Abstract
Phloem is a central conduit for the distribution of photoassimilate, nutrients, and signals among plant organs. A revised technique was used to collect phloem sap from small woody plants in order to assess changes in composition induced by water deficit and flooding. Bled phloem sap δ13C and sugar concentrations were compared to δ13C of bulk material, soluble carbon extracts, and the neutral sugar fraction from leaves. Amino acid composition and inorganic ions of the phloem sap was also analysed. Quantitative, systematic changes were detected in phloem sap composition and δ13C in response to altered water availability. Phloem sap δ13C was more sensitive to changes of water availability than the δ13C of bulk leaf, the soluble carbon fraction, and the neutral soluble fraction of leaves. Changes in water availability also resulted in significant changes in phloem sugar (sucrose and raffinose), inorganic nutrient (potassium), and amino acid (phenylalanine) concentrations with important implications for the maintenance of phloem function and biomass partitioning. The differences in carbohydrate and amino acid composition as well as the δ13C in the phloem, along with a new model system for phloem research, offer an improved understanding of the phloem-mediated signal, nutrient, and photoassimilate transduction in relation to water availability.
Keywords: Amino acids, Eucalyptus, flooding, phloem sap, raffinose, sucrose, water deficit
Introduction
Phloem is the major conduit for the transport of solutes and signalling among tissues of higher plants. The composition of phloem sap therefore offers considerable promise for use in diagnostic assessments of plant health. The development of such assessments is limited by our ability to sample phloem easily, with the characterization of phloem contents limited to a handful of studies of mainly herbaceous species (a for a review see Turgeon and Wolf, 2009).
Recently, several authors have begun investigating the nature and composition of phloem sap in tree species (Gessler et al., 2004, 2007; Scartazza et al., 2004; Tausz et al., 2008) using a phloem collection technique originally developed by Hartig (1860) and further developed for E. globulus by Pate et al. (1974, 1984). Using a razor blade, a small incision is made in the stem to the depth of the cambium and phloem ‘bleeds’ from the surface of the cut. This method has considerable advantages over more traditional techniques of phloem sap collection (highlighted by Turgeon, 2006) in that it is fast and it avoids the need for chelating reagents and extraction procedures, thus reducing contamination from companion cell contents (for review see Turgeon and Wolf, 2009).
Using this technique, significant changes have been detected in phloem sap carbon content and the abundance of the carbon isotope 13C compared to 12C (δ13C), both of which were strongly coupled to measures of water availability (Pate et al., 1998; Keitel et al., 2003, 2006). The abundance of carbon isotopes in plant components are often used as a surrogate estimation of water use efficiency (Seibt et al., 2008), and are likely to be improved by the analysis of carbon pools that have been recently assimilated. Phloem sap δ13C is positively related to growth under conditions where stomata play a major role in determining the rate of carbon diffusion into leaves (Cernusak et al., 2003; Tausz et al., 2008) and has also been shown to be a valid integrator of whole tree crown δ13C (Scartazza et al., 2004) indicating potential for markers of whole plant and ecosystem function (West et al., 2006; Bowling et al., 2008).
Whilst phloem sap δ13C has been shown to reflect leaf level processes, changes in the composition of solutes may dampen such relationships. Major changes in the abundance of compounds transported in the phloem may arise as part of plant acclimation strategies. Regulation of osmotic potential is a common response to water limitation in leaves of Eucalyptus species via the accumulation of low molecular weight amino acids (Shvaleva et al., 2006), sugars (Shvaleva et al., 2008), sugar alcohols (Merchant et al., 2006b), or inorganic ions (Liu et al., 2001). Changes in phloem sap carbon concentration (Cernusak et al., 2003), combined with the potential for changes in inorganic ions and amino acids (Lalonde et al., 2003) may play a similar role in regulating osmotic potential of the phloem. Identifying major changes in phloem composition, along with the potential for influencing measures of δ13C, is crucial for attaining valid assessments of plant health and physiological processes through the analysis of phloem sap.
The phloem bleeding technique was used on small trees of Eucalyptus globulus, facilitating studies under controlled experimental conditions. This technique samples phloem sap from the trees over a period of hours using the positive pressure generated in the phloem. This technique enables the collection of the transported solute pool with minimal contamination from wounding or modified exchange with the surrounding tissues (for a review see Turgeon and Wolf, 2009).
To evaluate the use of phloem sap as a diagnostic tool, it was investigated whether systematic differences exist in phloem sap composition compared to leaf carbon pools in young E. globulus plants treated with water deficit or flooding. An answer was sought to the following questions in relation to water-related plant stress. (i) Is phloem sap a more sensitive early indicator of stress than leaf carbon pools? (ii) What chemical components of the phloem sap and leaf carbon pools are the most sensitive early indicators of stress? (iii) Is the carbon isotope composition of phloem sap and leaf carbon a better early indicator of stress than their chemical composition? Our results are also discussed in relation to the possible phloem-loading mechanism of E. globulus and its importance for the allocation of carbon within the plant.
Materials and methods
Seedlings/saplings of E. globulus were germinated in a commercial nursery and raised under greenhouse conditions in Perth, Australia (31°57’ S, 115°52’ E). In spring 2006, approximately four months prior to the commencement of treatments, seedlings were transplanted into 9.0 l pots and placed into a greenhouse. Plants were planted into a 50:50 mix of peat and dry river sand. On each day, plants were watered to the field capacity of the soil by drip irrigation. By the time of sampling, individual plants were 12 months old, ∼1.5 m in height and the stems were approximately 2 cm in diameter.
Treatments
Twelve plants were allocated to each of a control treatment (well watered), a flooded treatment, and a water-deficit treatment. Flooded plants were submerged to a depth equal to the surface of the soil for a period of 2 weeks. The water in this bucket was replaced every third day. For drought treatment, plants were given 30% of the water used by well-watered plants by a method previously described by Merchant et al. (2006b) for a period of 2 weeks.
Phloem collection
Phloem sap collection was made using a single-sided razor blade. An incision was made in the stem of the tree at a height of approximately 3 cm above the base. The depth of the cut was determined on the basis of experience. Cutting too deep resulted in puncturing of the xylem; the outcome of which was negative pressure and loss of phloem sap into the stem. Cuts were made at approximately 10 am to avoid the effects on δ13C of transitory starch remobilization during the night identified by Gessler et al. (2007). Whilst each plant was different, phloem sap bled from incisions for a period of 4–6 h, mostly between 11.00 h and 14.00 h. Phloem sap formed a droplet on the surface of the cut that was progressively collected in a glass disposable pipette and bulked into a single microtube for each tree. Samples were immediately transferred to 4 °C during the collection period and then frozen at –80 °C. Not all trees bled sufficient phloem sap for analysis and thus the number of replicates varied throughout the study.
Xylem sap collection was made by cutting the main stem, stripping the bark, and fitting a plastic tube (2 cm in length) onto the cut xylem surface. Over a period of 2 h, xylem sap exuded from the cut surface and was transferred to 4 °C and then frozen at –80 °C.
Extractions from leaf material for carbohydrate and isotopic analysis
First, fully expanded leaf material was collected at midday on each of the sampling days. Ten first fully expanded leaves were collected from the canopy with leaves taken from several heights. Leaves were immediately microwaved as per Popp et al. (1996) and then oven dried at 75 °C. Samples were then ground to a powder and kept at –80 °C awaiting analysis.
For carbohydrate analysis, soluble compounds were extracted from approximately 40 mg of dried leaf material with methanol–chloroform–water (MCW, 12:5:3 by vol.) as detailed in Merchant et al. (2006a). The water fraction of the extraction solution included an internal standard of 0.1% xylitol. Leaf extracts or phloem sap were then deionized by the addition of 300 μl of mixed bed resin consisting of 1 part Dowex 1×8 (anion exchange, Cl– form) and 1 part Dowex 50W (cation exchange, formate form). Samples were agitated for a period of 2 h at room temperature. Following pulse centrifugation, 400 μl of the supernatant was transferred to a clean microtube and stored at –80 °C.
For the analysis of the carbon isotope composition of the soluble extract in leaves (δ13Csol), 40 mg of ground leaf material was weighed into a 2 ml microtube to which 1 ml of hot, deionized water was added and incubated for 1 h at 75 °C. Samples were centrifuged at 11 400 g for 3 min and 800 μl of the supernatant transferred to a 2 ml microtube.
Analysis of phloem sap and leaf extracts for soluble carbohydrates
Carbohydrates were separated and quantified using gas chromatography according to Merchant et al. (2006a). Sixty μl of deionized MCW extracts were dried and resuspended in 400 μl anhydrous pyridine to which 50 μl of trimethylchlorosilane (TMCS)/bis-trimethylsilyl-trifluoroacetamide mix (1:10 v/v, Sigma Aldrich) was added. Samples were incubated for 1 h at 75 °C and analysed by gas chromatography (GC) within 24 h. GC analysis was performed using a Shimadzu 17A series gas chromatograph using a DB1 (30 m) column. Split injection was made at 300 °C with an initial oven temperature programme of 60 °C for 2 min ramping to 300 °C at 10 °C min−1 and maintained for 10 min. Column flow rate was maintained at 1.5 ml min−1. Peak integration was made using Class VP analysis software (Shimadzu Corporation Limited).
Carbon isotope analysis
Four tissues or plant extracts were measured for isotopic composition in this study. For bulk leaf material (δ13Cleaf), approximately 1 mg of dried material was weighed out into tin cups and kept over a desiccant awaiting analysis. For δ13Csol, 200 μl soluble extract was transferred into tin cups and dried at 45 °C awaiting analysis. For leaf sugars (δ13Csug), 500 μl of the hot water extract was deionized on a mixed bed resin as described for soluble carbohydrate analysis (Merchant et al., 2006a) thus yielding a neutral sample consisting mainly of sugar alcohols and carbohydrates. 60 μl of the sample was then dried into tin cups at 45 °C for 12 h and kept over a desiccant awaiting analysis. For δ13Cphl, 5 μl of phloem sap was placed into tin cups and dried at 45 °C for 12 h and then kept over desiccant until analysed.
Isotope ratio mass spectrometry (IRMS) was used to determine the ratio of 13C and 12C in samples. Samples were analysed on an Isochrom mass spectrometer (Micromass, Manchester, UK) coupled to a Carlo Erba elemental analyser (CE Instruments, Milan). Samples were dropped from an AS200 auto-sampler and combusted by Dumas-combustion in a furnace kept at 1060 °C. Carbon isotope ratios are expressed in delta-notation, where δ13C=Rsample/Rstandard–1, and R is the ratio of 13C to 12C in a sample and standard (VPDB), respectively. The precision for the standard materials was between 0.06‰ and 0.11‰.
Amino acid analysis
Sample preparation for amino acid analysis followed that described for phloem sap and leaf sugars. For leaf extracts, samples for amino acid analysis were removed prior to deionizing with the mixed bed resin. For amino acids in phloem, 25 μl of sap was dried under vacuum into a 2 ml glass vial.
Five μl of internal standard (0.1 mg ml−1 norleucine) was added to samples, they were re-dried and then taken up in 100 μl of N,N-dimethylformamide. 50 μl of N-methyl-N-[tert-butyldimethyl-silyl]trifluoroacetimide was added and samples were derivatized by heating at 80 °C for 45 min. Amino acid derivatives were separated by capillary gas chromatography on a 5% diphenyl–95% dimethyl polysiloxane stationary phase (30 m long×0.25 mm ID×0.25 μm film thickness; Rtx-5SilMS, Restek, Bellfonte, USA). The column eluent was ionized by electron impact (70 eV) and mass spectra were collected from 100 –600 amu (GCMS-QP2010Plus, Shimadzu, Kyoto, Japan). Amino acids were identified based on retention times and mass spectra of authentic standards run under the same conditions. Quantification was based on the dominant M−57 ions (ions with a mass 57 less than the intact molecular ion—corresponding to loss of a t-butyl group), with the exception of arginine which was quantified based on the dominant M−188 ion (corresponding to loss of a t-butyl group plus a guanidino N).
Inorganic ion analysis
Major cations in leaf extracts were determined using inductively coupled plasma-optical photoemission spectroscopy (ICP-OES). For solid samples, approximately 40 mg of ground dried leaves were placed into 1 ml of hot (80 °C) deionized water for 1 h and agitated during this time. Samples were allowed to cool and then centrifuged at 13 000 rpm for 2 min. The supernatant was then removed and placed into a 1.5 ml microtube. 250 μl of this extract was then taken and placed into a 15 ml tube and diluted with 6 ml of deionized water. Samples were then analysed immediately or frozen until analysis. Samples that were frozen were re-agitated upon thawing. Phloem and xylem-derived inorganic ions were also analysed by ICP-OES. Subsamples of phloem (30 μl) and xylem (300 μl) were diluted with deionized water to a total volume of 6 ml and analysed within 3 h.
Calculations of osmotic potential
Molar concentrations of solutes in phloem sap were converted to osmotic potential (πs) using the van't Hoff equation:
where R is the universal gas constant (8.32 J mol−1 K−1), T is the temperature in Kelvin (set at 293) and cs is the concentration of solutes in phloem sap (mol l−1).
Growth measurement
At the beginning of the experiment, 12 plants were harvested prior to the application of treatments (pretreatment) and the leaf, stem, and root dry weights were determined for each plant. At the end of the treatment period, leaf, stem, and root dry weights were determined for each plant subjected to water-deficit, flooding, and control treatments. Average growth measurements for each plant component are expressed as the average gain in dry mass per week calculated as the difference between a plant components dry weight and that of the pretreatment harvested plants.
Statistical analysis
Effects of treatments were analysed by Analysis of variance using ‘Statistica’ analytical software (Version 6 StatSoft, Tulsa, OK). P values were calculated using Tukey's honestly significant difference post-hoc test mean values. Linear regressions were calculated using a general linear model.
Results
Treating plants with water deficit or flooding caused significant changes in both the chemical and isotopic composition of all leaf fractions and phloem sap with the exception of bulk leaf carbon in water-deficit-treated plants and leaf soluble carbon in flooded plants (Fig. 1). By far the most significant changes were observed in phloem sap, especially regarding the isotope and sugar composition (Figs 1, 2).
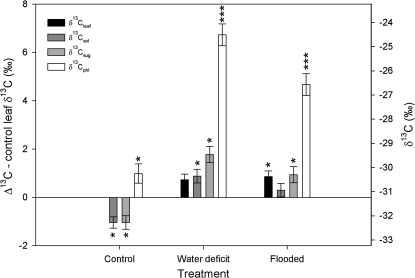
Fig. 1.
Bulk leaf carbon isotopic signature (δ13Cleaf, black column), leaf soluble carbon (δ13Csol, dark grey column), leaf ‘sugars’ (δ13Csug, light grey column), and phloem sap (δ13Cphl, white column) expressed as difference from control δ13Cleaf. 12-month-old E. globulus plants were either well watered or subject to water deficit or flooding for a period of 2 weeks (7≤n≤9). Error bars represent the standard error of mean values and are shown together with the significance of differences from control values (0) calculated using Tukey's honestly significant difference post-hoc test mean values where *P=0.05–0.01, ***P <0.001.
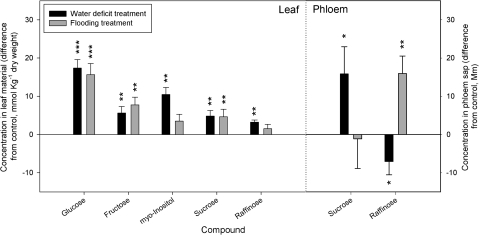
Fig. 2.
Sugars and sugar alcohols measured in leaves and phloem sap obtained from 12-month-old E. globulus plants subjected for 2 weeks to water deficit (black columns) and flood (grey columns) treatments (7≤n≤9). Average differences from control plants are presented. Error bars representing the standard error of mean values are shown together with the significance of differences from control values (0) calculated using Tukey's honestly significant difference post-hoc test mean values where *P=0.05–0.01, **P=0.01–0.001, ***P <0.001.
Both water deficit and flooding treatments resulted in less negative δ13C values of phloem sap carbon (δ13Cphl) than bulk leaf carbon (δ13Cleaf), leaf soluble carbon (δ13Csol), or leaf ‘sugars’ (δ13Csug) by 3–5‰, and δ13Cphl was 4–6‰ less negative in treated than control plants (Fig. 1). By contrast, in control plants δ13Cphl was only 1–2‰ less negative than δ13C of leaf carbon pools (Fig. 1). δ13C of leaf carbon pools were also less negative in treated than in control plants but the differences were much smaller than in phloem sap (Fig. 1).
The concentration of all measured carbohydrates and sugar alcohols in leaf material increased in both water-deficit and flood treated plants compared to controls (Fig. 2). Absolute concentrations of sugars ranged from 71–132 mmol kg−1 dry weight (total). The greatest absolute changes were observed in leaf glucose concentrations, which increased by more than 15 mmol kg−1 DW. As a percentage from levels detected in control plants, the largest increase in concentration was that of sucrose increasing by approximately 100% in both treatments.
The main sugars present in phloem sap were sucrose (≈180±19 mM) and raffinose (≈40±11 mM) Concentrations of sucrose increased significantly in plants under water deficit but not under flooding. By contrast, raffinose concentrations rose significantly in the phloem sap of flooded plants and fell in plants subjected to water deficit (Fig. 2).
Amino acids and measured ions were less sensitive to water stress, especially in waterlogged plants. There was no effect of treatment on the concentrations of measured ions in bulk leaf (Table 1A). For leaves, phloem and xylem, K+ and Na+ were present at the greatest concentrations amongst all the inorganic solutes analysed. Only K+ concentrations in the phloem of water-deficit-treated plants differed significantly from control plants (Table 1B); K+ increased by ∼30% in the phloem of water-deficit-treated plants. The concentration of measured ions in xylem sap was very small and no treatment effects were observed (Table 1C). Along with increased concentrations of sucrose, the increased concentration of potassium in the phloem of water-deficit-treated plants significantly increased phloem osmotic potential (Table 1D).
Table 1.
Concentrations of measured elements in leaf (A), phloem (B), and xylem (C) tissues along with the major contributors to phloem osmotic potential (D)
| Control | Water deficit |
Flooded |
|||
| x± se x± se | Sig | x±se | Sig | ||
| (A) Leaf (mmol kg-1 dry weight) | |||||
| Calcium | 22.3±1.9 | 23.2±2.8 | – | 23.8±2.6 | – |
| Potassium | 520±28 | 584±37 | – | 672±53 | – |
| Magnesium | 95.5±7.9 | 96.9±8.6 | – | 97.1±8.3 | – |
| Sodium | 308.6±13 | 330±14 | – | 323±17 | – |
| Phosphorus | 39.3±3.3 | 44.0±4.5 | – | 31.2±3.2 | – |
| Sulphur | 16.9±1.2 | 22.0±0.7 | – | 34.0±8.4 | – |
| (B) Phloem (mM) | |||||
| Calcium | 0.62±0.07 | 0.60±0.11 | – | 0.38±0.06 | – |
| Potassium | 35.1±1.5 | 43.4±1.8 | * | 38.2±4.6 | – |
| Magnesium | 1.17±0.07 | 1.04±0.12 | – | 1.26±0.15 | – |
| Sodium | 23.1±2.5 | 21.1±4.2 | – | 20.2±3.6 | – |
| Phosphorus | 3.98±0.21 | 3.50±0.22 | – | 4.86±0.70 | – |
| Sulphur | 1.34±0.17 | 1.93±0.16 | – | 1.46±0.35 | – |
| (C) Xylem (mM) | |||||
| Calcium | 0.28±0.03 | n/a | – | 0.18±0.05 | – |
| Potassium | 1.77±0.19 | n/a | – | 2.37±0.73 | – |
| Magnesium | 0.28±0.04 | n/a | – | 0.39±0.08 | – |
| Sodium | 2.61±0.32 | n/a | – | 3.22±0.49 | – |
| Phosphorus | 0.20±0.01 | n/a | – | 0.54±0.15 | – |
| Sulphur | 0.06±0.01 | n/a | – | 0.35±0.02 | – |
| (D) Contribution to phloem πs (MPa) | |||||
| Sucrose | 0.43±0.01 | 0.47±0.01 | ** | 0.42±0.01 | – |
| Raffinose | 0.08±0.01 | 0.06±0.00 | – | 0.12±0.01 | ** |
| Potassium | 0.09±0.00 | 0.11±0.01 | ** | 0.09±0.01 | – |
| Sodium | 0.05±0.00 | 0.05±0.01 | – | 0.05±0.01 | – |
Concentrations of measured ions in leaf tissues, phloem and xylem sap from E. globulus subjected to well-watered, water-deficit or flooding treatments. Treatments were imposed for a period of 2 weeks. Major contributors to phloem osmotic potential are also given in MPa. Average values are presented along with the associated standard error (7≤n ≤9). Significant differences in composition compared to control plants (well-watered, not flooded) are shown. P values were calculated using Tukey's honestly significant difference post-hoc test mean values where *P=0.05–0.01, **P=0.01–0.001, ***P <0.001.
Amino compounds increased in concentration in phloem sap collected from both water-deficit-treated (164±34.6 μg ml−1) and flooded (208±36.0 μg ml−1) plants compared with control plants (110±34.4 μg ml−1), albeit with greater variability than other constituents. The major amino acid in phloem sap was glutamine, representing up to 50% of the total amino-N pool in control and flood treated plants (Fig. 3). For water-deficit-treated plants, the concentration of phenylalanine increased (Fig. 3), reaching up to 41 μg ml−1 phloem sap. Phenylalanine appeared to accumulate at the expense of glutamine. Changes in amino acid concentration of the phloem sap were not reflected in leaf concentrations (data not shown).
Fig. 3.
Amino acid composition (% of total), of phloem sap for well-watered, water-deficit, and flooded E. globulus (7≤n≤9). Asterisks represent significant differences in abundance compared to well-watered plants (P <0.05). Significance testing was calculated using Tukey's honestly significant difference post-hoc test mean values. Error bars have been omitted for clarity.
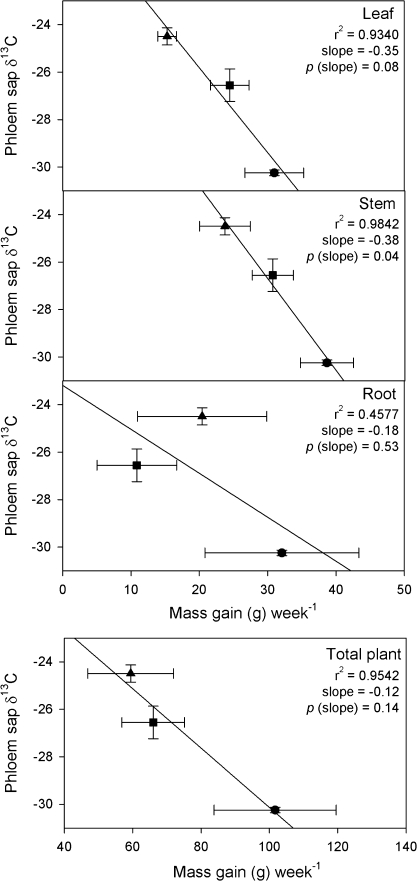
Both water-deficit-treated and flooded plants grew more slowly than control plants (Fig. 4). Of all tissues and extracts analysed, δ13C of phloem was best correlated with growth (Fig. 4). For leaf tissues, a negative relationship between δ13Cphl and the increase in leaf mass (Fig. 4) was detected. A similar relationship was observed for δ13Cphl and stem mass. Flood-treated plants had a reduction in root growth that was not reflected in δ13Cphl.
Fig. 4.
δ13Cphl versus the growth of leaves, stems, roots, and total biomass over the treatment period for well-watered (circles), water-deficit-treated (squares), and flooded (triangles) E. globulus (7≤n≤9). Treatments were imposed for a period of 2 weeks. Error bars represent the standard error of mean values. Regressions were calculated using a general linear model.
Discussion
Our study has provided strong evidence that carbon isotope discrimination measured in phloem sap is a very sensitive indicator of water stress in young E. globulus plants. It has also been shown that concentrations of sucrose and raffinose measured on the same phloem sap sample could be used to distinguish water deficit from the effects of flooding. By contrast, carbon pools, amino acid and nutrient concentrations in the leaf were poor predictors of water stress. The magnitude of changes in δ13Cphl compared with those in leaf carbon pools illustrates the greater sensitivity of the former to plant water status. Although δ13Csol and δ13Csug mirrored the development of the δ13Cphl, they had a much smaller response.
Previous experiments from field grown trees (Keitel et al., 2006) and controlled environment studies with herbaceous species (Gessler et al., 2008) suggest that δ13Cphl reflects environmental and physiological conditions during the previous 24–48 h, dependent on photosynthetic capacity, sugar pool size, transport distance, and time. For our study, dampening of changes in δ13Cphl as a result of mixing and variation in δ13C contributed by different source leaves was insufficient to override the isotopic signal of newly synthesized sugars. Although soluble leaf carbon has been regarded as the carbon pool most likely to reflect short-term physiological status (Gessler et al., 2007), it could be argued that, for the purposes of predicting physiological performance, δ13Cphl obtained from E. globulus is strongly coupled with the physiological status of fully expanded ‘source’ leaves due to fast rates of carbon export.
This conclusion is supported by the clear relationship between δ13Cphl and above-ground growth, and is further supported by field-based studies linking δ13Cphl with above ground growth in E. globulus (Tausz et al., 2008). Biomass accumulation is a long-term integrated measure of physiological performance. There was no relationship between δ13Cphl and below-ground components owing to the effect of flooding on carbon metabolism and biomass partitioning. Continuously flooded woody species have low root-to-shoot ratios and shallow rooting systems (Megonigal and Day, 1992). Because oxygen deprivation in the roots forces plants to switch from aerobic respiration to low-energy-yielding alcoholic fermentation, energy-intensive mechanisms like root-growth and nutrient uptake are reduced (Kreuzwieser et al., 2004). Simultaneously, photosynthesis, stomatal conductance, and phloem transport declines and sugar-supply to the roots is limited (Kreuzwieser et al., 2004). The less negative δ13C values in the flooded treatment indicate reduced stomatal conductance, albeit less reduced than in the water-deficit treatment. Moreover, increased sugar concentrations in the leaves points to increased storage of sugars in this part of the plant, also routinely observed for flooded trees (Kreuzwieser et al., 2004).
Cernusak et al. (2003) recently suggested that plants may adjust the relative concentrations of sucrose and raffinose in phloem to modify the osmotic potential of sap and thus maintain equilibrium with xylem water potential. In addition, high pressure is thought to offer advantages for the regulation of phloem function (Thompson, 2006; Turgeon, 2006) and irrespective of whether bulk flow is driven by source or sink characteristics, the regulation of osmotic potential in the phloem plays an important role in determining phloem function and flow. The observed concentrations of sucrose, raffinose, and potassium obtained here would give rise to an osmotic potential of around 0.6 MPa in the phloem, a valuable contribution given the range in water potentials observed for this species (White et al., 1996, 2003; Cernusak et al., 2003; Macfarlane et al., 2004). Despite this, changes in phloem sap composition did not give rise to significant changes in phloem sap osmotic potential owing largely to the molecular weights of the respective solutes. Phloem sap obtained from field-grown E. globulus have shown higher concentrations of sugars than those found here (Pate et al., 1998; Cernusak et al., 2003), thus systematic changes of a similar magnitude may influence osmotic potential under such conditions.
Large increases in sugar content, such as those observed in our water-deficit-treated and flooded plants, support previous findings of increased carbon content of phloem sap obtained from E. globulus trees during dry periods (Pate et al., 1998; Tausz et al., 2008). Changes in phloem carbon content of this magnitude could influence gene expression under various models proposed for assimilate partitioning (Paul et al., 2001; Halford and Paul, 2003; Rook and Bevan, 2003; Pourtau et al., 2004; Gupta and Kaur, 2005; Smith and Stitt, 2007) or the repression of photosynthesis (Paul and Driscoll, 1997; Paul et al., 2001; Halford and Paul, 2003). Many abiotic stresses cause major alterations in plant sugar status that affect gene expression (Gupta and Kaur, 2005). For example, sucrose-mediated transcriptional regulation of sucrose symporters involved in phloem loading has been well characterized in sucrose-fed sugar beet (Vaughn et al., 2002; Ransom-Hodgkins et al., 2003) suggesting that sugar signalling has important consequences for assimilate partitioning. The pathways by which plants export carbon from leaves differ among taxonomic groups and may be associated with environmental conditions (Turgeon et al., 2001).
The carbon content of phloem sap of E. globulus is dominated by sucrose and raffinose (Pate et al., 1998; Pate and Arthur, 2000; Tausz et al., 2008, and supported here) suggesting that, at least in part, a symplastic loading mechanism is employed (Turgeon, 1996). Symplastic loading has now been suggested as the predominant loading mechanism for woody species (Rennie and Turgeon, 2009). Whilst these measures of solute concentration are independent of flow, they suggest that changes in phloem sap composition arise at the site of phloem loading and are not solely a consequence of solute movement and/or degradation. Multiple mechanisms could lead to this phenomenon. The activity of raffinose synthase in a polymer trap model (Turgeon, 1996), may be influenced by changes in its cellular environment arising from water status and/or source and sink activities.
In addition, Turgeon and Medville (2004) suggested that plasmodesmatal aperture acting in such a system may be dictated by pressure differentials across the membrane as has been shown in trichomes (Oparka and Prior, 1992). Some plants are thought to move sugars exclusively via the apoplast, some exclusively via the symplast, and others using a combination of the two (van Bel et al., 1996; Voitsekhovskaja et al., 2009). Apoplastic loading is more prevalent in species from drier environments (Turgeon et al., 2001) and heterogeneity of phloem loading mechanisms has recently been detected in Alonsoa meridionalis (Voitsekhovskaja et al., 2009). Membrane potential of mesophyll cells is sensitive to sucrose and raffinose concentrations (van Bel et al., 1996) thus, changes in growth conditions (in this case dictated by water availability) may lead to changes in the contribution of the symplastic and apoplastic loading pathways.
Altered contributions of symplastic and apoplastic pathways may independently affect isotope composition thus introducing an additional factor in the enrichment of phloem carbon (Cernusak et al., 2009) compared to leaf tissues. It was found that phloem sap carbon obtained from control plants was enriched in 13C by approximately 1‰, compared to leaf soluble carbon, in agreement with a range of studies (Bowling et al., 2008; Cernusak et al., 2009). In an earlier study, Cernusak (2005) identified that 13C enrichment of sink tissues (compared to source leaves) does not result from an enriching process within the sink tissue of E. globulus and argued that the observed enrichment of sink tissues is attributable to isotope fractionation during the transport of carbon from the leaf. Whilst respiratory effects possibly play a role in the enrichment of the remaining carbon pool (Lanigan et al., 2008), it is presently unclear what proportion of these differences (if any) are attributable to the way carbon is exported from the leaf. Large, systematic changes in phloem composition may magnify intermolecular differences in 13C, thus introducing significant changes in phloem δ13C compared to leaf tissues. A logical step in assessing the use of phloem sap as a diagnostic tool is to characterize systematic variations in phloem composition further under conditions of varying water availability.
Significant accumulation of the amino acid phenylalanine, a known precursor for lignin biosynthesis in higher plants and a potential growth indicator for carbon allocation to stem structural components was also detected under water stress. Phenylalanine is a precursor to the first committed step in lignin biosynthesis (Rubery and Northcot, 1968) and may arise under periods of low sink strength due to decreased activity of the phenylalanine ammonia-lyase as has recently been suggested for tobacco (Fritz et al., 2006a, b). In our study, the accumulation of phenylalanine occurred at the expense of glutamine suggesting a change in resource partitioning among the free amino acid pool. Phenylalanine is also a strong antioxidant and may have (albeit less likely) secondary functions in maintaining the reduced (redox) state of the phloem sap. Equally phenylalanine may function as a form of nitrogen storage, although it is not commonly hypothesized for this purpose. Further investigations into the role of phloem sap (such as investigating sink strength and carbon allocation to woody tissues along the transport pathway) will provide a more detailed understanding of carbon fractionation and allocation in woody plants.
Acknowledgments
This work was supported by the Australian Research Council (ARC) linkage program LP 0562661 and discovery program DP 0988731, Great Southern Plantations, Oji paper, Integrated Tree Cropping (ITC), and Timbercorp. The authors would like to thank Ms Andrea Le-Page, Ms Kate Bowler, and Professor Pauline Grierson for assistance with the experimental set-up.
References
- Bowling DR, Pataki DE, Randerson JT. Carbon isotopes in terrestrial ecosystem pools and CO2fluxes. New Phytologist. 2008;178:24–40. doi: 10.1111/j.1469-8137.2007.02342.x. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Arthur DJ, Pate JS, Farquhar GD. Water relations link carbon and oxygen isotope discrimination to phloem sap sugar concentration in Eucalyptus globulus. Plant Physiology. 2003;131:1544–1554. doi: 10.1104/pp.102.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Farquhar GD, Pate JS. Environmental and physiological controls over oxygen and carbon isotope composition of Tasmanian blue gum, Eucalyptus globulus. Tree Physiology. 2005;25:129–146. doi: 10.1093/treephys/25.2.129. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Tcherkez G, Keitel C, et al. Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3plants? Review and synthesis of current hypotheses. Functional Plant Biology. 2009;36:199–213. doi: 10.1071/FP08216. [DOI] [PubMed] [Google Scholar]
- Fritz C, Mueller C, Matt P, Feil R, Stitt M. a. Impact of the C–N status on the amino acid profile in tobacco source leaves. Plant, Cell and Environment. 2006;29:2055–2076. doi: 10.1111/j.1365-3040.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- Fritz C, Palacios-Rojas N, Feil R, Stitt M. b. Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. The Plant Journal. 2006;46:533–548. doi: 10.1111/j.1365-313X.2006.02715.x. [DOI] [PubMed] [Google Scholar]
- Gessler A, Keitel C, Kodama N, Weston C, Winters AJ, Keith H, Grice K, Leuning R, Farquhar GD. delta C13of organic matter transported from the leaves to the roots in Eucalyptus delegatensis: short-term variations and relation to respired CO2. Functional Plant Biology. 2007;34:692–706. doi: 10.1071/FP07064. [DOI] [PubMed] [Google Scholar]
- Gessler A, Rennenberg H, Keitel C. Stable isotope composition of organic compounds transported in the phloem of European beech: evaluation of different methods of phloem sap collection and assessment of gradients in carbon isotope composition during leaf-to-stem transport. Plant Biology. 2004;6:721–729. doi: 10.1055/s-2004-830350. [DOI] [PubMed] [Google Scholar]
- Gessler A, Tcherkez G, Peuke AD, Ghashghaie J, Farquhar GD. Experimental evidence for diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant, Cell and Environment. 2008;31:941–953. doi: 10.1111/j.1365-3040.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Kaur N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. Journal of Biosciences. 2005;30:761–776. doi: 10.1007/BF02703574. [DOI] [PubMed] [Google Scholar]
- Halford NG, Paul MJ. Carbon metabolite sensing and signalling. Plant Biotechnology Journal. 2003;1:381–398. doi: 10.1046/j.1467-7652.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Hartig T. Beitrage zur physiologischen forstbotanik. Forst und Jagdzeitung. 1860;36:257–263. [Google Scholar]
- Keitel C, Adams MA, Holst T, Matzarakis A, Mayer H, Rennenberg H, Gessler A. Carbon and oxygen isotope composition of organic compounds in the phloem sap provides a short-term measure for stomatal conductance of European beech (Fagus sylvatica L.) Plant, Cell and Environment. 2003;26:1157–1168. [Google Scholar]
- Keitel C, Matzarakis A, Rennenberg H, Gessler A. Carbon isotopic composition and oxygen isotopic enrichment in phloem and total leaf organic matter of European beech (Fagus sylvatica L.) along a climate gradient. Plant, Cell and Environment. 2006;29:1492–1507. doi: 10.1111/j.1365-3040.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Papadopoulou E, Rennenberg H. Interaction of flooding with carbon metabolism of forest trees. Plant Biology. 2004;6:299–306. doi: 10.1055/s-2004-817882. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW. Phloem loading and unloading of sugars and amino acids. Plant, Cell and Environment. 2003;26:37–56. [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. Carbon isotope fractionation during photorespiration and carboxylation in. Senecio. Plant Physiology. 2008;148:2013–2020. doi: 10.1104/pp.108.130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Fairbairn DJ, Reid RJ, Schachtman DP. Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability. Plant Physiology. 2001;127:283–294. doi: 10.1104/pp.127.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane C, Adams MA, White DA. Productivity, carbon isotope discrimination and leaf traits of trees of Eucalyptus globulus Labill. in relation to water availability. Plant, Cell and Environment. 2004;27:1515–1524. [Google Scholar]
- Megonigal JP, Day FP. Effects of flooding on root and shoot production of bald cypress in large experimental enclosures. Ecology. 1992;73:1182–1193. [Google Scholar]
- Merchant A, Adams MA, Richter A, Popp M. Targeted metabolite profiling provides a functional link among eucalypt taxonomy, physiology and evolution. Phytochemistry. 2006a;67:402–408. doi: 10.1016/j.phytochem.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Merchant A, Tausz M, Arndt SK, Adams MA. Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant, Cell and Environment. 2006b;29:2017–2029. doi: 10.1111/j.1365-3040.2006.01577.x. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM. Direct evidence for pressure generated closure of plasmodesmata. The Plant Journal. 1992;2:741–750. [Google Scholar]
- Pate J, Shedley E, Arthur D, Adams M. Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus. Oecologia. 1998;117:312–322. doi: 10.1007/s004420050664. [DOI] [PubMed] [Google Scholar]
- Pate JS, Arthur DJ. Uptake, partitioning and utilization of carbon and nitrogen in the phloem bleeding tree, Tasmanian blue gum (Eucalyptus globulus. Australian Journal of Plant Physiology. 2000;27:869–884. [Google Scholar]
- Pate JS, Peoples MB, Atkins CA. Spontaneous phloem bleeding from cryopunctured fruits of a ureide producing legume. Plant Physiology. 1984;74:499–505. doi: 10.1104/pp.74.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS, Sharkey PJ, Lewis OAM. Phloem bleeding form legume fruits: technique for study of fruit nutrition. Planta. 1974;120:229–243. doi: 10.1007/BF00390291. [DOI] [PubMed] [Google Scholar]
- Paul M, Pellny T, Goddijn O. Enhancing photosynthesis with sugar signals. Trends in Plant Science. 2001;6:197–200. doi: 10.1016/s1360-1385(01)01920-3. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Driscoll SP. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant, Cell and Environment. 1997;20:110–116. [Google Scholar]
- Popp M, Lied W, Meyer AJ, Richter A, Schiller P, Schwitte H. Sample preservation for determination of organic compounds: microwave versus freeze drying. Journal of Experimental Botany. 1996;47:1469–1473. [Google Scholar]
- Pourtau N, Mares M, Purdy S, Quentin N, Ruel A, Wingler A. Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta. 2004;219:765–772. doi: 10.1007/s00425-004-1279-5. [DOI] [PubMed] [Google Scholar]
- Ransom-Hodgkins WD, Vaughn MW, Bush DR. Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta. 2003;217:483–489. doi: 10.1007/s00425-003-1011-x. [DOI] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R. A comprehensive picture of phloem loading strategies. Proceedings of the National Academy of Sciences, USA. 2009;106:14162–14167. doi: 10.1073/pnas.0902279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Bevan MW. Genetic approaches to understanding sugar-response pathways. Journal of Experimental Botany. 2003;54:495–501. doi: 10.1093/jxb/erg054. [DOI] [PubMed] [Google Scholar]
- Rubery PH, Northcot D. Site of phenylalanine ammonia-lyase activity and synthesis of lignin during xylem differentiation. Nature. 1968;219:1230. doi: 10.1038/2191230a0. [DOI] [PubMed] [Google Scholar]
- Scartazza A, Mata C, Matteucci G, Yakir D, Moscatello S, Brugnoli E. Comarisons of delta C-13 of photosynthetic products and ecosystem respiratory CO2 and their response to seasonal climate variability. Oecologia. 2004;140:340–351. doi: 10.1007/s00442-004-1588-1. [DOI] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 2008;155:441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- Shvaleva A, Silva FCE, Scotti P, et al. Physiological and biochemical responses to low non-freezing temperature of two Eucalyptus globulus clones differing in drought resistance. Annals of Forest Science. 2008;65 no. 204. [Google Scholar]
- Shvaleva AL, Silva FCE, Breia E, Jouve L, Hausman JF, Almeida MH, Maroco JP, Rodrigues ML, Pereira JS, Chaves MM. Metabolic responses to water deficit in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiology. 2006;26:239–248. doi: 10.1093/treephys/26.2.239. [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant, Cell and Environment. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- Tausz M, Merchant A, Kruse J, Samsa G, Adams MA. Estimation of drought-related limitations to mid-rotation aged plantation grown Eucalyptus globulus by phloem sap analysis. Forest Ecology and Management. 2008;256:844–848. [Google Scholar]
- Thompson MV. Phloem: the long and the short of it. Trends in Plant Science. 2006;11:26–32. doi: 10.1016/j.tplants.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Turgeon R. Phloem loading and plasmodesmata. Trends in Plant Science. 1996;1:418–423. [Google Scholar]
- Turgeon R. Phloem loading: How leaves gain their independence. Bioscience. 2006;56:15–24. [Google Scholar]
- Turgeon R, Medville R. Phloem loading. A re-evaluation of the relationship between plasmodesmatal frequencies and loading strategies. Plant Physiology. 2004;136:3795–3803. doi: 10.1104/pp.104.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Medville R, Nixon KC. The evolution of minor vein phloem and phloem loading. American Journal of Botany. 2001;88:1331–1339. [PubMed] [Google Scholar]
- Turgeon R, Wolf S. Phloem transport: cellular pathways and molecular trafficking. Annual Review of Plant Biology. 2009;60:207–221. doi: 10.1146/annurev.arplant.043008.092045. [DOI] [PubMed] [Google Scholar]
- van Bel AJE, Hendriks JHM, Boon EJMC, Gamalei YV, vandeMerwe AP. Different ratios of sucrose/raffinose-induced membrane depolarizations in the mesophyll of species with symplasmic (Catharanthus roseus, Ocimum basilicum) or apoplasmic (Impatiens walleriana, Vicia faba) minor-vein configurations. Planta. 1996;199:185–192. [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proceedings of the National Academy of Sciences, USA. 2002;99:10876–10880. doi: 10.1073/pnas.172198599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Rudashevskaya EL, Demchenko KN, Pakhomova MV, Batashev DR, Gamalei YV, Lohaus G, Pawlowski K. Evidence for functional heterogeneity of sieve element–companion cell complexes in minor vein phloem of Alonsoa meridionalis. Journal of Experimental Botany. 2009;60:1873–1883. doi: 10.1093/jxb/erp074. [DOI] [PubMed] [Google Scholar]
- West JB, Bowen GJ, Cerling TE, Ehleringer JR. Stable isotopes as one of nature's ecological recorders. Trends in Ecology and Evolution. 2006;21:408–414. doi: 10.1016/j.tree.2006.04.002. [DOI] [PubMed] [Google Scholar]
- White DA, Battaglia M, Macfarlane C, Mummery D, McGrath JF, Beadle CL. Selecting species for recharge management in Mediterranean south western Australia: some ecophysiological considerations. Plant and Soil. 2003;257:283–293. [Google Scholar]
- White DA, Beadle CL, Worledge D. Leaf water relations of Eucalyptus globulus ssp globulus and E. nitens: seasonal, drought and species effects. Tree Physiology. 1996;16:469–476. doi: 10.1093/treephys/16.5.469. [DOI] [PubMed] [Google Scholar]