more likely to exhibit physiological features characteristic of acclimation to prolonged drought stress, i.e. significantly more negative osmotic potential at full turgor (Ψπ,100) and greater cell wall hardening (ε) than green-leafed species (Table 1; Fig. 5B, D); the two groups did not differ in these characteristics during the summer when all leaves were green (Table 1; Fig. 1).
Low osmotic potential at full turgor is generally indicative of an increased accumulation of solutes (osmotic adjustment), which is a physiological strategy for retaining water osmotically during periods of water stress (Verslues et al., 2006). Solutes most commonly involved in osmotic adjustment are sugar alcohols, monosaccharides, amino acids, and inorganic ions (commonly K+) (Handa et al., 1983; Ranney et al., 1991; Wang and Stutte, 1992). In addition to lower osmotic potential, red-leafed species also had cell walls which were significantly harder (lower elasticity) than green-leafed species during summer and winter (Table 1). Briefly, a less elastic cell wall results in a rapid loss of turgor pressure as water is lost, and a faster decline in Ψ accordingly (as positive cell wall pressure, Ψp, is not maintained); this drop in Ψ allows the cell to avoid further water loss due to a less steep water potential gradient between adjacent cells and the mesophyll air space (Verslues et al., 2006). The loss of turgor pressure in high ε species appeared to account for relative declines in midday Ψ seen in both red and green-leafed species, as well as stomatal closure (Fig. 7).
Although red-leafed species as a group were more likely to have lower midday Ψ, higher ε, and more negative Ψπ,100 than green-leafed species, it should be noted that these attributes were not mutually exclusive. For example, the species which exhibited the greatest physiological acclimation to drought stress (i.e. the highest ε and lowest Ψπ, 100) during winter was a green-leafed evergreen (Vinca minor). Furthermore, several red-leafed evergreens had ε and Ψπ,100 which were comparable to those of green-leafed evergreens during winter (Table 1; Fig. 5). Similarly, although red-leafed species as a group did experience significantly lower midday Ψ than green-leafed species, some red-leafed species (L. fontanesiana and Rhododendron spp.) had only very mild declines in midday Ψ, similar to, or milder than, those of some green-leafed species (Fig. 3). It should be noted, however, that the red-leafed Rhododendron spp. was a horticultural variety of azalea, and it is unknown whether winter reddening was the result of artificial breeding. Regardless, it is clear that when Ψ, gas exchange, and pressure–volume curve data are combined, both red and green-leafed groups contain species exhibiting a broad range of drought tolerance. Therefore, although red-leafed species do appear more likely to correspond with those that tolerate the most negative Ψ during winter, this alone is not a satisfactory explanation for winter colour change as a general rule.
In addition to examining the relationship between leaf water status and reddening, other possible proximate explanations for winter reddening were also examined. Anthocyanin synthesis is known to be inducible by low Ψ, and also by the accumulation of specific solutes involved in osmotic adjustment (e.g. sugars) (Chalker-Scott, 1999, 2002); either of these might therefore function as a proximate mechanism for the induction of anthocyanin synthesis in evergreens. Our results were not consistent with the explanation that osmolarity alone is responsible for inducing reddening in angiosperm evergreens. It was found that the species with the most negative osmotic potential at full turgor during winter was a green-leafed species (V. minor), and there was a noticeable degree of overlap between green-leafed species’ Ψπ,100 and those of some red-leafed species during winter, inconsistent with a ‘threshold’ effect of solute accumulation on anthocyanin synthesis (Table 1; Fig. 5). Because sugars commonly play a role in osmotic adjustment, and are also known to induce anthocyanin synthesis in many species (Do and Cormier, 1991; Neta-Sharir et al., 2000; Schaberg et al., 2003; Nagira and Ozeki, 2004; Teng et al., 2005; Murakami et al., 2008), the levels of fructose, glucose, and sucrose were also measured in all species during summer and winter. During winter, no differences were found in fructose concentrations between red and green-leafed species, but there was a significantly greater amount of glucose in red-leafed species, and a significantly greater amount of sucrose in green-leafed species (Table 1; Fig. 5). However, because of the substantial overlap in relative amounts of all sugars between red and green species, it is clear that colour change is not predictable based on levels of one particular sugar alone. It is possible that elevated levels of these sugars do induce colour change in certain species but not others, although such a conclusion can not be drawn from these data alone. Also, other sugars or sugar alcohols may be affecting anthocyanin synthesis as well, which were not examined here.
Lastly, it has been suggested that anthocyanins may be directly involved in osmotic adjustment by functioning as an osmolyte (Chalker-Scott, 1999, 2002). Our results are generally not consistent with this explanation. If anthocyanins were contributing significantly to the osmotic pool, we might expect red-leafed species consistently to have more negative Ψπ,100 compared to green-leafed species, and anthocyanin content to correlate negatively with Ψπ,100 within individual species. Neither of these were evident in this study. Some green-leafed species had more negative Ψπ,100 without anthocyanin, and some red-leafed species had Ψπ,100 similar to those of green-leafed species (Fig. 5D). Furthermore, when anthocyanin concentration was plotted against Ψπ,100 for individual species, a negative correlation was only observed in one species (data not shown). Instead, anthocyanin concentration within red individuals appeared more strongly dictated by sun exposure (as shown in Hughes et al., 2005).
Conclusion
Our results indicate that winter leaf reddening can not be explained solely on the basis of drought stress. Only three of the six red-leafed species studied here appeared acclimated to very negative leaf Ψ (G. urceolata, G. procumbens, and L. fontanesiana), as did one green-leafed species entirely lacking anthocyanin in winter leaves (V. minor). The remaining species generally overlapped in terms of relative drought acclimation. However, although anthocyanin content did not correlate with Ψ within or between species, redness was strongly coupled with light environment—with the reddest leaves of an individual occurring in the sunniest microclimate, consistent with a high-light protective function.
Acknowledgments
Thanks to Sara Venables and Tanja Schuster for help in the field, and Drs Daniel M Johnson and Howard Neufeld for expertise and assistance with methodology. Funding for this project was provided by the Department of Biology (WKS) and the Graduate School of Wake Forest University.
Glossary
Abbreviations
- ROS
reactive oxygen species
- VPD
vapour pressure deficit
- Ψ
water potential
- Ψπ,100
osmotic potential at full turgor
- Ψπ,0
osmotic potential at the turgor loss point
- %RWC0
relative water content at the turgor loss point
- SWF
symplastic water fraction
- ε
bulk modulus of elasticity
- A
photosynthesis
- gs
stomatal conductance
- E
transpiration
References
- Adams WW, III, Zarter CR, Ebbert V, Demmig-Adams B. Photoprotective strategies of overwintering evergreens. Bioscience. 2004;54:41–49. [Google Scholar]
- Archetti M, Döring TF, Hagen SB, et al. Adaptive explanations for autumn colours: an interdisciplinary approach. Trends in Ecology and Evolution. 2009;24:166–173. doi: 10.1016/j.tree.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Balakumar T, Hani Babu Vincent V, Paliwal K. On the interaction of UV-B radiation (280–315 nm) with water stress in crop plants. Physiologia Plantarum. 1993;87:217–222. [Google Scholar]
- Beeson RC. Restricting overhead irrigation to dawn limits growth in container grown woody ornamentals. Horticultural Science. 1992;27:996–999. [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology. 1999;70:1–9. [Google Scholar]
- Chalker-Scott L. Do anthocyanins function as osmoregulators in leaf tissues? Advances in Botanical Research. 2002;37:104–129. [Google Scholar]
- Davis SD, Sperry JS, Hacke UG. The relationship between xylem conduit diameter and cavitation caused by freezing events. American Journal of Botany. 1999;86:1367–1372. [PubMed] [Google Scholar]
- Diamantoglou S, Rhizopoulou S, Kull U. Energy content, storage substances, and construction and maintenance costs of Mediterranean deciduous leaves. Oecologia. 1989;81:528–533. doi: 10.1007/BF00378964. [DOI] [PubMed] [Google Scholar]
- Do CB, Cormier F. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Reports. 1991;9:500–504. doi: 10.1007/BF00232105. [DOI] [PubMed] [Google Scholar]
- Dodd I, Critchley C, Woodall G, Stewart G. Photoinhibition in differently coloured juvenile leaves of Syzgium species. Journal of Experimental Botany. 1998;49:1437–1445. [Google Scholar]
- Dutt SK, Bal AR, Bandyopadhyay AK. Salinity induced chemical changes in Casuarina equisetifolia Forst. Egyptian Journal of Soil Science. 1991;31:57–63. [Google Scholar]
- Eryılmaz F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnology and Biotechnological Equipment. 2006;20:47–52. [Google Scholar]
- Feild TS, Lee DW, Holbrook NM. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of Red-Osier dogwood. Plant Physiology. 2001;127:566–574. [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Archives of Biochemistry and Biophysics. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Gould KS, McKelvie J, Markham KR. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant, Cell and Environment. 2002;25:1261–1269. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. London: Oxford University Press; 1986. [Google Scholar]
- Handa S, Bressan RA, Handa AK, Carpita NC, Hasegawa PM. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiology. 1983;73:834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes NM, Burkey KO, Neufeld HS. Functional role of anthocyanins in high-light winter leaves of the evergreen herb, Galax urceolata. New Phytologist. 2005;168:575–587. doi: 10.1111/j.1469-8137.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Smith WK. Seasonal photosynthesis and anthocyanin production in 10 broadleaf evergreen species. Functional Plant Biology. 2007;34:1072–1079. doi: 10.1071/FP07205. [DOI] [PubMed] [Google Scholar]
- Hüner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Kaliamoorthy S, Rao AS. Effect of salinity on anthocyanin in the root of maize. Indian Journal of Plant Physiology. 1994;37:169–170. [Google Scholar]
- Karageorgou P, Manetas Y. The importance of being red when young: anthocyanins and the protection of Quercus coccifera from insect herbivory and excess light. Tree Physiology. 2006;26:613–621. doi: 10.1093/treephys/26.5.613. [DOI] [PubMed] [Google Scholar]
- Knox GW. Water use and average growth index of five species of container grown woody landscape plants. Journal of Environmental Horticulture. 1989;7:136–139. [Google Scholar]
- Kyparissis A, Grammatikopoulos G, Manetas Y. Leaf morphological and physiological adjustments to the spectrally selective shade imposed by anthocyanins in Prunus cerasifera. Tree Physiology. 2007;27:849–857. doi: 10.1093/treephys/27.6.849. [DOI] [PubMed] [Google Scholar]
- Kytridis V-P, Karageorgou P, Levizou E, Manetas Y. Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. Journal of Plant Physiology. 2008;165:952–959. doi: 10.1016/j.jplph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Kytridis V-P, Manetas Y. Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. Journal of Experimental Botany. 2006;57:2203–2210. doi: 10.1093/jxb/erj185. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gould KS. Why leaves turn red. American Scientist. 2002;90:524–531. [Google Scholar]
- Lee DW, O'Keefe J, Holbrook NM, Feild TS. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research. 2003;18:677–694. [Google Scholar]
- Manetas Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora. 2006;201:163–177. [Google Scholar]
- Manetas Y, Petropoulou Y, Psaras GK, Drinia A. Exposed red (anthocyanic) leaves of Quercus coccifera display shade characteristics. Functional Plant Bioloy. 2003;30:265–270. doi: 10.1071/FP02226. [DOI] [PubMed] [Google Scholar]
- Murakami PF, Schaberg PG, Shane JB. Stem girdling manipulates leaf sugar concentrations and anthocyanin expression in sugar maple trees during autumn. Tree Physiology. 2008;28:1467–1473. doi: 10.1093/treephys/28.10.1467. [DOI] [PubMed] [Google Scholar]
- Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du Z, Kikuchi S. Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. Journal of Agriculture and Food Chemistry. 2003;51:2992–2999. doi: 10.1021/jf026179+. [DOI] [PubMed] [Google Scholar]
- Nagira Y, Ozeki Y. A system in which anthocyanin synthesis is induced in regenerated torenia shoots. Journal of Plant Research. 2004;117:377–383. doi: 10.1007/s10265-004-0170-6. [DOI] [PubMed] [Google Scholar]
- Neta-Sharir I, Shoseyov O, Weiss D. Sugars enhance the expression of gibberellin-induced genes in developing petunia flowers. Physiologia Plantarum. 2000;109:196–202. [Google Scholar]
- Oberbauer SF, Starr G. The role of anthocyanins for photosynthesis of Alaskan arctic evergreens during snowmelt. Advances in Botanical Research. 2002;37:129–145. [Google Scholar]
- Paine TD, Hanlon CC, Pittenger DR, Ferrin DM, Malinoski MK. Consequences of water and nitrogen management on growth and aesthetic quality of drought-tolerant woody landscape plants. Journal of Environmental Horticulture. 1992;10:94–99. [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annual Review of Plant Physiology. 1984;35:15–44. [Google Scholar]
- Ramanjulu S, Veeranjaneyulu K, Sudhakar C. Physiological changes induced by NaCl in mulberry var. Mysore local. Indian Journal of Plant Physiology. 1993;36:273–275. [Google Scholar]
- Ranney TG, Bassuk NL, Whitlow TH. Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees. Journal of the American Society for Horticultural Science. 1991;116:684–688. [Google Scholar]
- Sakomoto K, Kumiki I, Sawamura K, Kyoko H, Yoshihisa A, Takafumi Y, Tsutomu F. Anthocyanin production in cultured cells of Aralia cordata Thumb. Plant Cell. Tissue and Organ Culture. 1994;36:21–26. [Google Scholar]
- Schaberg PG, van den Berg AK, Murakami PF, Shane JB, Donnelly JR. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiology. 2003;23:325–333. doi: 10.1093/treephys/23.5.325. [DOI] [PubMed] [Google Scholar]
- Sherwin HW, Farrant JM. Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscose. Plant Growth Regulation. 1998;24:203–210. [Google Scholar]
- Smirnoff N. Plant resistance to environmental stress. Current Opinion in Biotechnology. 1998;9:214–219. doi: 10.1016/s0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- Spyropoulos CG, Mavrommatis M. Effect of water stress on pigment formation in Quercus species. Journal of Experimental Botany. 1978;29:473–477. [Google Scholar]
- Suzuki M. Enhancement of anthocyanin accumulation by high osmotic stress and low pH in grape cells (Vitis hybrids) Journal of Plant Physiology. 1995;147:152–155. [Google Scholar]
- Taneda H, Tateno M. Hydraulic conductivity, photosynthesis and leaf water balance in six evergreen woody species from fall to winter. Tree Physiology. 2005;25:299–306. doi: 10.1093/treephys/25.3.299. [DOI] [PubMed] [Google Scholar]
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75-PAP1 gene. Plant Physiology. 2005;139:1840–1852. doi: 10.1104/pp.105.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholakalabavi A, Zwiazek JJ, Thorpe TA. Osmotically-stressed poplar cell cultures: anthocyanin accumulation, deaminase activity, and solute composition. Journal of Plant Physiology. 1997;151:489–496. [Google Scholar]
- Turner N. Techniques and experimental approaches for the measurement of plant water status. Plant and Soil. 1981;58:339–366. [Google Scholar]
- Uemura M, Steponkus PL. Cold acclimation in plants: relationship between lipid composition and the cryostability of the plasma membrane. Journal of Plant Research. 1999;112:245–254. [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu J-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. The Plant Journal. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- Wang ZC, Stutte GW. The role of carbohydrates in active osmotic adjustment in apple under water stress. Journal of the American Society for Horticultural Science. 1992;117:816–823. [Google Scholar]
- Xiong L, Zhu J-K. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment. 2002;25:131–139. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Yang Z-M, Zheng S-J, Hu A-T, Zheng Y-F, Yan J-Y. Response of cucumber plants to increased UV-B radiation under water stress. Journal of Environmental Sciences (China) 2000;12:236–240. [Google Scholar]