Abstract
Background
Wide local excision of primary cutaneous melanoma usually provides clear margins and excellent local control. Nonclassical presentations of cutaneous melanoma, however, can challenge this treatment algorithm. Specifically, persistent melanoma-in-situ (MIS) at the margin not suspected clinically makes planning definitive wide local excision more difficult. We hypothesized that the use of punch biopsies as a mapping tool would allow us to obtain clear margins in these challenging cases.
Methods
Punch biopsies were performed at sites 1 to 2 cm from prior positive margins. Subsequent wide local excision was planned, including all punch biopsy sites with positive findings: atypical melanocytic hyperplasia, MIS, or invasive melanoma. The management of three patients was documented prospectively. Standard surgical techniques were used independent of an experimental protocol. Medical records were reviewed, and data were summarized under institutional review board protocol HIC 10803.
Results
The results of punch biopsies identified invasive melanoma, MIS, or atypical melanocytic hyperplasia in all three patients with MIS at the margins. All three mapping procedures were well tolerated and resulted in resection with negative margins in a single definitive resection.
Conclusion
Melanoma mapping with punch biopsy technique allows for definitive excision in cases when disease persists at the margins of the reexcision or in cases with unclear clinical examinations.
Wide local excision of a primary cutaneous melanoma usually provides clear margins around a primary melanoma and excellent local control. Nonclassical presentations of cutaneous melanoma, however, continue to challenge the physician and may not fit into the typical management algorithm. This is especially the case when the radial growth phase component of the melanoma is nonpigmented and not clinically evident.1 Occasionally, the radial growth phase component of a melanoma may extend well beyond the visible extent of the melanoma. The planning of a wide local excision is based on a reliable skin examination and a location where adequate margins can be achieved. In cases of extensive atypical melanocytic hyperplasia (AMH) and melanoma-in-situ (MIS), defining margins can be more difficult.
Standard management of MIS includes resection to a clear margin. Leaving MIS in place is associated with risk of subsequent development of invasive melanoma, with associated mortality risk.2–4 Especially when an invasive melanoma arises from an area of extensive MIS, it is possible that other areas of invasive melanoma may have arisen concurrently from a different location within that area of MIS. Thus, it is prudent to reexcise to clear margins if MIS is present at the original wide excision margin. In cases of AMH that are related to the primary melanoma, reexcision to clear margins is also indicated.
We have developed a melanoma mapping technique that permits tissue sampling to enable clear margins. This method minimizes morbidity and maintains a traditional surgical approach for obtaining adequate local control of primary or locally recurrent melanoma and is comparable to a technique described to define tumor margins in angiosarcoma.5 Although similar staged surgical procedures have been described in melanoma,6,7 our technique offers the advantage of performing simple punch biopsies without use of a Wood's lamp and the ability to evaluate the tissue at multiple distances from the tumor in a single procedure. We have used this melanoma mapping technique in the setting of extensive MIS or invasive melanoma at or beyond the initial surgical margins of resection. We present this methodology and three cases in which it has been applied at our institution.
Patients and Methods
During the past several years, three patients presented with invasive melanoma, MIS at initial margins, and one or more complexities in their clinical presentation for which there is no standard management recomendation to ensure a clear margin. We developed an approach for mapping the extent of melanoma that was successful in several patients and may have broader applicability. This mapping method uses standard surgical techniques and was not part of an experimental protocol. The management of three patients was documented prospectively with photographic and written records during their clinical care. The medical records for these patients were reviewed, and data were summarized under institutional review board protocol HIC 10803.
For patients with extensive radial growth phase melanoma manifested by MIS or invasive melanoma extending to surgical margins in the wide excision specimen, before additional surgical resection, the following steps were taken. Marks were made on the skin to identify biopsy sites 1 cm beyond the scar or residual melanoma lesion. They were placed 1 to 2 cm away from each other surrounding the scar or resection margins (Fig. 1A). Additional biopsy sites were marked in an outer concentric circle 1 cm peripheral to the inner sites. These were 2 cm away from the scar (Fig. 1B).
FIG. 1.
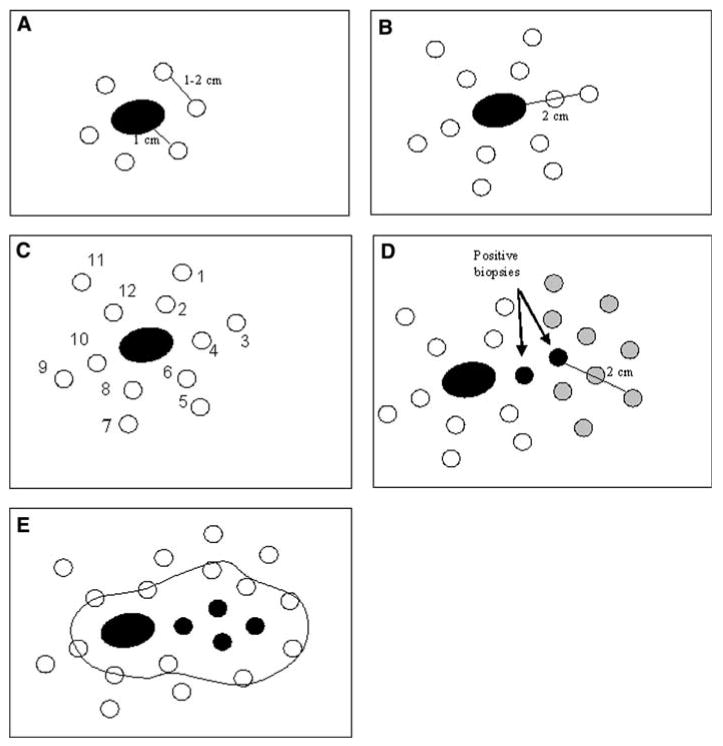
Schematic representation of melanoma mapping technique.
Each biopsy site was marked on the skin with a number, ideally in the order it would be sampled, moving from most peripheral to least peripheral. The biopsy site marks were documented by photographs and a drawing submitted with the pathology requisition. Punch biopsies were performed, and samples 2 mm in diameter were obtained at the identified sites. The same punch biopsy instrument was used for each of two separate biopsies. The first sample with each instrument was taken at the more peripheral location to avoid contamination from a tumor area to a tumor-free area (e.g., sites 1 and 2 with one instrument, then 3 and 4 with a second instrument). Each specimen was labeled unambiguously with the biopsy site number and was sent along with a sketch of the biopsy site locations (Fig. 1C).
For a positive punch biopsy report (AMH, MIS, or invasive melanoma) at a peripheral location (e.g., 2 cm from the prior scar or lesion), the mapping technique could be repeated on a subsequent day to sample surrounding tissue out to an additional 1 and 2 cm beyond that site (Fig. 1D).
After adequate mapping, wide local excision of skin extending at least 1 cm beyond all positive or borderline biopsy sample sites was performed (Fig. 1E). Margins were evaluated on the excision specimen in accord with standard practice.
Case Presentations: Application of Technique
We present three cases that illustrate the use of melanoma mapping technique to define surgical resections in situations not optimally treated by the standard algorithm.
Patient 1
The first patient is a 72-year-old white man who was referred to our institution for evaluation and treatment of locally recurrent melanoma on the glabrous skin of the palm of his right hand. Nine years before seeking care at our institution, he had been diagnosed with an acral lentiginous melanoma (Clark level IV, Breslow thickness 2.1 mm) at this site. He had received appropriate surgical treatment, including a wide local excision and a split-thickness skin graft, and he had negative sentinel lymph node biopsy findings (T3aN0M0, stage IIA). Forty months after this, and approximately 5 years before coming to this institution, he experienced local recurrence with an invasive melanoma (Clark level III, Breslow depth 1.17 mm). He again underwent a wide local excision and repeat sentinel lymph node biopsy at the same original cancer center. The sentinel node biopsy findings were negative, but there was atypical melanocytic proliferation at multiple margins. These positive margins were not reresected, but he was treated with adjuvant radiotherapy (30.0 Gy in five fractions) and high-dose systemic interferon treatment for 1 year.
Nine years after his initial diagnosis, the patient developed a 5-mm-diameter recurrent pigmented lesion at the ulnar edge of his original skin graft, which underwent biopsy and was read as MIS, and he sought care at our institution. We performed another wide local excision with approximately a 1-cm margin and applied a full-thickness skin graft. However, histologic evaluation of the excision margins revealed MIS at all margins. None of the surrounding skin appeared pigmented or otherwise suspicious, but an additional 1-cm margin was excised, including the peripheral edge of the recent skin graft. The defect was covered with a doughnut-shaped full-thickness skin graft, preserving the central part of the recently applied skin graft in the center (Fig. 2B). Despite now having excised margins approximately 2 cm beyond the clinically evident local recurrence, histologic examination of the specimen revealed focally invasive Clark level III malignant melanoma 0.9 mm in depth, with extensive MIS extending to the proximal and distal aspects of the radial (lateral) margins.
FIG. 2.
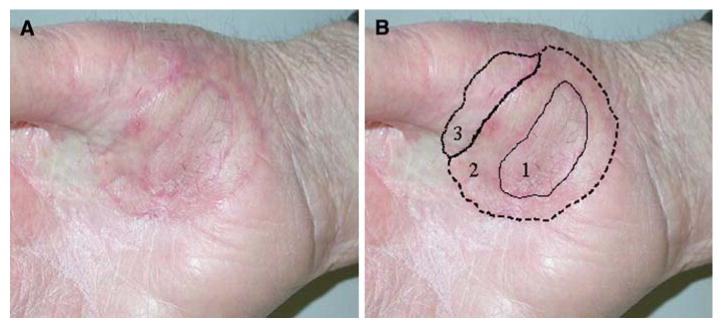
Patient 1. After 3 excisions and skin grafts (numbered and shown with solid, dashed, and dotted lines, respectively), there was still a positive margin at the radial aspect of excision 3. There is no clinically evident pigmented lesion. There is some fibrosis and scar from previous radiation and surgery.
The surrounding skin had no clinically evident melanocytic lesions (Fig. 2A). To determine the additional margin required while salvaging hand function, a skin-mapping technique was used. Under local anesthesia, in the outpatient clinic, eleven 2-mm punch biopsies were performed in the areas surrounding the graft margins (Fig. 3). Three of the 11 punch biopsy samples (procedures 4, 5, and 7) revealed MIS, and the results of biopsy 6 had superficially invasive, Clark level II, 0.35-mm malignant melanoma. Ten additional punch biopsies were then performed on a separate day, marking 1 and 2 cm out from original biopsy sites 4, 5, 6, and 7 (Fig. 3C, labels A–J). Of these second-tier biopsies, the only pathologic abnormality was atypical junctional melanocytic hyperplasia (Fig. 3C, labels A and B).
FIG. 3.
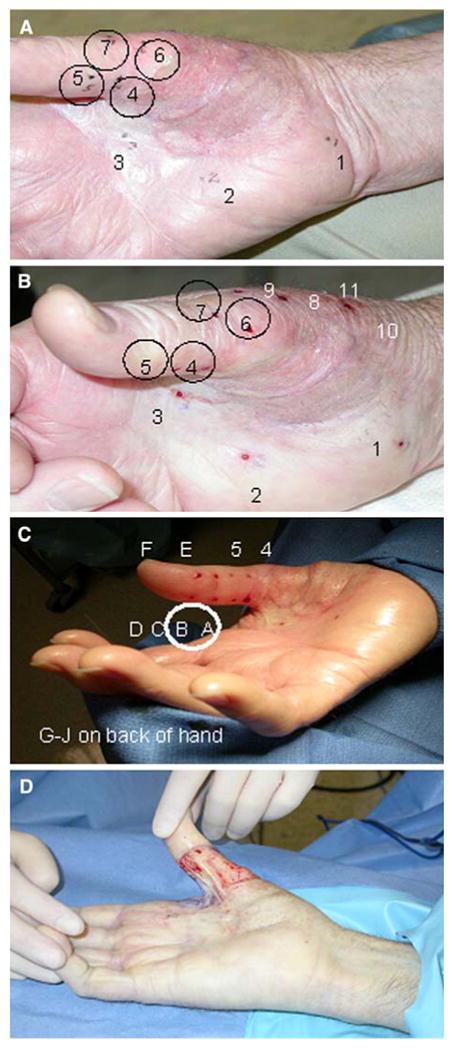
Patient 1. (A) Mapping of inner and outer circle, 1 and 2 cm from positive margin. Biopsy sites 4, 5, and 7 revealed melanoma-in-situ (MIS), and site 6 revealed invasive melanoma. (B) Additional biopsies, 8 to 11. (C) Secondary mapping procedure 2 cm from each positive biopsy site. The only pathologic abnormality was atypical junctional melanocytic hyperplasia at biopsies A and B. Wide local excision was performed to include all positive sites. (D) Intraoperative photograph after wide local excision.
Wide surgical reexcision was performed, taking 1-cm margins beyond all positive biopsy sites (Figs. 1E and 3D). A split-thickness skin graft was applied. The total width of this patient's mapped-out melanoma was approximately 6 cm in diameter, which was dramatically more extensive than the clinically evident lesion. He has had good functional recovery after skin grafting, and since the last repair, the patient has had regular follow-up with his primary dermatologist and has been without evidence of recurrence for 28 months.
Patient 2
The second patient is a 46-year-old white woman who presented with an ulcerated 2.1-mm, Clark level IV melanoma of her right upper back with microscopic metastasis to a right axillary sentinel lymph node (T3b, N1a, M0, stage IIIB). She underwent wide local excision with a 2-cm margin in October 2006. The original pathology review reported diffuse lentiginous melanocytic proliferation with some cellular atypia and hyperplasia extending to numerous margins. These concerning margins were reviewed and discussed with her surgeon and dermatologist, and she was advised that she likely had extensive melanocytic atypia all over her back for which meaningful margins would not likely be achievable. She was concerned about recurrence risk and sought a second opinion at our institution, and our dermatopathology review was that the atypical melanocytic changes extending throughout the wide excision specimen met criteria for diagnosis of MIS. This MIS extended to the superior and inferior margins and was continuous with the original biopsy scar. She had extensive sun damage and numerous nevi on the skin of her back, and there was irregular pigmentation, but there was no identifiable clinical correlate to the histologic findings of MIS (Fig. 4A). The original recommendation had been to follow her skin examinations clinically rather than perform additional surgery.
FIG. 4.
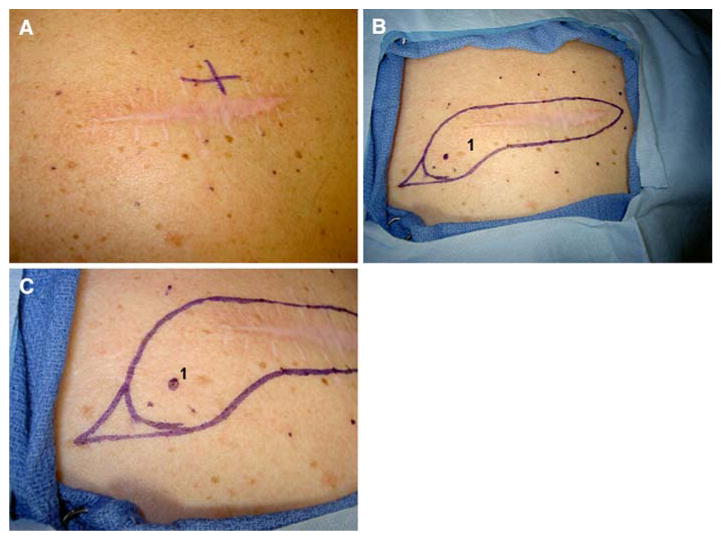
Patient 2. (A) Scar from previous melanoma excision with melanoma-in-situ (MIS) at multiple margins, multiple nevi, and extensive sun damage. (B) Mapping of biopsy sites, 1 and 2 cm away from the original scar and 1 cm apart from one another. Only biopsy 1 revealed any pathologic abnormality (MIS). (C) Magnified view of planned excision, including 1-cm margin around the scar and 1.5-cm margins around the positive biopsy site.
Her sister had died years before from melanoma while in her 30s, and our patient had thus been vigilant, with careful and routine dermatologic surveillance every 3 months. Despite that, her melanoma had been diagnosed at a relatively advanced stage, so she was uncomfortable with a watch-and-wait approach.
The decision was made to assess whether there was histologic evidence of MIS extending so wide that it would not be resectable, or whether a reasonable reresection margin could, in fact, be defined. The melanoma mapping technique was used to address this question and to guide surgical intervention for local control. Sixteen 2-mm punch samples were taken by punch biopsy 1 and 2 cm away from the scar (Fig. 4B). Despite the original clinical opinion that there would be extensive AMH/MIS well beyond a reasonable excision margin, histologic assessment of these punch biopsy specimens revealed no additional melanoma and showed atypical melanocytic proliferation at only 1 of 16 biopsy sites (Fig. 4B and C, site 1). Because the original margins were extensively positive for MIS, we recommended reexcision, but were able to limit the reexcision to 1-cm margins, with the exception of the single site of AMH, where we recommended 1.5 cm beyond this biopsy site (Fig. 4C). This was closed primarily. The final pathology review revealed clear margins, and the patient has remained free of locoregional recurrence for 17 months.
Patient 3
The third patient represents another case where an invasive melanoma with MIS at the margins was not reexcised and presented later as invasive disease at one margin. This patient was diagnosed with melanoma on her chest in 1990 and was treated with wide excision. In 2001 she developed a second primary melanoma of her left wrist, which was diagnosed as extensive lentigo maligna MIS with focal invasion to 0.45 mm, Clark level II, and with MIS at the margins. Reexcision was not performed. In 2007 she was diagnosed with an invasive Clark level V melanoma 4.35 mm deep at the edge of the prior skin graft on the left wrist, with MIS surrounding the invasive disease (Fig. 5), and was referred to our institution. MIS extended to multiple margins. Skin examination revealed multiple concerning areas beyond the original skin graft, but no gross melanocytic lesion was evident at the edge of the recent excision. Again, we used the melanoma mapping technique to plan for definitive resection. Twenty-three 2-mm punch biopsies were performed; analysis revealed that two had atypical junctional nevi and one had MIS. Wide local excision was performed, with 1-cm margins from the previous scar and from each of the three positive biopsy sites. The pathology review from this wide excision revealed multiple areas of MIS within the specimen, but the margins were widely clear of disease.
FIG. 5.
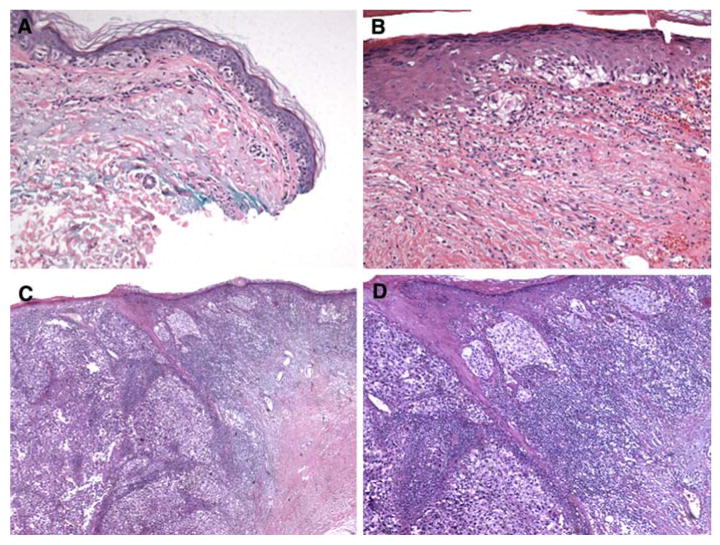
Histology from patient 3. (A) Melanoma-in-situ (MIS) at margin from biopsy in 2001. (B) Persistent MIS at biopsy in 2007 (C) Invasive-MIS junction in 2007. (D) Invasive disease at higher magnification in 2007, arising from prior MIS at lateral margin.
Discussion
Wide local excision of melanoma generally provides clear margins around a primary melanoma. In the case, however, of invasive melanoma or MIS at the margins of a wide excision, reexcision to clear margins is indicated; failure to do so can result in invasive recurrences, as highlighted by the histories of patients 1 and 3.
Our routine practice when there is MIS or invasive melanoma at margins of a wide excision is to reexcise the entire scar, with an additional 1-cm margin when original margins were positive. Additionally, when margins demonstrate AMH related to an MIS or invasive melanoma, reexcision to clear margins is indicated. Simple reexcision is adequate in most cases, especially those patients with disease at just one margin. Likewise, if the reexcision specimen demonstrated positive margins, most patients undergo adequate reexcision, with the second reexcision planned in a similar fashion. We do not propose that the punch biopsy technique be used routinely, even in the case of positive margins on second reexcisions.
We do recommend the proposed technique, however, in select cases. As illustrated in this report, when there is extensive MIS throughout the specimen or at numerous margins without pigmentation or other clinical findings to guide further reexcision, there is no standard approach to ensure clear margins with minimal morbidity. In cases where there is extensive sun damage and associated pathological changes, there is no standard approach to sample the field and to obtain a map of which areas have concerning pathology (such as AMH, MIS, or malignant melanoma) versus benign skin changes (such as melanocytic hyperplasia). In these clinical scenarios, we would recommend that this punch biopsy technique be considered. Stronger consideration would be recommended in anatomic locations where minimizing the size of resection is critical, as illustrated by patients 1 and 3 in this report. The face may in fact be an appropriate location, as the punch biopsy sites heal rapidly and scar minimally.
The present report illustrates a skin mapping technique that enables definitive resection of extensive occult melanoma at surgical margins. Mapping may enable definition of the appropriate margin when the extent of MIS or atypia may be unclear clinically. In all three cases, adequate margins were obtained in a single definitive resection, sparing the patient multiple additional procedures. This technique uses simple 2-mm punch biopsies and standard histological examination. This provides several advantages: low morbidity outpatient procedure, rapid healing, simultaneous evaluation at multiple distances from the original excision, and no requirement for specialized training or technology.
There are other alternatives to consider in managing difficult cases such as those presented here. These include systemic and local therapies.1,8–10 High-dose interferon alfa is the only approved adjuvant treatment for melanoma; however, it has not been evaluated for local control of radial growth phase melanoma and has clinically important morbidity.11–15 Radiotherapy has been used for intransit metastases, nodal disease, and palliation, though limited data exist to guide its use as an adjuvant for incomplete surgical excision of local disease.16
Topical imiquimod cream, an immune stimulator acting as a Toll-like receptor 7 agonist, has been reported to cause regression of MIS.10 In the cases described, however, use of topical imiquimod would have been problematic without histologic mapping of the extent of tumor to guide the application. Furthermore, where there is clinically invisible MIS or invasive melanoma, there is no way to be confident of the success of imiquimod treatment without biopsy.
Surgical options include Mohs micrographic surgery and modified Mohs procedures. Although traditional Mohs surgery in some experienced centers has been reported to provide excellent margin control in melanoma,17,18 its widespread use is limited by the training and expertise required of the dermatopathologists and Mohs surgeon. Its role in management of melanoma is also generally debated.
Variants of the Mohs technique—“slow Mohs”19 and other similar staged excision techniques20–22—have been developed to aid in treatment. These approaches address the challenge of the important background of junctional melanocytic hyperplasia in chronically sun-damaged skin23 by allowing for evaluation of tissue fixed in formalin. Further modifications to the “slow” or staged Mohs technique have been described as the “perimeter technique”6 and the “square” procedure.7 These approaches enable sampling of the entire margin, but use a substantially more invasive initial procedure than in our mapping approach and leave a greater defect, pending definitive resection. Additionally, in these methods, only a single margin width is evaluated with each procedure, potentially requiring a greater number of procedures. One series reported up to four staging procedures before definitive reexcision.6 No technique is perfect, and the punch biopsy technique does not evaluate the entire margin; there is a limit to the extent of mapping that is realistic. In cases of non-continuous field cancerization, the punch biopsy technique may miss small areas of troubling pathology. In cases of continuous disease or near-continuous disease, however, the punch biopsy technique permits sampling of multiple areas at various distances from the original lesion in a single procedure with low morbidity.
Another approach uses confocal laser scanning microscopy to diagnose excised lesions24 or in vivo tumor margins25 as a means of distinguishing benign from malignant tissue. Ex vivo accuracy rate is reported to be >90%.24 This is a useful approach but requires special expertise and capabilities that are not widely available. It does not include direct histology assessment.
Use of punch biopsies for mapping melanoma in combination with ultraviolet (UV) imaging has been reported previously.1 The UV light permits identification of melanin in the dermal layer and preliminarily suggests the border of the melanocytic lesion. In experienced hands, UV imaging seems to be useful, and the punch biopsies aid in histologic confirmation of margins. Some melanomas, however, are not pigmented and therefore may not be highlighted by UV light. In patients with extensive melanocytic changes independent of melanin production, such as patient 2 described above, the extent of melanin production may not correspond to the extent of tumor. Thus, we propose that punch biopsy mapping can be useful independent of specialized imaging technology.
We have found that the described melanoma mapping approach is very well tolerated, definitive, and consistent with standard management approaches for definitive therapy of primary melanoma. This technique is simple to perform and is based on a standard punch biopsy technique. It does not require specialized equipment or experience and may be helpful to surgeons and dermatologists to add to their armamentarium for management of selected cases. We hope that the experience of others with this technique will further define its role.
In our clinical experience, this punch biopsy technique has proven useful in defining the extent of disease in patients with extensive melanoma that is not clinically distinguishable on examinations. Punch biopsies were performed in the outpatient setting with minimal morbidity. We did not analyze frozen sections, and we would not recommend them for these 2-mm punch biopsies. The subsequent surgical resection can use the precise landmarks created by the biopsy sites, maximizing the opportunity to obtain disease-free margins and minimizing patient morbidity in selected patients with extensive radial growth phase melanoma extending to margins of excision.
References
- 1.Jeneby TT, Chang B, Bucky LP. Ultraviolet-assisted punch biopsy mapping for lentigo maligna melanoma. Ann Plast Surg. 2001;46:495–9. doi: 10.1097/00000637-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JW, Sagebiel RW, Calderon W, et al. The frequency of local recurrence and microsatellites as a guide to reexcision margins for cutaneous malignant melanoma. Ann Surg. 1984;200:759–63. doi: 10.1097/00000658-198412000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicker TJ, Kavanagh GM, Herd RM, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. Br J Dermatol. 1999;140:249–54. doi: 10.1046/j.1365-2133.1999.02657.x. [DOI] [PubMed] [Google Scholar]
- 4.Pitman GH, Kopf AW, Bart RS, et al. Treatment of lentigo maligna and lentigo maligna melanoma. J Dermatol Surg Oncol. 1979;5:727–37. doi: 10.1111/j.1524-4725.1979.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 5.Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. Report of three cases and review of the literature. Dermatol Surg. 1998;24:1105–10. doi: 10.1111/j.1524-4725.1998.tb04083.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney MH, Joseph M, Temple CL. The perimeter technique for lentigo maligna: an alternative to Mohs micrographic surgery. J Surg Oncol. 2005;91:120–5. doi: 10.1002/jso.20284. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TM, Headington JT, Baker SR, et al. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol. 1997;37:758–64. doi: 10.1016/s0190-9622(97)70114-2. [DOI] [PubMed] [Google Scholar]
- 8.Barlow RJ, White CR, Swanson NA. Mohs' micrographic surgery using frozen sections alone may be unsuitable for detecting single atypical melanocytes at the margins of melanoma in situ. Br J Dermatol. 2002;146:290–4. doi: 10.1046/j.1365-2133.2002.04661.x. [DOI] [PubMed] [Google Scholar]
- 9.Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656–65. doi: 10.1046/j.1524-4725.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- 10.Ray CM, Kluk M, Grin CM, et al. Successful treatment of malignant melanoma in situ with topical 5% imiquimod cream. Int J Dermatol. 2005;44:428–34. doi: 10.1111/j.1365-4632.2005.02582.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 12.Kirkwood JM, Manola J, Ibrahim J, et al. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–7. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–80. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 14.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818–25. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 15.Wheatley K, Ives N, Hancock B, et al. Interferon as adjuvant treatment for melanoma. Lancet. 2002;360:878. doi: 10.1016/S0140-6736(02)09986-5. [DOI] [PubMed] [Google Scholar]
- 16.Delaney G, Barton M, Jacob S. Estimation of an optimal radiotherapy utilization rate for melanoma: a review of the evidence. Cancer. 2004;100:1293–301. doi: 10.1002/cncr.20092. [DOI] [PubMed] [Google Scholar]
- 17.Bene NI, Healy C, Coldiron BM. Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases. Dermatol Surg. 2008;34:660–4. doi: 10.1111/j.1524-4725.2007.34124.x. [DOI] [PubMed] [Google Scholar]
- 18.Zitelli JA, Mohs FE, Larson P, et al. Mohs micrographic surgery for melanoma. Dermatol Clin. 1989;7:833–43. [PubMed] [Google Scholar]
- 19.Moller MG, Pappas E, Zager JS, et al. Management of melanoma in situ with staged marginal and subsequent central tumor excision. Ann Surg Oncol. 2008;15(Suppl 2):63. [Google Scholar]
- 20.Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142–8. doi: 10.1016/j.jaad.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Hill DC, Gramp AA. Surgical treatment of lentigo maligna and lentigo maligna melanoma. Australas J Dermatol. 1999;40:25–30. doi: 10.1046/j.1440-0960.1999.00311.x. [DOI] [PubMed] [Google Scholar]
- 22.Huilgol SC, Selva D, Chen C, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140:1087–92. doi: 10.1001/archderm.140.9.1087. [DOI] [PubMed] [Google Scholar]
- 23.Barlow JO, Maize J, Sr, Lang PG. The density and distribution of melanocytes adjacent to melanoma and nonmelanoma skin cancers. Dermatol Surg. 2007;33:199–207. doi: 10.1111/j.1524-4725.2006.33039.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329–33. doi: 10.1111/j.1365-2133.2007.08389.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen CS, Elias M, Busam K, et al. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153:1031–6. doi: 10.1111/j.1365-2133.2005.06831.x. [DOI] [PubMed] [Google Scholar]


