Abstract
Diel (24 h) leaf growth patterns were differently affected by temperature variations and the circadian clock in several plant species. In the monocotyledon Zea mays, leaf elongation rate closely followed changes in temperature. In the dicotyledons Nicotiana tabacum, Ricinus communis, and Flaveria bidentis, the effect of temperature regimes was less obvious and leaf growth exhibited a clear circadian oscillation.These differences were related neither to primary metabolism nor to altered carbohydrate availability for growth. The effect of endogenous rhythms on leaf growth was analysed under continuous light in Arabidopsis thaliana, Ricinus communis, Zea mays, and Oryza sativa. No rythmic growth was observed under continuous light in the two monocotyledons, while growth rhythmicity persisted in the two dicotyledons. Based on model simulations it is concluded that diel leaf growth patterns in mono- and dicotyledons result from the additive effects of both circadian-clock-controlled processes and responses to environmental changes such as temperature and evaporative demand. Apparently very distinct diel leaf growth behaviour of monocotyledons and dicotyledons can thus be explained by the different degrees to which diel temperature variations affect leaf growth in the two groups of species which, in turn, depends on the extent of the leaf growth control by internal clocks.
Keywords: Circadian clock, elongation, expansion, image analysis, photosynthesis, starch, sucrose
Introduction
Leaf growth is controlled by a complex network of factors. Some of these factors are endogenous regulatory mechanisms that determine leaf shape (Wyrzykowska et al., 2002; Rolland-Lagan et al., 2003), the progression of the cell cycle (Tsukaya and Beemster, 2006), and the relationship of leaf growth to the circadian clock (Nozue and Maloof, 2006). Other factors can be regarded as external, such as the recurring changes of day and night, alterations in temperature, or further physical, chemical, or biotic parameters, which can increase or decrease growth at various time-scales (Granier and Tardieu, 2009; Walter et al., 2009). All growth-controlling environmental effects regulate endogenous mechanisms, but to a different extent. Light quality and quantity are sensed by the phytochrome and cryptochrome photoreceptors (Somers et al., 1998; Devlin and Kay, 2000, 2001) and affect photosynthesis and plant primary metabolism, which is partly controlled by the circadian clock (Harmer et al., 2000). Altered carbohydrate metabolism, in turn, leads to instantaneous and often very specific growth reactions in leaves (Wiese et al., 2007; Sulpice et al., 2009). Temperature changes also induce immediate and clear responses of growth, but it is still uncertain to what extent this is mediated via signalling cascades (Penfield, 2008; Franklin, 2009) or mere thermodynamic laws which affect the rate of all biochemical reactions (Parent et al., 2010). It is well known that the duration of each phase in the plant life cycle is inversely related to temperature (Amir and Sinclair, 1991). This knowledge is at the base of a number of models predicting the progression of the plant life cycle in any climatic scenario (Keating et al., 2003; Yan et al., 2004). It also led to the definition of thermal time: a concept that can be used for the quantitative description of plant or even single leaf development (Granier and Tardieu, 1998; Granier et al., 2002). It has been proposed recently that the rates of most developmental processes are co-ordinated in such a way that temperature-compensated rates and durations can be calculated (Parent et al., 2010).
While, on a long time-scale, leaf growth of different species is tightly correlated to temperature, the response to short-term temperature alterations seems to vary strongly when growth reactions are investigated during a 24 h day (the diel cycle) as indicated by Walter et al. (2009). In monocotyledonous species, leaf elongation rate largely follows temperature alterations (Ben-Haj-Salah and Tardieu, 1995; Pietruszka et al., 2007), whereas in dicotyledonous species, growth patterns do not seem to be related to the daily pattern of temperature variations. In the dicot model species Arabidopsis thaliana, leaf growth, as well as hypocotyl growth, seems to be controlled by the circadian clock (Dodd et al., 2005; Nozue et al., 2007).
The aim of this study was to investigate to what extent the 24 h pattern of leaf growth rate is linked to changes in temperature and/or to endogenous rhythms. Hence, leaf growth variations of several species in fluctuating temperature regimes were compared to (i) leaf growth patterns in a stable temperature and (ii) to the pattern which would be expected if short-term variations in leaf expansion rate followed the response curve to temperature established over longer periods. The involvement of the circadian clock in these patterns was investigated by analysing leaf growth in plants transferred from regular day–night regimes to continuous light. Investigations were done in two monocotyledons, Zea mays and Oryza sativa, and four dicotyledons, Ricinus communis, Nicotiana tabacum, Arabidopsis thaliana, and Flaveria bidentis. Two of these species are C4 (Z. mays and F. bidentis); the others are C3.
Materials and methods
Plant material, climate conditions, and experimental outline
Maize (Zea mays, hybrid Helix), flaveria (Flaveria bidentis), tobacco (Nicotiana tabacum, ecotype Samsun), and castor bean (Ricinus communis, ecotype Carmencita) plants were germinated and grown in the greenhouse for 3 weeks and were then moved to the growth chamber 1 week before experiments started. Plants were grown in pots (0.1 l) filled with a pre-fertilized soil (ED73, Balster Einheitserdewerk, Fröndenberg, Germany). Thale cress (Arabidopsis thaliana, ecotype Ler), rice (Oryza sativa cv. Azucena) and tobacco (Nicotiana tabacum, ecotype Samsun) plants were grown for specific experiments. During all experiments, soil water content was kept at retention capacity.
A growth chamber was set to 22 °C air temperature, 12/12 h day/night and 60% relative humidity. Plants were given 1 week to acclimate to those conditions before the experiments started. Unless specified otherwise, day 1 was the last day with constant air temperature. Between days 2 and 4, air temperature was switched to follow an approximately sine-shaped function centred on 22 °C with a minimum at 17 °C and a maximum at 27 °C. Minimum temperature was reached at the end of the night and maximum temperature at the end of the day. In experiments with A. thaliana and O. sativa, only daylength was varied.
Light intensity measured with a quantum light sensor (LI-190SB; Li-Cor Biosciences GmbH, Bad Homburg Germany) was 600 μmol m−2 s−1 photosynthetically active radiation (PAR). The light was provided by an array of high pressure sodium lamps (MASTER SON-T PIA Agro 400W; Phillips Deutschland GmbH, Hamburg, Germany). In experiments with A. thaliana, light intensity was 120 μmol m−2 s−1 and light was provided by fluorescent lamps (Osram; Fluora, Munich, Germany). Air temperature and relative humidity settings of the climate chamber were measured with a portable data logger placed at canopy level (Testo 175-H1; Testo AG, Lenzkirch, Germany). The experiment with rice was carried out in the Phenodyn phenotyping platform (Sadok et al., 2007). Plants were first grown in the greenhouse under naturally fluctuating light. They were then transferred in a growth chamber under a continuous light intensity of 500 μmol m−2 s−1 at leaf level, measured with a light sensor (LI-190SB, Li-Cor Quantum PAR, Lincoln, NE USA). The meristem temperature, measured with fine copper-constantan thermocouples inserted in the meristematic zones of four non-measured plants per treatment, was kept constant at 26±2 °C.
Growth analysis
R. communis, N. tabacum, and F. bidentis
Investigated leaves were fixed in a stationary position (Walter et al., 2002) by using a strip of Parafilm (Parafilm M, Pechiney Plastic Packaging Company) and kept flat with five nylon threads, clamped to the edge of the leaf using shortened hair clips and fabric tape to protect the leaf. Each of the threads was pulled with a weight (R. communis: 12 g; N. tabacum: 1.5 g; F. bidentis: 5 g) and spun over a metal ring surrounding the leaf (Fig. 1F), a procedure which does not affect temporal or spatial growth patterns (Walter et al., 2002). Leaf images were acquired with high resolution, monochrome CCD cameras (Scorpion SCOR-20SO; Point Grey Research, Vancouver, BC, Canada), positioned above the plants and equipped with a standard objective lens (25 mm; Cosmicar/Pentax, The Imaging Source, Bremen, Germany) and an infrared interference filter (880 nm; Edmund Optics, Karlsruhe, Germany). Constant illumination throughout day and night was provided by six infrared diode clusters (880 nm; Conrad Electronics, Hirschau, Germany). Grey value images were taken every 180 s and saved in a multi-tiff format. Image sequences were evaluated with algorithms based on a structure tensor approach that calculates optical flow via the brightness constancy constraint equation (BCCE) (Bigün and Granlund, 1987; Schmundt et al., 1998). Using the structure tensor approach, velocities of all visible and moving structures at the leaf surface within the image sequence of a growing leaf were calculated. Area relative growth rates (RGR) were calculated as the divergence of the estimated velocity field by selecting an area of interest (AOI) within the image and tracking the structure within this AOI with time. (For more details, see Walter et al., 2002; Schmundt et al., 1998; and Matsubara et al., 2006.)
Fig. 1.
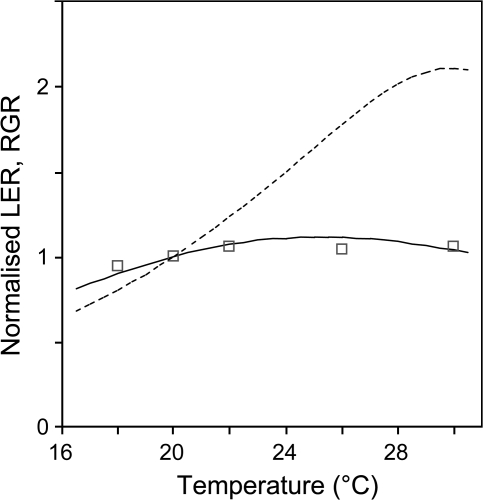
Response curves of leaf expansion rate of Z. mays (dotted line) and N. tabacum (solid lines). Data originate from Parent et al. (2010) for maize and from Raper et al. (1976) for N. tabacum. Symbols show experimental data of Raper et al. (1976). LER, leaf elongation rate. RGR, relative growth rate. Values at 20 °C are by convention set to 1 for normalization (see Parent et al., 2010).
Z. mays, O. sativa, and A. thaliana
Leaf elongation rate of maize was measured with a rotation resistance transducer (RRT, low torque potentiometers Novotechnik, Ostfildern, Germany). A string was fixed to the tip of a growing leaf using shortened hair clips and fabric tape, spun over the pulley of the RRT sensor above the leaf and attached to a 25 g weight. Strings for the measurement of A. thaliana leaf length growth were glued (Pattex, Henkel, Düsseldorf, Germany) to the leaf tip of a growing leaf with c. 1 cm length and spun over the RRT sensor attached in length growth direction to a 1.8 g weight. RRT sensors were interfaced using a Multifunction Data Acquisition device (CompactDAQ National Instruments NI, Austin, Texas, USA) connected to a laptop PC. A custom made LabView 8.5 (National Instruments NI, Austin, Texas, USA) program allowed the wheel rotation to be measured and recorded every 10 s. In the rice leaf, elongation rate was continuously monitored with rotating displacement transducers (RDTs, 601–1045 Full 360 Smart Position Sensor; Spectrol Electronics, Ltd, Wiltshire, England), both in the greenhouse and in the growth chamber. Leaf elongation was transmitted to the sensor via a pulley attached to it, which carried a thread attached to the leaf tip and to a 20 g counterweight (Sadok et al., 2007). Both elongation and climatic data were stored every 15 min in the Phenodyn database (http://bioweb.supagro.inra.fr/phenodyn/).
Modelling the temperature response
Leaf expansion rate under fluctuating temperature was compared to that expected from the response curves to temperature (Fig. 1). For maize, the response curve corresponding to several developmental processes was published by Parent et al. (2010). For N. tabacum, they originate from the study of Raper et al. (1976). All data were fitted to a Johnson et al. (1942) function, as in Parent et al. (2010).
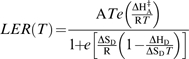 |
where LER is leaf expansion rate, T is temperature, A is a proportionality constant, ΔHA‡ (kJ mol−1) is a fitted parameter representing the enthalpy of activation of the equivalent reaction. ΔHD and ΔSD are fitted parameters representing the enthalpy and entropy between the catalytically active and inactive states of the enzyme or enzymatic system. Parameters of response curves were calculated in three steps using the R language (R_Development_Core_Team, 2005). Data were first smoothed by a second order polynomial equation in a range of 5 °C at steps of 1 °C each. The inflexion point was determined where the slope of the linear regression on three consecutive points was maximum. This step was omitted for the tobacco data which lacked datapoints at low temperatures. The parameters of the numerator were determined by linear regression on transformed variables (ln (J/T), 1/T) in the range of temperatures lower than the inflexion point. The parameters of the denominator of equation 1 were determined by linearization in the range of temperatures above the inflection point following the method presented in Parent et al. (2010). The resulting parameters were ΔHA=3.71 and 1.5, ΔHD=285 and 135, and ΔSD=0.93 and 0.46, for Z. mays and N. tabacum, respectively.
Carbohydrates
In the main experiment, leaves were harvested for carbohydrate analysis when the lamps were switched on and off (every 12 h), respectively. For each species, two plants were sampled and from each plant, two leaf discs (diameter 8 mm) were punched out of growing leaves (the same position as the leaves taken for growth analyses). Leaf discs were weighed, pooled, frozen in liquid nitrogen, and stored at –80 °C for further extraction. Soluble carbohydrates were extracted from frozen leaf material and glucose, fructose, sucrose, and starch concentrations were analysed in a coupled enzyme assay (Jones et al., 1977) using a multiplate spectrophotometer (ht II; Anthos Mikrosysteme GmbH, Krefeld, Germany) as described in Walter et al. (2002) and Wiese et al. (2007).
Gas exchange
Gas exchange of growing leaves of each investigated species was measured using a portable, open path design, infrared gas-exchange system (Li-6400; Li-Cor Biosciences GmbH, Bad Homburg, Germany). The area of the leaf chamber was 6 cm2. Light intensity and temperature inside the leaf chamber were allowed to follow the ambient conditions of the growth chamber. To avoid large fluctuations of the reference CO2, the air that went to the analyser inside the growth chamber was buffered using a 50 l plastic tank linked to the greenhouse with a nozzle.
Results
Leaf expansion of dicotyledonous species did not follow temperature variations while leaf elongation of maize did
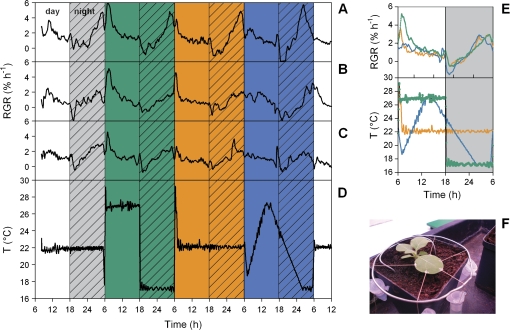
Similar diel leaf growth patterns occurred in tobacco plants regardless of the temperature regime (Fig. 2A–D). After having been grown at a constant temperature of 22 °C until the start of the experiment, plants were exposed on day 1 to a constant daytime temperature of 27 °C, followed by a night temperature of 17 °C. On day 2, temperature was set to a constant value of 22 °C again and on day 3, temperature was modulated between an afternoon peak temperature of 27 °C and a minimum of 17 °C at the end of the night. The average diel leaf growth pattern was almost identical during all three days (Fig. 2E).
Fig. 2.
Relative growth rates (RGR) of leaves of N. tabacum grown in different temperature regimes. (A, B, C) The RGR of three replicate plants, respectively. The temperature regime to which these plants were exposed is depicted in (D). Average values are shown in (E) with colours coding for the three different temperature regimes to which the plants were subsequently exposed. Positioning of the leaf for growth analysis is shown in (F).
In a second experiment; daily leaf growth cycles of four species were investigated with plants being exposed either to a constant temperature of 22 °C or to a fluctuating temperature regime with an afternoon maximum of 27 °C and a night minimum of 17 °C (Fig. 3). On day 1, with constant temperature, pronounced diel leaf growth variations were observed with maxima in the early morning or late at night for N. tabacum, R. communis, and F. bidentis. Despite variations between individuals, RGR varied significantly between the times of maximal and minimal growth activity during the diel cycle. Between days 2 and 4, plants were exposed to the fluctuating temperature regime. Diel growth cycles of the dicot species still reached maximal growth at the same time of the day, but the amplitude was damped and differences between maxima and minima were not significant any longer. By contrast, Zea mays leaves grew with a constant rate throughout the day and night when temperature was constant and developed a pronounced variation in the fluctuating temperature regime. On day 1, leaf elongation rate only changed transiently when the light was switched on or off. On day 2, leaf elongation rate decreased sharply in the morning and increased throughout the day, reaching a maximum value in the early hours of the night, when temperature was still high. During the night, leaf elongation rate declined, reaching a plateau value at the end of the night, when temperature also reached a low plateau value. On day 3 and day 4, these patterns were repeated and the correlation between leaf elongation rate and temperature became even more prominent. Maximal leaf elongation occurred as soon as temperature reached its maximum value, without any appreciable delay, taking into account the temporal definition used in this experiment (10 s). When data from the two different temperature treatments was averaged (Fig. 3, right panels), it became obvious that growth patterns in the two dicot species were far less related to temperature than in Z. mays.
Fig. 3.
Leaf growth of various plants from different species in different temperature regimes. Temperature regime was switched from constant to fluctuating at the beginning of day 2 (lower panels). For N. tabacum, R. communis, and F. bidentis, relative growth rates (RGR) were measured, while for Z. mays, leaf elongation rate (LER) was analysed. Right panels show average values; different line colours show different replicate plants. Asterisks indicate the significance level of growth differences in consecutive time intervals (framed in boxes; T-test): **P <0.01, *P >0.05; n.s. not significant.
Fluctuations in carbohydrate availability and gas exchange did not account for differences in diel leaf expansion patterns between species
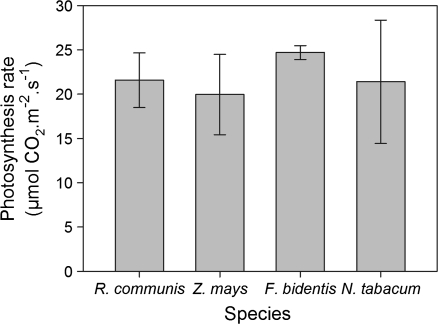
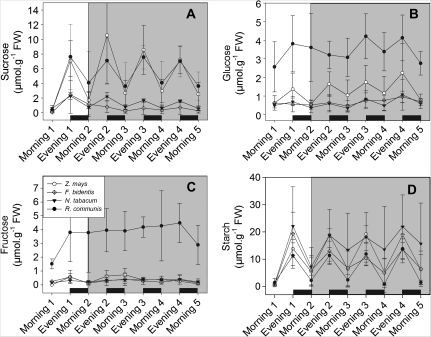
Gas exchange and carbohydrate analyses were performed in Z. mays, F. bidentis, N. tabacum, and R. communis. Assimilation rates of all the species studied were high (20 μmol m−2 s−1 CO2 and more; Fig. 4) reflecting near optimal growth conditions and thus a good vegetative status. Carbohydrate analyses showed increasing contents of starch and sucrose in all species during the day and a decrease at night (Fig. 5). On day 1 (constant temperature) the nocturnal decrease in starch was similar in all species. On days 2, 3, and 4 (fluctuating temperature regime) starch fluctuations in N. tabacum and F. bidentis decreased. Flaveria bidentis has a very low overall soluble sugar concentration with values ranging between 0 and 1 μmol g−1 FW, which do not fluctuate strongly throughout the day. For sucrose, the strongest fluctuations were seen in Z. mays and R. communis. Overall, no pronounced differences in carbohydrate fluctuations between the two different temperature regimes were observed for any of the species studied.
Fig. 4.
Assimilation rate of growing leaves. Mean value and SD (n=4).
Fig. 5.
Carbohydrate concentrations of growing leaves. (A) Sucrose, (B) glucose, (C) fructose, and (D) starch. During day 1, plants were exposed to constant temperature, thereafter (grey background) temperature fluctuated as shown in Fig. 2. Mean value and SD (n=4).
In continuous light, dicotyledonous species revealed diel oscillations of leaf expansion that were not observed in monocotyledonous species
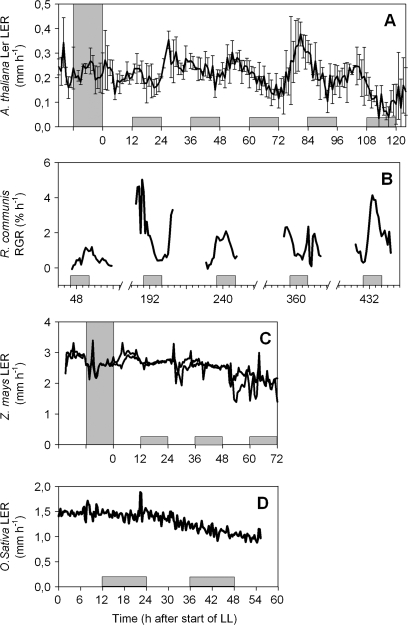
To investigate the effect of the circadian clock on the rhythmicity of leaf growth patterns, the leaf elongation rates of the monocotyledonous species Z. mays and O. sativa and of the dicotyledonous species R. communis and A. thaliana were investigated in continuous light (Fig. 6). Plants that had been exposed to fluctuating light until the first day of analysis were transferred to constant light (LL) for at least 3 d. In A. thaliana and R. communis, the diel leaf growth cycle clearly continued. In R. communis, leaves from different plants were followed over a long time period (18 d) after transition. In A. thaliana, leaf growth was followed in the same leaves from the day before light transition throughout some days of LL, demonstrating a higher period length than 24 h. By contrast, leaf growth of Z. mays and of O. sativa did not oscillate markedly in a diel manner until the end of the experiment.
Fig. 6.
Leaf growth of (A) A. thaliana, (B) R. communis, (C) Z. mays, and (D) O. sativa transferred from 12/12 h day/night to a continuous light regime. Hatched bars represent subjective night in the continuous light treatment. For Z. mays, replicate plants are shown with different lines; for A. thaliana, mean value and SD (n=4) are shown.
An addition of the effects of endogenous rhythms and temperature accounted for experimentally observed patterns in all studied species
Maize and Nicotiana tabacum were tested to find out to what extent the experimental patterns observed under fluctuating temperature (days 2–4; Fig. 3) could be accounted for by the additive effects of endogenous rhythms (day 1; Fig. 3) and temperature dependency of leaf growth (presented in Fig. 1). The endogenous rhythm was considered as flat in maize, consistent with Fig. 3 (day 1). The endogenous rhythm of N. tabacum was inferred from the mean pattern on day 1 (Fig. 3), with a minimum expansion rate at 12.00 h. Although maize and tobacco were subjected to the same temperature scenarios, simulated temporal patterns of leaf expansion rate deduced from the response curves presented in Fig. 1 differed markedly (Fig. 7). Because the response curve reached a plateau in tobacco at temperatures which still caused an increase in elongation rate of maize, the simulated pattern of temperature effect was much more pronounced in maize than in tobacco (Fig. 7B, D).
Fig. 7.
Addition of the effects of temperature and endogenous rythms in Z. mays (A, B) and N. tabacum (C, D). (A, C) Effect of temperature (solid line) and of endogenous rhythms (dotted line). (B, D) Experimental diel pattern (mean of experiments on days 2–4, solid lines) and simulation from the superposition of the two effects presented in (A) and (C).
In maize, the addition of the two effects correctly simulated the experimental pattern, with two exceptions: (i) a marked peak of leaf elongation rate followed light extinction, (ii) observed elongation rates were lower than the simulated ones during the day, and higher during the night. These two exceptions are probably linked to the effect of evaporative demand which lowers maize leaf expansion rate during the day (Sadok et al., 2007). In tobacco, the additive effects of endogenous rhythms and temperature also simulated the observed leaf growth pattern correctly. Observed and simulated patterns were consistent throughout the day, except for (i) a marked oscillation of RGR at 12 h, as in the case of maize, when the lights were turned off and (ii) an unexplained discrepancy 4 h after the lights were turned on. Overall, the additive effects of endogenous rhythms and temperature account for the observed leaf growth patterns, but a supplementary effect of evaporative demand was noted.
Discussion
The rhythmic variation of leaf growth in A. thaliana and N. tabacum with a slightly extending periodicity under continuous light (Fig. 3B) shows that this diel pattern is controlled by the circadian clock. This confirms findings on the overall growth performance of Arabidopsis plants (Dodd et al., 2005) and on the circadian control of hypocotyl elongation (Dowson-Day and Millar, 1999; Nozue et al., 2007). From an evolutionary point of view, a correct phasing between plant metabolism and forthcoming environmental conditions represents a crucial advantage compared with organisms with a coarser regulation (Dodd et al., 2005; Kobayashi and Weigel, 2007; Resco et al., 2009). Because temperature compensation is an essential feature of the circadian clock (Harmer, 2009), the periodicity and amplitude of rhythmic processes that are controlled by the circadian clock are affected only to a minor extent by temperature variations (Nakajima et al., 2005). When measured leaf growth patterns are compared with patterns calculated from the effects of temperature on leaf growth at the longer time-scale (Fig. 4), it becomes clear that diel leaf growth variations in all species can be considered as the added effects of circadian-clock-controlled processes and temperature-related processes, with an additional effect of evaporative demand consistent with Sadok et al. (2007). The main difference between leaf growth in monocotyledonous and dicotyledonous species is that, in dicotyledons, circadian effects are much more pronounced than in monocotyledons, where they can be neglected. In addition, the temperature effect was lower in N. tabacum than in maize because of its lower sensitivity to temperature in the range considered. Although many processes throughout the plant kingdom are controlled by the circadian clock, the degree to which the circadian clock affects other processes differs between tissues, organs, and species (McClung, 2006; Hotta et al., 2007; James et al., 2008; Jones, 2009). More precisely, the circadian system is a network of more than one single clock inside the plant with independent oscillators in each cell (Jones, 2009) that can elucidate a multitude of output responses in a multicellular organism. Hence, it comes as no surprise that in a recent DNA-microarray study, roots were found to be much less affected by the circadian clock than shoots (James et al., 2008).
A reason for the observed difference in the significance of the circadian clock for leaf growth processes in monocot and dicot species might be found in the different organization and architectural location of their growth zones. The cellular organization of monocot leaf growth zones is very similar to that of roots, which is probably related to the similar constraints to which these tissues have been exposed in an evolutionary context (Walter et al., 2009). In contrast to dicotyledonous leaf growth zones, root apices and growth zones of monocotyledonous leaves are protected from atmospheric temperature variations by being embedded in the soil or within the sheaths of older leaves, respectively. Moreover, the growth zones of roots and monocotyledonous leaves are not involved in photosynthesis, which is itself a process strongly controlled by the circadian clock (Harmer et al., 2000).
Primary metabolism does not differ strongly between monocot and dicot species, which is reflected in prominent differences between starch and sucrose concentrations in the morning and evening in young leaves of both plant types under both temperature regimes. The periodic fluctuations of starch and sucrose in Z. mays in constant temperature suggest that light–dark cycles exert a stronger effect than available carbohydrates on growth patterns in monocotyledonous leaves.
The homologues of genes of the central oscillator of the plant circadian clock are conserved in monocots (Miwa et al., 2006). Nevertheless, our data shows that maize and rice leaf growth proceeds constantly throughout the diel cycle when plants are transferred from a day–night growth regime into continuous light (Fig. 5A). The fact that leaf growth of the C4 dicot species F. bidentis responds similarly to temperature alterations as the investigated C3 dicot species indicates that differences between maize and the investigated dicot species are related to differences in the two families (possibly their growth zone organization) rather than to differences in photosynthetic metabolism. This is confirmed by the growth pattern of O. sativa, which as a C3 monocot species does not show internal growth rhythms.
Acknowledgments
We thank Beate Uhlig and B Suard for their support during the growth of the plants. We acknowledge receipt of Flaveria bidentis seeds from Robert Furbank. RP thanks the International Helmholtz Research School of Biophysics and Soft Matter for stimulating discussions, and RP and MM acknowledge the support of their PhD theses at the Heinrich-Heine-Universität Düsseldorf.
References
- Amir J, Sinclair TR. A model of the temperature and solar radiation effects on spring wheat growth and yield. Field Crops Research. 1991;28:47–58. [Google Scholar]
- Ben-Haj-Salah H, Tardieu F. Temperature affects expansion rate of maize leaves without change in spatial distribution of cell length: analysis of the coordination between cell division and cell expansion. Plant Physiology. 1995;109:861–870. doi: 10.1104/pp.109.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigün J, Granlund GH. Optimal orientation detection of linear symmetry. International Cconference on Computer Vision. 1987:433–438. [Google Scholar]
- Devlin P, Kay S. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. The Plant Cell Online. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin P, Kay S. Circadian photoperception. Annual Review of Physiology. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. The Plant Journal. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- Franklin KA. Light and temperature signal crosstalk in plant development. Current Opinion in Plant Biology. 2009;12:63–68. doi: 10.1016/j.pbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Granier C, Massonnet C, Turc O, Muller B, Chenu K, Tardieu F. Individual leaf development in Arabidopsis thaliana: a stable thermal-time-based programme. Annals of Botany. 2002;89:595–604. doi: 10.1093/aob/mcf085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Is thermal time adequate for expressing the effects of temperature on sunflower leaf development? Plant, Cell and Environment. 1998;21:695–703. [Google Scholar]
- Granier C, Tardieu F. Multi-scale phenotyping of leaf expansion in response to environmental changes: the whole is more than the sum of parts. Plant, Cell and Environment. 2009;32:1175–1184. doi: 10.1111/j.1365-3040.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- Harmer SL. The circadian system in higher plants. Annual Review of Plant Biology. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H, Han B, Zhu T, Wang X, Kreps JA, Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR. Modulation of environmental responses of plants by circadian clocks. Plant, Cell and Environment. 2007;30:333–349. doi: 10.1111/j.1365-3040.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- James AB, Monreal JA, Nimmo GA, Kelly CL, Herzyk P, Jenkins GI, Nimmo HG. The circadian clock in Arabidopsis roots is a simplified slave version of the clock in shoots. Science. 2008;322:1832–1835. doi: 10.1126/science.1161403. [DOI] [PubMed] [Google Scholar]
- Johnson FH, Eyring H, Williams RW. The nature of enzyme inhibitions in bacterial luminescence: sulfanilamide, urethane, temperature and pressure. Journal of Cellular and Comparative Physiology. 1942;20:247–268. [Google Scholar]
- Jones MA. Entrainment of the Arabidopsis circadian clock. Journal of Plant Biology. 2009;52:202–209. [Google Scholar]
- Jones MGK, Outlaw WH, Jr, Lowry OH. Enzymic assay of 10−7to 10−14moles of sucrose in plant tissues. Plant Physiology. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating BA, Carberry PS, Hammer GL, et al. An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy. 2003;18:267–288. [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it's time for change: mobile signals controlling photoperiod-dependent flowering. Genes and Development. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Hurry V, Druart N, Benedict C, Janzik I, Chavarria-Krauser A, Walter A, Schurr U. Nocturnal changes in leaf growth of Populus deltoides are controlled by cytoplasmic growth. Planta. 2006;223:1315–1328. doi: 10.1007/s00425-005-0181-0. [DOI] [PubMed] [Google Scholar]
- McClung CR. Plant circadian rhythms. The Plant Cell. 2006;18:792–803. doi: 10.1105/tpc.106.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Serikawa M, Suzuki S, Kondo T, Oyama T. Conserved expression profiles of circadian clock-related genes in two Lemna species showing long-day and short-day photoperiodic flowering responses. Plant and Cell Physiology. 2006;47:601–612. doi: 10.1093/pcp/pcj027. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- Nozue K, Maloof JN. Diurnal regulation of plant growth. Plant, Cell and Environment. 2006;29:396–408. doi: 10.1111/j.1365-3040.2005.01489.x. [DOI] [PubMed] [Google Scholar]
- Parent B, Turc O, Gibon Y, Stitt M, Tardieu F. Modelling temperature-compensated physiological rates, based on the coordination of responses to temperature of developmental processes. Journal of Experimental Botany. 2010 doi: 10.1093/jxb/erq003. doi:10.1093/jxb/erq003. [DOI] [PubMed] [Google Scholar]
- Penfield S. Temperature perception and signaltransduction in plants. New Phytologist. 2008;179:615–628. doi: 10.1111/j.1469-8137.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- Pietruszka M, Lewicka S, Pazurkiewicz-Kocot K. Temperature and the growth of plant cells. Journal of Plant Growth Regulation. 2007;26:15–25. [Google Scholar]
- R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2005. R: a language and environment for statistical computing. [Google Scholar]
- Raper CD, Weeks WW, Wann M. Temperatures in early post-transplant growth: influence on carbohydrate and nitrogen utilization and distribution in tobacco. Crop Science. 1976;16:753–757. [Google Scholar]
- Resco V, Hartwell J, Hall A. Ecological implications of plants' ability to tell the time. Ecology Letters. 2009;12:583–592. doi: 10.1111/j.1461-0248.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Rolland-Lagan A, Bangham JA, Coen E. Growth dynamics underlying petal shape and asymmetry. Nature. 2003;422:161–163. doi: 10.1038/nature01443. [DOI] [PubMed] [Google Scholar]
- Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F. Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant, Cell and Environment. 2007;30:135–146. doi: 10.1111/j.1365-3040.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Schmundt D, Stitt M, Jähne B, Schurr U. Quantitative analysis of the local rates of growth of dicot leaves at a high temporal and spatial resolution, using image sequence analysis. The Plant Journal. 1998;16:505–514. [Google Scholar]
- Somers DE, Devlin PF, Kay SA. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science. 1998;282:1488–1490. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, et al. Starch as a major integrator in the regulation of plant growth. Proceedings of the National Academy of Sciences, USA. 2009;106:10348–10353. doi: 10.1073/pnas.0903478106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Beemster GTS. Genetics, cell cycle and cell expansion in organogenesis in plants. Journal of Plant Research. 2006;119:1–4. doi: 10.1007/s10265-005-0254-y. [DOI] [PubMed] [Google Scholar]
- Walter A, Feil R, Schurr U. Restriction of nyctinastic movements and application of tensile forces to leaves affects diurnal patterns of expansion growth. Functional Plant Biology. 2002;29:1247–1258. doi: 10.1071/PP01255. [DOI] [PubMed] [Google Scholar]
- Walter A, Silk WK, Schurr U. Environmental effects on spatial and temporal patterns of leaf and root growth. Annual Review of Plant Biology. 2009;60:279–304. doi: 10.1146/annurev.arplant.59.032607.092819. [DOI] [PubMed] [Google Scholar]
- Wiese A, Christ MM, Virnich O, Schurr U, Walter A. Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle. New Phytologist. 2007;174:752–761. doi: 10.1111/j.1469-8137.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- Wyrzykowska J, Pien S, Hui Shen W, Fleming AJ. Manipulation of leaf shape by modulation of cell division. Development. 2002;129:957–964. doi: 10.1242/dev.129.4.957. [DOI] [PubMed] [Google Scholar]
- Yan HP, Kang MZ, Reffye P, Dingkun M. A dynamic, architectural plant model simulating resource-dependent growth. Annals of Botany. 2004;93:591–602. doi: 10.1093/aob/mch078. [DOI] [PMC free article] [PubMed] [Google Scholar]