Abstract
Therapeutic hypothermia has become an accepted part of post-resuscitation care. Efforts to shorten the time from return of spontaneous circulation to target temperature have led to the exploration of different cooling techniques. Convective-immersion uses a continuous shower of 2°C water to rapidly induce hypothermia. The primary purpose of this multi-center trial was to evaluate the feasibility and speed of convective-immersion cooling in the clinical environment. The secondary goal was to examine the impact of rapid hypothermia induction on patient outcome.
24 post-cardiac arrest patients from 3 centers were enrolled in the study; 22 agreed to participate until the 6-month evaluations were completed. The median rate of cooling was 3.0°C/h. Cooling times were shorter than reported in previous studies. The median time to cool the patients to target temperature (<34°C) was 37 min (range 14–81 min); and only 27 min in a subset of patients sedated with propofol. Survival was excellent, with 68% surviving to 6 months; 87% of survivors were living independently at 6 months.
Conductive-immersion surface cooling using the ThermoSuit® System is a rapid, effective method of inducing therapeutic hypothermia. Although the study was not designed to demonstrate impact on outcomes, survival and neurologic function were superior to those previously reported, suggesting comparative studies should be undertaken. Shortening the delay from return of spontaneous circulation to hypothermic target temperature may significantly improve survival and neurologic outcome and warrants further study.
Keywords: Hypothermia, Cardiac arrest
1. Introduction
Mild therapeutic hypothermia reduces mortality and morbidity after cardiac arrest. Two large multi-center randomized controlled trials1,2 have shown that reducing a patient's core temperature to a target range of 32–34°C following resuscitation from cardiac arrest can improve important intermediate and long-term outcomes compared to supportive therapy without cooling. A third trial showed improvements in metabolic endpoints for patients who present with pulseless electrical activity or asystole. Experimental studies3–13 suggest that the period of therapeutic hypothermia reduces neuronal damage by minimizing oxygen consumption, reducing inflammation and edema, and possibly by stimulating the release of protective proteins.
The clinical benefits of therapeutic hypothermia are thought to be greatest when administered early after return of spontaneous circulation. The 2005 AHA/ILCOR Guidelines14 recommend that therapeutic hypothermia be considered early after a cardiac arrest (class 2). The European Council on Resuscitation recommends that “cooling should be started as soon as possible”15 and the Canadian Association of Emergency Physicians recommends that for selected patients “cooling should begin as soon as the clinical situation allows.”16,17
The challenge of cooling patients rapidly is a difficult one. Commonly employed cooling methods include cold saline infusion, ice-packs, and fan-and-misting techniques. There are a variety of approved devices available that offer both whole body and partial body cooling using external or intravascular methods, all developed in an effort to speed and simplify the cooling process.15 The interval from the return of spontaneous circulation (ROSC) to achieving target temperatures of therapeutic hypothermia is highly variable. If cooling strategies require complex or invasive equipment, require transfer to higher levels of care, or if they incur delays because of slow cooling rates, the therapeutic benefit achieved may be suboptimal.16
Ice water immersion has previously been demonstrated to produce very rapid cooling in humans,18,19 particularly when the water is circulating and providing an additional convective heat loss. We refer to this method of hypothermia induction as convective-immersion surface cooling. The ThermoSuit® System (TSS) (Life Recovery Systems) is a whole body cooling device which has been described previously.20 It uses convective-immersion by circulating ice water from a perforated top-sheet and an under-blanket across the skin surface at a rapid rate (14 L/min).
The primary purpose of this trial was to observe the feasibility and speed of cooling of the ThermoSuit® System in the clinical environment. The secondary goal was to examine the impact of rapid hypothermia induction on patient outcome.
2. Methods
This was a multi-center observational study. Patients were recruited from the Vienna General Hospital, Vienna, Austria; St. Michael's Hospital, Toronto, Canada; and Kingston General Hospital, Kingston, Canada. The study protocol received ethical approval from each institutional review board. Study monitoring and database management was performed by i3 Innovus (Burlington Ontario, Canada).
We included patients who had a cardiac arrest of presumed cardiac origin who were successfully resuscitated to a spontaneous rhythm but had persistent neurological dysfunction (Glasgow Coma Score<8). We excluded patients with an estimated or known age<18 years, patients who could not have an oesophageal temperature probe successfully inserted, patients for whom the resuscitation lasted longer than 60 min, patients with a core temperature less than 35°C after return of spontaneous circulation (ROSC), patients with a comatose state prior to the cardiac arrest, pregnant patients, patients with a known terminal illness, patients with known enrollment in another study, patients requiring urgent surgery, patients with a coagulopathy and active bleeding, patients more than 4 h after ROSC and patients with haemodynamic instability (SBP < 90 mmHg or MAP < 60 mmHg for >30 min after ROSC despite vasopressors). Patients with a height greater than 188 cm or elbow-to-elbow length greater than 60 cm were excluded due to size limitations of the cooling device.
Study patients meeting inclusion/exclusion criteria were placed in the device (Fig. 1) in accordance with the product's instructions for use (Life Recovery Systems, Waldwick, NJ, USA). In accordance with the local ethics committee requirements and regulations a waiver of consent was used to prevent delays in patient care, with confirmation of consent for use of study data from the patient or patient's substitute decision-maker when they became available. Sedation, analgesia, and paralysis (if used) were administered prior to the start of cooling according to standard institutional procedure for post-resuscitative therapeutic hypothermia. Medications and their dosages were recorded from the medical record. Adhesive defibrillation pads were applied to the patient in the conventional locations and an esophageal temperature probe was inserted for continuous temperature monitoring. Wounds, catheterization sites and defibrillation electrodes were covered with occlusive dressings prior to activating the device. Defibrillation was provided if required without discontinuation of the cooling process.
Fig. 1.
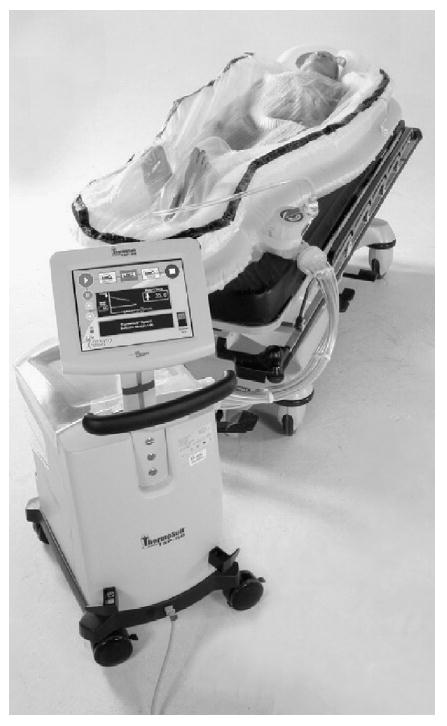
The Life Recovery Systems ThermoSuit® System, which uses convective-immersion to cool patients.
Cooling was initiated by circulating ice-cold water (2.0 ± 2.0°C) through the TSS. The clinician was prompted to purge the fluid from the suit when the patient's oesophageal temperature reached 34.5°C. This was intended to produce a final patient oesophageal temperature of 32–34°C, which we used to define our target temperature. Cooling time was defined as time from activation of water flow over the patient to core temperature reaching the target range.
Patients were removed from the device and maintained at 32–34°C using warming or cooling techniques at the discretion of the treating physician for 12–24 h. The patients were then re-warmed at a target rate of 0.2–0.5°C/h using passive blankets or a conventional heating blanket.
Patient data collected included time of start of cooling, time of purging of the TSS, temperature of water infused through the suit, oesophageal temperature, blood pressure, heart rate and rhythm, and electrocardiogram (ECG). Episodes of shivering and drugs administered to control shivering or discomfort were recorded.
The primary outcome measure was time from collapse to a core temperature of 34.0°C. Other data collected included cardiac arrest time, drug administration, operator interaction with the device, and adverse events. Safety parameters measured included survival at 24 h, hospital discharge, 30 days, and 6 months. Mini-Mental Status Exam (MMSE), Modified Rankin Score (MRS) and Cerebral Performance Category (CPC) were collected 30 ± 7 days and 6 months ± 15 days post-event. MMSE was also collected at hospital discharge ± 3 days. Information was collected at 6 months regarding whether the surviving patients were living independently or required long-term care.
3. Results
A total of 24 patients with post-resuscitative coma were enrolled. Two patients were excluded after declining to give consent for neurological assessments. Table 1 shows the characteristics of the patients enrolled, while Table 2 shows the cooling time and rate, survival, and neurologic outcomes.
Table 1.
Patient characteristics.
| Height | 175(172–180) cma |
| Age | 65(50–80) years |
| Presenting rhythm | |
| VF/VT | 14/22(63.6%) |
| PEA | 7/22(31.8%) |
| Asystole | 1/22(4.5%) |
| Duration of resuscitation | 18.0(15.3–25.0) mina |
| Starting temperature | 36.1(35.5–6.8) °Ca |
Median (interquartile range).
Table 2.
Outcomes.
| Cooling time (min) | All patients (n: 22) | Sedated with propofol (n: 7) | Sedated with other agents (n: 15) |
|---|---|---|---|
| Minimum | 14.0 | 14.0 | 21.0 |
| Maximum | 81.0 | 39.0 | 81.0 |
| Median (interquartile range) | 37.0(27.25–48.75) | 27.0(22.5–32.5) | 46.0(31.0–57.5) |
| Mean (std. dev.) | 40.0(17.3) | 27.1(8.4) | 45.9(17.0) |
| Cooling rate (°C/h) | |||
| Minimum | 1.3 | 2.5 | 1.3 |
| Maximum | 8.8 | 8.8 | 7.9 |
| Median (interquartile range) | 3.0(2.3–3.9) | 4.2(2.9–5.9) | 2.7(2.0–3.5) |
| Mean (std. dev.) | 3.5(2.0) | 4.7(2.5) | 3.0(1.6) |
| Patient outcomes (n: 22) | |||
| Survival to 6 months | 15(68.1%) | ||
| Living independently | 13(59.1%) | ||
| Neurologic testing (at 6 months) | CPC | MMSE | MRS |
| Median (interquartile range) | 1(1–1) | 28(28–29) | 0(0–1) |
| Mean (std. dev.) | 1.23(.60) | 27.9(2.23) | 0.61(1.12) |
Std. dev.: standard deviation; CPC: Cerebral Performance Category; MMSE: Mini-Mental Status Exam; MRS: Modified Rankin Score.
The median time from initiation of cooling to the target core temperature (34°C) was 37 min. 87% of the patients reached target temperature in less than 60 min, and 33% reaching target in 30 min or less. A subgroup of patients who received propofol as their sedating agent had a significantly shorter cooling time. The median rate of cooling for all patients was 3°C/h, with the propofol group achieving a median rate of 4.2°C/h. Fig. 2 demonstrates the mean esophageal temperature change in the first 30 min of cooling.
Fig. 2.
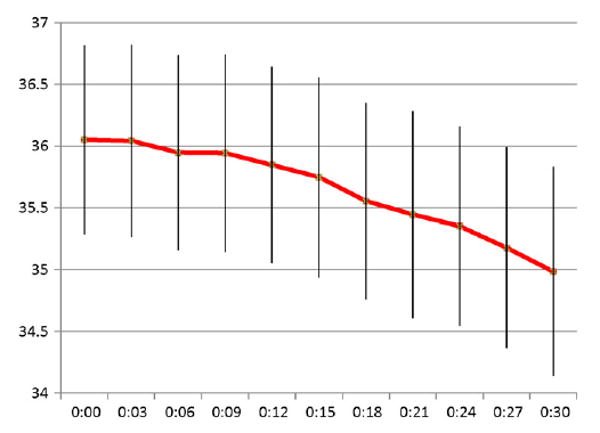
Mean esophageal temperature with standard deviations for the first 30 min of cooling.
There were no serious adverse patient events related to the device. There was one reported device malfunction that did not alter the care of the patient. Thirteen patients had core temperatures that dropped below 32°C, and the median time spent below 32°C was 180 min (75% quartile 317 min). There was no association between the minimum patient temperature and patient outcome in this study.
Only five of the study patients required additional cooling to maintain them in the therapeutic range for the entire 24 h of therapy, the remaining 17 patients remained in the target core temperature range passively.
Of the 22 patients who consented to follow-up, 68% survived to 6 months. 87% of these survivors were living independently. Time from ROSC to target temperature was significantly lower in survivors than non-survivors (Table 3).
Table 3.
Survival and time to 34°C.
| Time from ROSC to 34°Ca (min) | All patients (22) | Survivors (15) | Non-survivors (7) |
|---|---|---|---|
| Minimum | 109 | 109 | 114 |
| Maximum | 422 | 348 | 422 |
| Median (interquartile range) | 204(145–246) | 184(145–234) | 220(155–256) |
| Mean (standard deviation) | 210(85) | 196(73) | 232(101) |
ROSC: return of spontaneous circulation.
Time interval includes time to stabilize patient, transfer to definitive care, set-up and induction of therapeutic hypothermia.
4. Discussion
Convective-immersion cooling resulted in significantly shorter cooling times than previously reported. There was no control group in this study using an alternative cooling device, but this method appears to be much better than those reported in previous trials. The cooling rate of 3.0°C/h achieved with the device compares quite favorably to the rate of 0.9 °C/h reported in the Bernard trial.2 In the HACA trial,1 which used a combination of methods, had a median time to target temperature of 480 min, while the Bernard trial2 achieved 120 min—still more than three times the 37 min achieved with conductive-immersion using the ThermoSuit® System.
In 2007 Hoedemaekers et al. published a prospective comparison of methods of hypothermia induction (Table 4). The most rapid cooling rate they achieved was with an intravascular cooling device at 1.46°C/h, half the rate achieved by the ThermoSuit.20
Table 4.
Comparison of available cooling methods.
Skin surface cooling using water slightly above the freezing point produces mild cutaneous vasodilatation. This phenomenon has been described as the “Lewis Reaction” or “hunting effect”, a reversal of the normal vasoconstrictive response to cold in part due to reduced contractility of the walls of the arterioles at temperatures below 8°C.21–23 This enhances the rate of heat transfer through the skin and accelerates core cooling. Use of propofol as a sedating agent seemed to shorten cooling times further, possibly due to the vasodilatory effect of the drug.
Although the study is too small to make conclusions about outcome, there is an apparent correlation between shorter time to target temperature and survival on subgroup analysis within the study (Table 3). Wolff et al.24 had a similar finding when they studied 49 patients cooled with intravascular catheters and concluded that a 1-h delay in reaching target temperature tended to worsen the likelihood of a favorable outcome by 31%. Direct evidence of survival benefit from more rapid induction of therapeutic hypothermia in a controlled human study may no longer possible due to a loss of clinical equipoise but the results of this study support the commonly held belief that the sooner therapeutic hypothermia is achieved, the better the patient's neurologic outcome.
Time from ROSC to target temperature involves a number of system issues in addition to the effectiveness of the cooling methods. Stabilization of the patient, interfacility and interdepartmental transfers can significantly delay the initiation of cooling and the time to target temperature. As more rapid cooling methods are developed, these delays will comprise a greater proportion of the overall time interval. As we move the time it takes to cool patients from 8 h toward 30 min, a 1-h delay in cooling initiation becomes much more significant. Issues of who will initiate cooling and where it will be initiated may need to be reexamined in some systems.
The high survival rates achieved in this study were accompanied by a high percentage of patients living independently at 6 months. Although survival is often considered the ultimate outcome measure, consideration of quality of life will continue to be of concern to clinicians and patients alike. In addition to objective, numeric neurologic assessments like the MMSE, MRS and CPC, the ability to live independently is a very meaningful measure that should continue to be a part of the long-term evaluation of these interventions.
Once the patients were cooled to the target temperature they tended to remain in the target range when left exposed to room air. Only 5 of the study patients required any additional cooling to maintain them in the therapeutic range for the entire 24 h of therapy. Those that did require temperature maintenance could be maintained at the desired level with simple means such as conventional cooling blankets or temperature-controlled air. This is in contrast to studies that used the application of cold fluids, where most of the patients re-warmed spontaneously and needed additional cooling.25 This might be explained by the higher heat mass that can be removed by conductive-immersion cooling.
The study has a number of limitations. As a feasibility study, there was not a comparison group using another method of cooling. Comparison to historical controls is always difficult due to variations in patient populations and other treatment provided. The study is too small to make conclusions about outcomes, although the results are hypothesis generating.
This study was designed primarily to assess the feasibility of cooling with the ThermoSuit and secondarily to assess outcomes. Convective-immersion surface cooling by the ThermoSuit was rapid and effective. The measured outcomes are among the best reported, despite the inclusion of patients who presented with pulseless electrical activity and asystole. Further efforts to shorten the time from ROSC to therapeutic hypothermia, and further study of the impact of this simple, effective cooling method are essential.
5. Conclusion
Conductive-immersion surface cooling using the ThermoSuit® System is a rapid, effective method of inducing therapeutic hypothermia. Although the study was not designed to demonstrate impact on outcomes, survival and neurologic function were superior to those previously reported, suggesting comparative studies should be undertaken. Shortening the delay from return of spontaneous circulation to hypothermic target temperature may significantly improve survival and neurologic outcome and warrants further study.
Acknowledgments
This study was funded by Life Recovery Systems HD, LLC. The ThermoSuit® System was developed with the assistance of funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant number 5R44HL072542-03. Drs. Freedman, Ohley, Schock, and Klock participated in review of the study data and manuscript, and in the decision to submit the manuscript for publication. LRS provided funds to i3 Innovus for study monitoring and independent auditing of study data under GLP (Good Laboratory Practice) guidelines.
Footnotes
Conflict of interest: Drs. Howes, Dorian and Holzer received research grants for this study. Drs. Freedman, Ohley, and Schock are co-inventors of the ThermoSuit® System and own stock in Life Recovery Systems HD, LLC (LRS). Dr. Freedman is employed by Freedman Memorial Cardiology, Alexandria, LA, and serves as President of LRS. Dr. Ohley is employed by the University of Rhode Island (URI), Kingston, RI, and is a part-time employee of LRS. Dr. Schock is employed by LRS. Dr. Klock received financial assistance from LRS while a student at URI and is now employed by Northern Arizona University, Flagstaff, AZ. Danica Krizanac worked as scientific collaborator and received honoraria for this study by Life Recovery Systems. Michael Holzer has received honoraria for lectures from KCI Medical and Medivance, he worked as medical advisor for KCI Medical and Emcools, he received travel grants for scientific conferences from Alsius, KCI Medical, Emcools and Life Recovery Systems. The Department of Emergency Medicine received research grants from Alsius, KCI Medical, Medivance Inc., Life Recovery Systems, Benechill Inc. and Medcool Inc.
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2009.12.025.
References
- 1.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard S, Gray T, Buist M, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Chopp M, Chen H, Dereski MO, Garcia JH. Mild hypothermic intervention after graded ischemic stress in rats. Stroke. 1991;22:37–43. doi: 10.1161/01.str.22.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989;101:299–304. doi: 10.1016/0304-3940(89)90549-1. [DOI] [PubMed] [Google Scholar]
- 5.Inglefield JR, Perry JM, Schwartz RD. Postischemic inhibition of GABA reuptake by tiagabine slows neuronal death in the gerbil hippocampus. Hippocampus. 1995;5:460–8. doi: 10.1002/hipo.450050508. [DOI] [PubMed] [Google Scholar]
- 6.Siemkowicz E, Haider A. Post-ischemic hypothermia ameliorates ischemic brain damage but not post-ischemic audiogenic seizures in rats. Resuscitation. 1995;30:61–7. doi: 10.1016/0300-9572(94)00859-e. [DOI] [PubMed] [Google Scholar]
- 7.Sterz F, Leonov Y, Safar P, et al. Multifocal cerebral blood flow by Xe-CT and global cerebral metabolism after prolonged cardiac arrest in dogs. Reperfusion with open-chest CPR or cardiopulmonary bypass. Resuscitation. 1992;24:27–47. doi: 10.1016/0300-9572(92)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Lei B, Tan X, Cai H, et al. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25:147–52. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Clark RS, Kochanek PM, Marion DW, et al. Mild posttraumatic hypothermia reduces mortality after severe controlled cortical impact in rats. J Cereb Blood Flow Metab. 1996;16:253–61. doi: 10.1097/00004647-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Chopp M, Knight R, Tidwell CD, et al. The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab. 1989;9:141–8. doi: 10.1038/jcbfm.1989.21. [DOI] [PubMed] [Google Scholar]
- 11.Zhu C, Wang X, Cheng X, et al. Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res. 2004;996:67–75. doi: 10.1016/j.brainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Roelfsema V, Bennet L, George S, et al. Window of opportunity of cerebral hypothermia for postischemic white matter injury in the near-term fetal sheep. J Cereb Blood Flow Metab. 2004;24:877–86. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- 13.Hachimi-Idrissi S, Van Hemelrijck A, Michotte A, et al. Postischemic mild hypothermia reduces neurotransmitter release and astroglial cell proliferation during reperfusion after asphyxial cardiac arrest in rats. Brain Res. 2004;1019:217–25. doi: 10.1016/j.brainres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–21. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 15.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council Guidelines for Resuscitation 2005 Section 4. Adult advanced life support. Resuscitation. 2005;67S1:S39–86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Howes D, Green R, Gray S, Stenstrom R. Easton D for the Canadian Association of Emergency Physicians Critical Care group. Guidelines for the use of hypothermia after cardiac arrest. Can J Emerg Med. 2006;8:106–8. doi: 10.1017/s1481803500013579. [DOI] [PubMed] [Google Scholar]
- 17.Howes D, Green R, Gray S, Stenstrom R. Easton D for the Canadian Association of Emergency Physicians Critical Care group. Evidence for the use of hypothermia after cardiac arrest. Can J Emerg Med. 2006;8:109–15. doi: 10.1017/s1481803500013579. [DOI] [PubMed] [Google Scholar]
- 18.Proulx CI, Ducharme MB, Kenny GP. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94:1317–23. doi: 10.1152/japplphysiol.00541.2002. [DOI] [PubMed] [Google Scholar]
- 19.Taylor N, Caldwell J, Heuvel A, Patterson M. To cool, but not too cool: that is the question-immersion cooling for hyperthermia. Med Sci Sports Exerc. 2008;40:1962–9. doi: 10.1249/MSS.0b013e31817eee9d. [DOI] [PubMed] [Google Scholar]
- 20.Hoedemaekers C, Ezzahti M, Gerritsen A, van der Hoeven J. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11:R91. doi: 10.1186/cc6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman R, Cote M, Schock R, Schofield L, Ohley W. Rapid non-invasive whole body cooling via a skin contacting circulating thin layer of cold water. Circulation. 2003;108:419–20. [Google Scholar]
- 22.Lewis T. The nocisensor system of nerves and its reactions. BMJ. 1937;1:491–4. doi: 10.1136/bmj.1.3974.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daanen H, Van de Linde F, Romet T, Ducharme M. The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol. 1997;76:538–43. doi: 10.1007/s004210050287. [DOI] [PubMed] [Google Scholar]
- 24.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133:223–8. doi: 10.1016/j.ijcard.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 25.Kliegel A, Janata A, Wandaller C, et al. Cold infusions alone are effective for induction of the rapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation. 2007;73:46–53. doi: 10.1016/j.resuscitation.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Uray T, Malzer R, Sterz F, Holzer M, Laggner A, Behringer W. Out-of-hospital surface cooling to induce mild hypothermia in human cardiac arrest: a feasibility trial. Resuscitation. 2008;77:331–8. doi: 10.1016/j.resuscitation.2008.01.005. [DOI] [PubMed] [Google Scholar]


