Abstract
AMP-activated protein kinase (AMPK) is a serine threonine kinase that is highly conserved through evolution. AMPK is found in most mammalian tissues including the brain. As a key metabolic and stress sensor/effector, AMPK is activated under conditions of nutrient deprivation, vigorous exercise, or heat shock. However, it is becoming increasingly recognized that changes in AMPK activation not only signal unmet metabolic needs, but also are involved in sensing and responding to ‘cell stress', including ischemia. The downstream effect of AMPK activation is dependent on many factors, including the severity of the stressor as well as the tissue examined. This review discusses recent in vitro and in vivo studies performed in the brain/neuronal cells and vasculature that have contributed to our understanding of AMPK in stroke. Recent data on the potential role of AMPK in angiogenesis and neurogenesis and the interaction of AMPK with 3-hydroxy-3-methy-glutaryl-CoA reductase inhibitors (statins) agents are highlighted. The interaction between AMPK and nitric oxide signaling is also discussed.
Keywords: angiogenesis, endothelium, ischemia, neuronal metabolism, nitric oxide, statins
Introduction
AMP-activated protein kinase (AMPK), a serine threonine kinase, is a key metabolic and stress sensor/effector that is activated under conditions of nutrient deprivation, vigorous exercise, or heat shock (Turnley et al, 1999). Over the past 10 years the importance of AMPK and the central role that it has in both peripheral and central nervous system metabolism have become increasingly recognized. In peripheral tissue, including cardiac and skeletal muscle, adipose tissue, pancreas, and liver, activation of AMPK signals unmet metabolic demand. Increasing cellular levels of AMP or, to a lesser extent, falling levels of ATP, lead to activation of AMPK through phosphorylation by an upstream kinase. Phosphorylation represents the active state of this protein kinase (Hawley et al, 1996; Ramamurthy and Ronnett, 2006; Ronnett et al, 2009). Activation of AMPK leads to numerous downstream effects on a multitude of metabolic pathways, with subsequent enhancement of ATP-generating, catabolic pathways (i.e., fatty acid oxidation), and inhibition of energy ‘storing' pathways such as lipid, fatty acid (Liu et al, 2006), cholesterol (Clarke and Hardie, 1990), and protein synthesis (Horman et al, 2002) through phosphorylation of key regulatory proteins (Scharf et al, 2008) making ATP more readily available. Once activated, AMPK also controls energy metabolism by directly regulating metabolic enzymes and gene transcription (Figure 1).
Figure 1.
AMPK signaling when energy balance is unmet. Green arrow: activation; red arrow: inhibition. AMPK is activated when the energy demand is higher than the supply and inhibited when the supply is higher than demand. CaMKK β, Ca2+/calmodulin-dependent protein kinase β; TAK1, TGF β-activated kinase 1; PP2Cα, protein phosphatase-2Cα; GS, glycogen synthase; HMG-CoAR, 3-hydroxy-3-methy-glutaryl-CoA reductase; PFK-2, phosphofructokinase-2; TF, transcription factors; P53, tumor protein 53; mTOR, mammalian target of rapamicin; eEF2K, eukaryotic elongation factor-2 kinase; GLUT-3,4, glucose transporter 3 and 4. (The color reproduction of this figure is available on the html full text version of the manuscript.)
The role played by AMPK in the central regulation of energy balance by mediating food intake has been well described (Lage et al, 2008; Xue and Kahn, 2006). However, it is becoming increasingly recognized that changes in AMPK activation not only signal unmet metabolic needs, but are also involved in sensing and responding to ‘cell stress.' The downstream effect of AMPK activation on cell survival differs depending on (1) the tissue examined, (2) the degree of stress (mild versus severe), and (3) the metabolic capacity of the cells examined. In peripheral organs such as the heart, activation of AMPK during low-energy states such as ischemia reduces damage (Miller et al, 2008). What occurs in the brain in the setting of an ischemic injury is less clear. As neurons, unlike other mammalian cells, lack key glycolytic enzymes (Cidad et al, 2004), and have limited ability to store nutrients, the response to AMPK activation when oxygen and glucose are unavailable leads to increased lactate production and acidosis (Li and McCullough, unpublished observations). The overall effect of manipulating AMPK levels in the ischemic brain likely depends on the metabolic setting, and remains a matter of debate. We have found that inhibition of AMPK reduces damage induced by middle cerebral artery occlusion (MCAO), as does genetic deletion of one of the catalytic isoforms (Li et al, 2007; McCullough et al, 2005). However, others have proposed that AMPK represents an endogenous neuroprotective pathway (Kuramoto et al, 2007). This review discusses the work done to date in both in vitro and in vivo ischemic models and also discusses the possible interaction and contribution of AMPK in the cerebral vasculature and nitric oxide signaling to the AMPK response.
The regulation of AMPK isoforms
AMPK is a heterotrimer that consists of three subunits; the catalytic α subunit, which has two isoforms α1 and α2, the β subunit that also has two isoforms, and the γ subunit, which has three known isoforms (Ofir et al, 2007). Each subunit appears to have distinct functions and tissue localization. The best studied of the three subunits is the catalytic α subunit. This subunit contains the threonine phosporylation site that when phosphorylated leads to AMPK activation (Hawley et al, 1996; Ofir et al, 2007). This phosphorylation site is regulated by three known upstream AMPK kinases, LKB1 (serine threonine kinase 11), Ca2+/calmodulin-dependent protein kinase (CaMKK), and transforming growth factor β-activated kinase 1 (TAK1) (Figure 2).
Figure 2.
Regulation of AMPK phosphorylation. Green arrow: activation; red arrow: inhibition. CaMKK β, Ca2+/calmodulin-dependent protein kinase β; TAK1, TGF β-activated kinase 1; PP2C, protein phosphatase 2C. (The color reproduction of this figure is available on the html full text version of the manuscript.)
The phosphorylation of AMPK by LKB1 is enhanced by AMP (Towler and Hardie, 2007) and this may be an important pathway by which AMPK can be activated by energy demand to maintain metabolic homeostasis. LKB1 appears to selectively target AMPK α2 (Long and Zierath, 2006; Sakamoto et al, 2005; Tzatsos and Tsichlis, 2007). In Caenorhabditis elegans and in ischemic mouse heart, AMPK α2 activation is dependent on LKB1 (Lee et al, 2008). In addition, in the heart, the LKB1-AMPK α2 pathway may become more significant when ATP depletion is severe (Hardie, 2008). This pathway may also be important in the ischemic brain, as a dramatic increase in phosphorylation of LKB1 (in whole brain homogenates) is seen after MCAO (Li et al, 2007).
AMPK can also be activated by the upstream AMPK kinase Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKβ), which has a role in both normal cerebral physiology (i.e., neuronal firing) as well as in pathologic conditions (i.e., ischemia-induced calcium influx through NMDA receptor activation). CaMKKβ functions as AMPK kinase in the hypothalamus to control food intake (Anderson et al, 2008), showing this pathway's contribution to whole body energy balance. Injection of the glutamate receptor agonist kainic acid (KA) into mouse hippocampus induced an increase in CaMKKβ activity and AMPK activation as well as enhancement of hippocampal cell death (Lee et al, 2009). At present there are no studies that have directly investigated CaMKK or LKB1's regulation of AMPK during cerebral ischemia.
In vitro studies using cell-free assays in yeast (Saccharomyces cerevisae) have also identified a third putative upstream AMPKK named TAK1 (transforming growth factor-β-activated kinase 1) (Figure 2), although it is unclear if this has any physiologic relevance in the brain (Momcilovic et al, 2006). It is interesting to note that TAK1 is activated by TGF β, which has been shown to have neuroprotective properties against ischemia-induced neuronal death (Buisson et al, 2003). Ongoing work in our lab is investigating the role of each of these upstream kinases in the response to ischemia and the downstream effects on AMPK signaling on stroke outcome.
Recently, the importance of regulation of the rate of dephosphorylation of AMPK has gained attention (Sanders et al, 2007; Suter et al, 2006). AMP binding can directly inhibit the dephosphorylation of AMPK by protein phosphatase 2Cα, which is responsible for removing the phosphate at the Thr-172 site, thus keeping AMPK in its active state (Sanders et al, 2007) (Figure 2). In addition, any decrease in endogenous phosphatases preserves AMPK phosphorylation and prolongs its activation (Sanders et al, 2007; Suter et al, 2006). Hurley has also recently discovered that each α subunit may have additional serine phosphorylation sites (α1 at 485 and α2 at residue 491) that are controlled by cAMP signaling and lead to a reduction in AMPK activity (Hurley et al, 2006). This novel regulation pathway has not been explored in vivo.
AMPK subunit function and localization
The function and regulation of the subunits of AMPK is an area of intense investigation. Most of the data regarding the individual subunit function has been derived from studies of genetically manipulated mice (Viollet et al, 2007). The subunit isoforms (α1 and α2) are distinct although highly homologous. The C-terminal region of the subunit is required for its association with the β and γ subunits. Each catalytic isoform forms a complex with noncatalytic β and γ subunits for AMPK activity (Gao et al, 1996). Studies have indicated that AMPK α2, not α1, is induced under hypoxic conditions in human glioma cells (Neurath et al, 2006). Mice deficient in AMPK α2 show glucose intolerance and reduced insulin sensitivity, whereas the AMPK α1 null mice do not show such changes (Viollet et al, 2003a, 2003b). In addition, AMPK α2 knockout mice are protected against MCAO-induced injury, whereas the AMPK α1 knockout mice are indistinguishable from wild type (Li et al, 2007). This suggests that most of the detrimental effects of AMPK activation in stroke are mediated by this isoform.
The β subunit has a central AMPK-binding domain which may be a regulatory domain that allows AMPK to act as a sensor of energy status by monitoring glycogen levels (McBride et al, 2009; McBride and Hardie, 2009; Polekhina et al, 2005). However, as neurons have limited glycogen storage pools, this mechanism of regulating AMPK activity may be less important in the central nervous system than in the periphery. Interestingly, in Drosophila, knockout of the β subunit (a single subunit of each isoform is found in flies) leads to rapid degeneration in the retina and optic lobe, most likely through autophagic cell death. Neuronal loss was dependent on neuronal excitation, as it was ameliorated when flies were raised in the dark (Spasic et al, 2008). Similar developmental brain abnormalities have been recently described in mice lacking the β1 subunit ((Dasgupta and Milbrandt, 2009), however, the role of AMPK β in stroke remains unexplored.
The γ subunit contains four tandem cystathionine B-synthase motifs, which bind AMP or ATP. This subunit has three isoforms (1, 2, and 3). Mutations in PRKAG2, the gene for the γ2 regulatory subunit, leads to cardiac hypertrophy and electrophysiologic abnormalities, including pre-excitation (Wolff–Parkinson–White syndrome) and atrioventricular conduction block (Arad et al, 2002). However the mechanism for this is unclear. The regulation and function of these complexes in the brain is not clear, nor is the importance of the specific isoform combinations. Humans have seven genes encoding AMPK subunits (α1, α2; β1, β2; γ1, γ2, γ3) that can form at least 12 αβγ heterotrimers, increasing the potential for diversification of function (Jorgensen et al, 2005). The possibility that different combinations of isoforms can regulate the specificity for AMP, ATP, and downstream phosphorylation targets is of great interest and is under investigation (Viollet et al, 2009). Differences in the distribution of the isoforms in the periphery have been well described (Viollet et al, 2009) but much less is known regarding the localization, regulation, or function of these isoforms in the brain.
AMPK expression in the brain
AMPK expression in the adult brain was reported in 1995 (Gao et al, 1995). The most systematic study of AMPK isoform localization was subsequently conducted by Turnley (Turnley et al, 1999). In this work, both the developmental expression and the specific cell type expressing AMPK were assessed. Each of the AMPK subunits was expressed in the central nervous system throughout early development into adulthood. Using northern analysis, the α1 catalytic subunit showed a fairly low but consistent level of expression at all ages, whereas expression of the α2 subunit had a dramatic increase in expression between E10 and E14, during the period of neuronal differentiation.
Using a nonspecific pan-α antibody, AMPK α was found predominantly in neurons with minimal staining in astrocytes in adult brain (McCullough et al, 2005). Using isoform (α1 versus α2)-specific antibodies, Turnley found that the α2 isoform was distributed extensively throughout the mouse brain and is the predominant catalytic subunit. AMPK α2 localizes more to the neuronal nucleus compared with α1, but when in astrocytes AMPK α2 was found in the cytoplasm. The α1 subunit was barely detectable in astrocytes and was not found in great quantities in the brain (Turnley et al, 1999). For the noncatalytic AMPK subunits, γ1 was also largely confined to neurons, occasionally seen in oligodendrocytes, and rarely expressed in astrocytes. Both β isoforms were found expressed predominantly in neurons; β1 and γ1 also localize predominately to the nucleus (Turnley et al, 1999).
It is likely that each isoform has different functions, as we have shown for stroke using knockout models. The AMPK α2 isoform, not AMPK α1, has a significant role in the detrimental response of AMPK activation in ischemic brain (Li et al, 2007). Research in human embryonic kidney cells has found a similar distinction between the two AMPK α catalytic isoforms. Although oxidative stress and 2-DG stimulated phosphorylation of insulin receptor substrate-1, a proapoptotic molecule, primarily through the AMPK α1 subunit, AMPK activation during energy depletion was mediated by AMPK α2 (Tzatsos and Tsichlis, 2007).
AMPK complexes containing the α2 rather than the α1 isoform have a greater dependence on AMP for activation (∼five-fold stimulation compared with ∼two-fold) (Salt et al, 1998). Similar findings were seen in human glioblastoma cells where the AMPK α2 isoform, not AMPK α1, mediated the effect of hypoxia by inducing vascular endothelial growth factor (VEGF) expression (Neurath et al, 2006). The AMPK isoform usage may reflect the specificity of the AMPK upstream kinases. As complete deletion of the catalytic subunits leads to embryonic lethality (Viollet et al, 2007), studies are currently underway using tissue-specific knockouts (i.e., α2 deletion in astrocytes, neurons, or endothelium in α1 knockout mice) to determine the role that each has in the response to stroke.
AMPK expression and activation in the vasculature: interaction with nitric oxide
AMPK is expressed in both smooth muscle and endothelium (Zou and Wu, 2008). Both α1 and α2 catalytic subunits are expressed in pulmonary arterial smooth muscle cells (Evans et al, 2006) and both isoforms are expressed in endothelial cells, although α1 predominates (Davis et al, 2006; Zou et al, 2004). AMPK α1 may mediate the endothelial response to oxidant stress as silencing AMPK α1 in human umbilical vein cultures leads to reductions in the transcription of genes involved in antioxidant defense, mitochondrial content, endothelial nitric oxide synthase (eNOS) generation, and cell proliferation. This leads to an accumulation of reactive oxygen species (ROS) and subsequent apoptosis (Colombo and Moncada, 2009). It is likely that both catalytic isoforms of AMPK have a role in the endothelial response to hypoxia. Although AMPK α2 is barely detectable in the endothelium, angiogenesis after hypoxic stress requires AMPK α2 in human umbilical vein cultures (Nagata et al, 2003; Zou and Wu, 2008). Whether stroke-induced angiogenesis requires AMPK is under investigation.
Examination of AMPK in the in the vasculature often uses Metformin, one of the most commonly used drugs for the treatment of type II diabetes, as an AMPK activator. Metformin exerts its therapeutic effects, at least in part, by activating AMPK. In the UK Prospective Diabetes Study treatment with metformin decreased macrovascular morbidity and mortality independently of glycemic control (UK Prospective Diabetes Study (UKPDS) Group, 1998). Many studies now show that the beneficial effects of metformin in protecting the peripheral vasculature, such as reducing vascular inflammation, enhancing endothelial function, and protecting endothelium, are mediated by AMPK (Davis et al, 2006). Metformin inhibits TNFα-induced nuclear factor-kappa B expression (NF-κB) activation in vascular endothelial cells, leading to an inhibition of NF-κB-dependent gene expression of various inflammatory and cell adhesion molecules. This effect appears to be mediated by AMPK as transfection of AMPK siRNA significantly attenuated metformin-induced inhibition of NF-κB activation (Huang et al, 2009). AMPK activation induced by metformin also improved endothelial function in diabetic mice by blunting diabetes-induced reduction of AMPK phosphorylation and normalized acetylcholine-induced endothelial relaxation, an effect mediated by AMPK α2 (Wang et al, 2009).
In addition, many of these beneficial effects of AMPK on the peripheral vasculature are mediated by eNOS activation (Chen et al, 1999; Zou and Wu, 2008). AMPK can phosphorylate eNOS at two sites (Ser 633 and Ser 1177) in the endothelium (Morrow et al, 2003). Interestingly, NO and AMPK seem to reciprocally regulate each other as rising levels of NO can also activate AMPK, most likely through oxidant pathways (Hou et al, 2008). It appears that metformin also requires NO to initiate the activation of AMPK in the vasculature, given the fact that metformin no longer activates AMPK when NO is directly inhibited in bovine endothelial cells (Zou et al, 2003).
Little is known regarding AMPK's effects in the cerebral vasculature. It is important to note that many differences exist in the peripheral and cerebral blood vessels in the organization and function of the endothelium. Even within the brain itself, the microvasculature endothelium is heterogeneous (Ge et al, 2005). These facts make extrapolation of the findings in the periphery to the brain difficult. Expression of AMPK in the cerebral vasculature has only been investigated over the past several years. AMPK α has been detected in the endothelium of the basilar artery (Osuka et al, 2009). In vitro studies have showed AMPK activation by low glucose levels. AMPK activation also contributes to downstream hypoxic responses as a DN mutant AMPK, partially ameliorated hypoxia-induced VEGF production (Lopez-Lopez et al, 2007). AMPK activation with 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR) enhanced VEGF expression in cultured rat brain endothelium, which was ameliorated by DN AMPK, showing the specificity of this response to AMPK activation (Lopez-Lopez et al, 2007). The physiologic consequences of AMPK activation in vivo are less clear, but an intriguing new study has shown increased phosphorylation of AMPK and eNOS in the basilar artery 2 days after experimental subarachnoid hemorrhage when vasospasm begins. Subarachnoid hemorrhage might thus induce temporary activation of AMPKα, leading to phosphorylation of eNOS at Ser1177 (Osuka et al, 2009). Whether this is detrimental and exacerbates or ameliorates injury is unclear, but suggests that AMPK may be a target for treatment of subarachnoid hemorrhage-induced vasospasm. Interestingly, high levels of phosphorylated AMPK are seen in the double mutant amyloid precursor protein/presenilin 2 mouse, a transgenic model of AD with a low density of cerebral vessels (Lopez-Lopez et al, 2007). Reduced cerebral vascular density likely led to a decreased glucose and oxygen supply, which may activate AMPK in the endothelium (Lopez-Lopez et al, 2007). Activated AMPK may then stimulate eNOS in the cerebral vasculature (Osuka et al, 2009). The consequence of AMPK or eNOS activation on cerebral blood flow is not known. However, AMPK may have important physiologic and pathologic functions, as in the periphery: it is likely that AMPK produces its regulatory effects in the brain vasculature through eNOS-mediated acute vasodilatation or VEGF-mediated chronic increase in vascular generation.
In the periphery, NO is an important activator of AMPK. Activation by NO may also be an important mechanism by which AMPK is activated in the brain during ischemia, as ONOO– is produced by calcium-mediated activation of neuronal NOS (nNOS) (Heales et al, 1999). Ischemia robustly increases the phosphorylation of AMPK in brain, as in the periphery and vasculature, presumably in an attempt to restore ATP levels. We have found that ischemia-induced AMPK activation is muted in mice lacking nNOS, suggesting that nNOS (or ONOO–) is an important trigger for AMPK phosphorylation (McCullough et al, 2005). We have also shown that administration of Compound C, an AMPK inhibitor, is neuroprotective, but this effect is lost in nNOS-deficient mice (Li et al, 2006). This suggests that the protective effect of AMPK inhibition in the brain may also be mediated in part by NO signaling.
Stroke and AMPK
AMPK's Role in Neuronal Survival/Death
Most of the work performed to date on AMPK in the brain has investigated AMPK's regulation of hypothalamic energy balance. Given the high metabolic energy demands of the brain and the relative intolerance to ischemia, hypoxia, and energy depletion (Ramamurthy and Ronnett, 2006), it is likely AMPK has an important function in neuronal cell survival (Ronnett et al, 2009). Initially, the hypothesis that AMPK has a neuroprotective role in cerebral ischemia seemed attractive as activation of this kinase increases ATP production, potentially providing energy for energy depleted neurons. However, unlike peripheral tissues, neurons lack the enzymes necessary for glycolysis, the major ATP-generating pathway activated during hypoxia, making them exquisitely sensitive to ischemia. AMPK activates phosphofructokinase (PFK-2), which catalyzes the rate-limiting step in glycolysis. There is minimal, if any, activation of PFK-2 in neurons, making them unable to produce ATP through glycolysis (Figure 3). Astrocytes can metabolize glucose into lactate through glycolytic pathways (Figure 3), and do store some glycogen (Vilchez et al, 2007) providing a short-term energy supply for ischemic neurons (Bouzier-Sore et al, 2002; Pellerin and Magistretti, 1994). However, progressive lactic acidosis leads to neuronal death after periods of prolonged injury such as seen in stroke.
Figure 3.
The differential effects of AMPK in regulating glycolysis in neurons and astrocytes. Ischemia/hypoxia-induced AMPK phosphorylation activates phosphofructokinase 2 (PFK2) in astrocytes, leading to enhanced glycolysis and increased glycolytic products (pyruvate, lactate, and ATP). In neurons where there is minimal PFK-2 activity, the ability to produce ATP through glycolysis is limited. F6P, fructose-6-phosphate; F2, 6P, fructose-2,6-bisphosphate. Green arrow: activation; red arrow: inhibition. (The color reproduction of this figure is available on the html full text version of the manuscript.)
Whether AMPK is beneficial or detrimental in the ischemic brain has engendered considerable controversy (Hardie and Frenguelli, 2007). One of the first studies to examine neuronal tissue showed that the AMPK activator AICAR enhanced survival under conditions of reduced energy availability (glucose deprivation, glutamate excitotoxicity) or increased cellular stress (amyloid exposure) in cultured hippocampal neurons (Culmsee et al, 2001). Several studies performed in neuronal cell lines/tissue also support the hypothesis that AMPK activation is detrimental. AICAR promotes apoptosis in undifferentiated human neuroblastoma cells (SH-SY5Y), inducing an increase in caspase-3 activity (Garcia-Gil et al, 2003). Overexpression of active AMPK or treatment with AICAR enhanced oxidative stress and induced apoptosis in mouse neuroblastoma cells through NF-κB activation (Jung et al, 2004). Most recently, it was found that AMPK activation in primary cortical cell cultures induced by the environmental pollutant Tributyltin chloride led to neuronal death and Compound C reduced Tributylin's neurotoxic effects (Nakatsu et al, 2008).
The role of AMPK in vivo is also very likely different to that seen in vitro, especially in neurons. Neurons are post mitotic, have poor energy stores, and are intolerant to cellular stress, especially when examined without the supporting glial cells and vasculature that characterize the in vivo state. The metabolic functions of neurons and astrocytes are distinct and there are complex interactions between these two types of cells during energy depletion. Therefore, the most appropriate way to investigate the role of AMPK in neuroprotection after stroke is to examine outcomes in a whole animal system. To date, only three such studies have been performed and were all in rodents, two by our group showing that AMPK activation is deleterious, and one by Kuramoto et al (2007) that suggested that AMPK activation is protective.
In the first study to examine stroke outcome, pharmacological agents were used to manipulate AMPK phosphorylation status. We found that treatment with Compound C or with the fatty acid synthase inhibitor C75 (which reduces AMPK phosphorylation indirectly) led to a reduction in pAMPK levels and reduced damage in a reversible MCAO model. These results suggest that after cerebral ischemia, AMPK activation is detrimental and reducing AMPK activation is protective. It has been noted by others that these original studies were limited, as we used pharmacological approaches that could certainly have ‘off-target' effects (Hardie and Frenguelli, 2007). For example, the AMPK activator, AICAR has proapoptotic effects independent of AMPK (Lopez et al, 2003) and we found that it led to systemic vasodilatation and lowered Mean Arterial Pressure, most likely through its known effects on adenosine. More recently we have used a more direct approach by examining animals with selective gene deletion of either AMPK α1 or AMPK α2 (Li et al, 2007). AMPK α2 knockout mice were protected compared with wild-type controls, whereas there was no effect of AMPK α1 deletion. The beneficial effect of the AMPK inhibitor Compound C was lost in AMPK α2 knockout mice implying that the α2 isoform mediates the detrimental effect of AMPK after stroke. The genetic approach combined with the pharmacological approach further confirmed that inhibition of AMPK in the brain is neuroprotective.
In contrast, others (Kuramoto et al, 2007) have suggested that activation of AMPK leads to neuroprotection through phosphodependent functional modulation at serine 783 of the GABA(B) receptor. This interaction between AMPK and the R2 subunit of GABA(B) led to activation of postsynaptic K+ channels, resulting in prolonged hyperpolarization This could potentially lead to suppression of neurotransmitter release at presynaptic sites, reducing neuronal activity when energy stores are low (Kuramoto et al, 2007). In support of this hypothesis, when S783A mutants of R2, which cannot be phosphorylated by AMPK, were expressed in hippocampal neurons, there was a significant decrease in survival after a 15 mins anoxic insult in the cells expressing the mutant. However, the effect was quite small (11%) and no studies were performed to confirm that this protection was specifically mediated by AMPK. This could have been evaluated with a reversal of the effect by AMPK siRNA, AMPK inhibitors, or AMPK knockout cultures. It is also unknown whether this occurs with an actual ischemic challenge (i.e., oxygen/glucose deprivation). These investigators went on to show that GABA(B) R2 expression increased in rat brain 4 h after MCAO. However, ischemia-induced phosphorylation of GABA was seen primarily in the CA3 and dentate gyrus rather than in the cortex and striatum, where a dramatic downregulation of staining was seen. This suggests that GABA receptor modulation may only occur at sites distant from the ischemic damage. Again, a definitive link showing that AMPK was responsible for the ischemia-induced rise in GABA(B) phosphorylation, or the downstream effect of this enhanced phosphorylation (no histologic studies were performed) on stroke outcome remains to be investigated.
As described above, studies investigating the role of AMPK in neuronal survival/death have generated much controversy. The discrepancies, seen in studies performed in vitro, are most likely because of the differences in models, culture conditions (Kleman et al, 2008), and the cell type used (transformed versus primary cells). Mimicking neuronal metabolism in vitro is often difficult, and can lead to erroneous conclusions. For instance, neuronal cell culture media typically contains 25 mmol/L glucose, which is significantly higher than physiologic levels (between 0.82 and 2.4 mmol/L) (Ronnett et al, 2009). In a study comparing the cellular response of neurons cultured in media with different glucose concentrations (Kleman et al, 2008), cultures maintained at 3 mmol/L glucose responded in a much more similar manner to what is observed in vivo.
In vivo studies suggest that the duration and severity of the cellular stress determine the cells response to AMPK activation. Dagon et al found that mild to moderate (40%) dietary restriction increased hippocampal AMPK activity, induced neurogenesis, and improved cognition. It is likely moderate caloric restriction may enhance cell survival through transient AMPK activity. Similarly, ischemic preconditioning, which also produces transient moderate energy deprivation, may also be protective against stroke injury through activating AMPK. In contrast, 60% caloric restriction activated AMPK further, but led to reduced cognition and enhancement of neuronal apoptosis. Similar findings have been seen with KA injections, which dramatically increase AMPK activity, seizure rates, and cell death (Lee et al, 2009). Moreover, chronic corticosteroid administration in mice leads to a sustained increase in AMPK activation, leading to hippocampal cell death (Zhao et al, 2008). It is likely that the duration and level of AMPK activation, as well as the cell type examined and model used, determine the downstream effect of AMPK. Under conditions of severe energy deficiency such as in cerebral ischemia, prolonged overactivation of AMPK may indeed exacerbate brain injury, as confirmed by our studies in focal stroke model using both pharmacological agents and genetic tools (Li et al, 2007; McCullough et al, 2005).
Potential Downstream Targets of AMPK in Neuronal Survival/Death After Ischemia
Under physiologic conditions in periphery, AMPK activation promotes ATP production and inhibits ATP consumption. This is achieved by inactivating ACC (which provides substrates for the biosynthesis of fatty acid) and 3-hydroxy-3-methy-glutaryl-CoA (HMG-CoA) reductase (the rate controlling enzyme in cholesterol synthesis) to inhibit fatty acid and cholesterol synthesis. In addition, AMPK can increase glycolysis by activating PFK-2, increase fatty acid oxidation, activate glucose transportation (through glucose transporter 4) and inhibit glycogen synthesis (Ronnett et al, 2009). However, neuronal metabolism is very different than that of any other organ system. Neurons cannot perform glycolysis, can not oxidize fatty acid efficiently, and have no glycogen stores and are therefore exquisitely sensitive to hypoxia and hypoglycemia. Within a short period of energy deficiency, AMPK activation enhances astrocytic glycolysis and ketosis to provide energy to ischemic neurons. However, prolonged glycolysis in astrocytes leads to progressive acidosis and inhibits the ability of neurons to use lactate as energy sources (Blazquez et al, 1999; Pellerin and Magistretti, 1994) leading to neuronal death. We have found exacerbated lactic acidosis and enhanced neuronal injury after MCAO in mice treated acutely with AMPK activators (Li and McCullough, unpublished observations).
Glucose is the major fuel for the brain. In the periphery, AMPK activation of glucose transporters (GLUTs) facilitates glucose transport into the cell. AMPK activation increases translocation of GLUT4 onto the cell surface in the heart (Li et al, 2004) and increases the expression of GLUT4 in skeletal muscle (Jessen et al, 2003). Little is known regarding AMPK-induced glucose transport in the brain, or if it even occurs under physiologic conditions. Different isoforms of GLUTs are expressed in brain compared with the periphery (Simpson et al, 2007); GLUT1 in astrocytes and GLUT3 in neurons (Simpson et al, 2007; Vannucci et al, 1997). As glucose is an obligatory fuel for neurons, glucose transporter function is crucial for neuronal survival. SiRNA knockdown of GLUT3 increased the sensitivity of cultured neurons to glutamate excitotoxicity (Weisova et al, 2009), possibly by reducing available glucose. Inhibition of AMPK (with compound C or SiRNA) also reduced glutamate-induced surface GLUT3 expression (Weisova et al, 2009). However, what effects, if any, that AMPK-mediated GLUT upregulation has in the ischemic brain are unknown.
Conceivably, attempts to increase glucose levels during conditions of low glucose such as stroke could lead to more rapid metabolic failure as cells attempt to ineffectively increase glucose influx when there is no available substrate. Cell damage could also be exacerbated in the reperfusion phase, when glucose is restored and floods into the cells secondary to GLUT upregulation, leading to poststroke hyperglycemia. Poststroke hyperglycemia is known to be associated with poorer functional outcomes in stroke patients independent of hemorrhage risk (Alvarez-Sabin et al, 2003). Hyperglycemia induces blood–brain barrier dysfunction, increases edema, and increases the risk of hemorrhagic transformation (Saposnik et al, 2004). The endothelium is sensitive to glucose levels, and high glucose induces ROS in the endothelium, damaging the vascular wall, possibly contributing to hemorrhagic transformation (Zou and Wu, 2008). At this point, the functional consequence of GLUT upregulation in the ischemic and reperfused brain is unknown, but reducing poststroke hyperglycemia by manipulation of AMPK-induced GLUT function is an area of active investigation in our laboratory.
During severe energy depletion, neurons attempt to increase energy supply to survive. Autophagy is a process that cells use to produce energy (Du et al, 2009) during nutrient starvation that is characterized by the engulfment of cytoplasmic material and organelles to recycle amino acid and other nutrients. Autophagic pathways can be activated by rising levels of AMPK, in response to energy need (Wang and Guan, 2009). However, recent work has shown that autophagy contributes to neuronal cell death after ischemia (Du et al, 2009). Therefore, activating AMPK-dependent energy-producing cascades after stroke seems to only further stress the ischemic brain, enhancing metabolic dysfunction and worsening outcome.
A number of other important enzymes energy-producing pathways (glycolysis, tricarboxylic acid cycle) are potential targets of AMPK including glyceraldehyde-3-phosphate dehydrogenase andcitrate synthase. Although the tricarboxylic acid cycle has the potential to generate much more ATP per mole of glucose than produced by glycolysis, it is believed that the latter process is primarily responsible for meeting the increased energy demands during times of high activity in the brain during normal physiologic conditions (Tuerk et al, 2007). However, under conditions of extreme energy deficiency such as ischemia, activation of these enzymes is of little benefit.
Therefore, the down stream mediator(s) by which AMPK activation exacerbates brain injury during stroke are unknown. Research has suggested that it may involve overstimulation of AMPK-mediated energy-producing cascades such as glycolysis, GLUT, and autophagy all of which could further stress the ischemic brain and exacerbate metabolic failure. Further studies will be needed to determine if AMPK is an appropriate target for stroke therapy.
Chronic Changes Induced by AMPK
Besides direct effects on metabolism and acute cell survival/death, emerging data suggest that AMPK activation could be an important contributor to recovery after stroke.
eNOS has been implicated as an important contributor to poststroke neurogenesis through activation of brain-derived neurotrophic factor expression (Chen et al, 2005). As AMPK is a direct activator of eNOS, AMPK may also have a role in poststroke neuronal regeneration and repair. AMPK activation mediates the KA-induced increase in brain-derived neurotrophic factor expression and transcriptional activation of NF-κB, both of which are likely involved in neurogenesis and repair post stroke (Yoon et al, 2008). The expression pattern of AMPK α2 during development as described earlier, in addition, implies the role of AMPK in neurogenesis. Interestingly, AMPK is the major upstream regulator of the enzyme HMG-CoA reductase, which is the primary enzyme involved in cholesterol biosynthesis. When AMPK is phosphorylated and active, it inhibits HMG-CoA reductase by phosphorylation, ensuring that cholesterol biosynthesis is halted in times of acute energy demand. It has been well described that HMG-CoA reductase inhibitors (statins) improve outcome after stroke in both animal models and in clinical trials (Sillesen et al, 2008). Statins have a key role in secondary stroke prevention, as administration of high-dose atorvastatin has been shown to significantly reduce future ischemic events (Goldstein et al, 2009).
Statins have numerous beneficial effects on the brain and vasculature that are independent of lipid lowering (Brookes et al, 2009). Statins augment eNOS release, an effect that is mediated by AMPK (Sun et al, 2006). Atorvastatin treatment in human umbilical vein cultures increased phosphorylation of AMPK as well as AMPK activity and increased angiogenesis in vitro. These effects were abolished by Compound C or with a dominant-negative mutant of AMPK (Sun et al, 2006). Mice administered oral atorvastatin had increased levels of AMPK in the aorta and myocardium (Sun et al, 2006). As mentioned previously, both AMPK α1 and α2 are expressed in endothelial cells, although α1 predominates (Davis et al, 2006; Zou et al, 2004). However, AMPK α2 although expressed in low levels in the endothelium, may be the more important isoform in the endothelial response to hypoxia. The aorta of AMPK α2 knockout mice showed attenuated atorvastatin-induced eNOS phosphorylation (Chen et al, 2009b). Interestingly, statin-induced AMPK activation is lost in eNOS knockout mice (Choi et al, 2008), implying that the presence of both AMPK and eNOS is necessary for the full effect of statins in the peripheral vasculature.
Of the two major upstream kinases of AMPK, LKB1 is accepted as an important contributor to statin-mediated induction of AMPK. Levels of phosphorylated LKB1 increase with statin treatment (Kou et al, 2009). Nuclear LKB1 translocation occurs after statin treatment and before the increase in AMPK activation (Choi et al, 2008). The potential contribution of CaMKKβ to statin-mediated increase in AMPK is currently under debate. One group has found that administration of the CaMKKβ inhibitor, STO609 had no effect on statin-induced AMPK activation (Choi et al, 2008), but others have found that siRNA-mediated knockdown of CaMKKβ completely blocked simvastatin-induced endothelial cell migration and ameliorated statin-induced phosphorylation of both AMPK and LKB1, as did pharmacological inhibition with STO-609 (Kou et al, 2009). Differences in culture conditions and the endothelial cell type examined may account for these differences.
Statin-induced AMPK phosphorylation appears to require the presence of ROS (Choi et al, 2008). Statin treatment significantly increased ROS (similar to the action of metformin) whereas preincubation with mito-TEMPOL, a superoxide dismutase mimetic, abolished statin-enhanced phosphorylation of both AMPK and its downstream target ACC-Ser(79). In vivo administration of statin increased AMPK phosphorylation C57BL/6J mice but not in mice deficient in eNOS, suggesting that NO and the oxidants produced by NOS activation are the triggering factors for statins effects on AMPK in the vasculature (Choi et al, 2008). The actual mechanism by which statins activate AMPK remains unclear. It is possible that the inhibition of HMG-CoA reductase by pharmacological agents sets up a feedback loop in which AMPK senses this inhibition and enhances AMPK to compensate. As we have shown that acute activation of AMPK is deleterious in stroke models, it is likely that prolonged treatment will have different effects on AMPK in the vasculature in the brain; detrimental in the acute ischemic period, beneficial for later repair after the ischemic insult is complete.
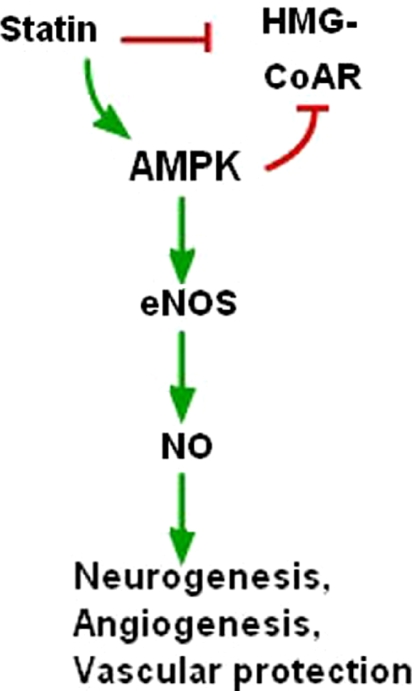
The effect of AMPK on cerebral angiogenesis is currently unknown, but an area of intense investigation. Accumulating data has shown that statins enhance angiogenesis, neurogenesis, and increase functional outcome in animal models of stroke (Chen et al, 2003; Zheng and Chen, 2007) and traumatic brain injury (Wu et al, 2008) (Figure 4). Statins may increase neurogenesis by activating eNOS, which is known to be involved in neuronal regeneration after stroke (Chen et al, 2005). Many of the effects of statins on neurogenesis and angiogenesis are mediated through VEGF (Chen et al, 2006; Uruno et al, 2008) or HIF1α (Nishimoto-Hazuku et al, 2008), both of which are downstream targets of AMPK (Jung et al, 2008; Reihill et al, 2007). AICAR enhances whereas Compound C attenuates the angiogenesis of endothelial progenitor cells in vitro and in vivo (Li et al, 2008). Statin-induced increases in AMPK phosphorylation accelerated the development of collateral vessels and angiogenesis in response to hindlimb ischemia, an effect that was dampened after treatment with Compound C (Izumi et al, 2009). More direct evidence regarding the effect of AMPK on the cerebral vasculature has been recently reported in cultured rat brain endothelium. AMPK activation by AICAR increased VEGF levels and subsequently increased endothelial proliferation and metabolism (Lopez-Lopez et al, 2007). The possibility that AMPK could mediate poststroke repair is intriguing and presents an exciting new target for investigators studying AMPK or stroke.
Figure 4.
Statins regulate neurogenesis, angiogenesis, and vascular protection through AMPK, eNOS, and NO pathways. HMG-CoAR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Green arrow: activation; red arrow: inhibition. (The color reproduction of this figure is available on the html full text version of the manuscript.)
Conclusions and possible future directions
Using AMPK as a target to develop therapeutic treatments for stroke is promising. Both pharmacological and genetic approaches have shown that AMPK activation is detrimental in stroke, at least in rodent models, whereas AMPK inhibition during the acute stroke phase provided sustained neuroprotection. Inhibiting AMPK during the acute energy depletion phase of stroke may induce a type of ‘neuronal hibernation' similar to what is seen with hypothermia, reducing energy demand and subsequent metabolic failure. The down stream mechanism by which AMPK worsens stroke outcome during the acute phase is not clear but it may involve lactic acidosis, glucose transporter upregulation, and autophagy activation. Astrocytes and neurons are metabolically distinct cell types. It will be important to dissect out the effect of AMPK activation in each component of the neurovascular unit. One appropriate way to do this in vivo is to use neuronal or astrocytic conditional AMPK null mice. These are currently under evaluation. In addition, of the three potential upstream AMPK kinases, LKB1 may be the most critical in the response to cerebral ischemia. Accumulating data indicate that NO is essential for AMPK activation, and interactions between NO, NOS, and ONOO– warrant further investigation.
Interestingly, during the chronic recovery phase, AMPK activation may enhance neurogenesis, angiogenesis, and improve brain function. One way to chronically activate AMPK is through the use of statins or metformin. Both drugs are used clinically and found to reduce the stroke incidence (Chaturvedi et al, 2009; UK Prospective Diabetes Study (UKPDS) Group, 1998; Selvin et al, 2008), possibly owing to the positive effect of chronic AMPK activation in cerebral vasculature. This is in agreement with experimental data suggesting a beneficial effect of chronic AMPK activation in the vasculature. However, a cautionary note should be made; recent evidence suggests that there may be a downside to chronic AMPK activation, at least that which is induced with metformin. Although no clinical data are currently available that metformin enhances neurodegeneration even when widely applied in patients at risk for diabetic neuropathy, mice administered chronic oral metformin at doses that lead to activation of AMPK had significantly increased generation of β amyloid, which has a major causal role in Alzheimer's Disease (Chen et al, 2009a). Compound C inhibited metformin-induced β-amyloid accumulation, implicating AMPK. Future research using more selective AMPK activators, examination of the effects of acute and chronic inhibition of AMPK, and the use of selective genetic models will provide important information on this pathway and its potential therapeutic potential for stroke patients.
Acknowledgments
This work was supported by NIH R01 NS050505 and NS055215 (to LDM). The authors thank Dr Lisa Christy Turtzo for her critical reading of the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator—treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations causeglycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez C, Woods A, de Ceballos ML, Carling D, Guzman M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J Neurochem. 1999;73:1674–1682. doi: 10.1046/j.1471-4159.1999.731674.x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Merle M, Magistretti PJ, Pellerin L. Feeding active neurons: (re)emergence of a nursing role for astrocytes. J Physiol (Paris) 2002;96:273–282. doi: 10.1016/s0928-4257(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, McGown CC, Reilly CS. Statins for all: the new premed. Br J Anaesth. 2009;103:99–107. doi: 10.1093/bja/aep149. [DOI] [PubMed] [Google Scholar]
- Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D. Transforming growth factor-beta and ischemic brain injury. Cell Mol Neurobiol. 2003;23:539–550. doi: 10.1023/A:1025072013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Zivin J, Breazna A, Amarenco P, Callahan A, Goldstein LB, Hennerici M, Sillesen H, Rudolph A, Welch MA. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology. 2009;72:688–694. doi: 10.1212/01.wnl.0000327339.55844.1a. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006;141:737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA. 2009a;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009b;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cidad P, Almeida A, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5′-AMP-activated protein kinase. Biochem J. 2004;384:629–636. doi: 10.1042/BJ20040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival followingglucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell. 2009;16:256–270. doi: 10.1016/j.devcel.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, Chu CT, Kagan VE, Clark RS. Starving neurons show sex difference in autophagy. J Biol Chem. 2009;284:2383–2396. doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Hardie DG, Galione A, Peers C, Kumar P, Wyatt CN.2006AMP-activated protein kinase couples mitochondrial inhibition by hypoxia to cell-specific Ca2+ signalling mechanisms in oxygen-sensing cells Novartis Found Symp 272234–252.discussion 252–238, 274–239 [PubMed] [Google Scholar]
- Gao G, Fernandez CS, Stapleton D, Auster, Widmer J, Dyck JR, Kemp BE, Witters LA. Non-catalytic beta- and gamma-subunit isoforms of the 5′-AMP-activated protein kinase. J Biol Chem. 1996;271:8675–8681. doi: 10.1074/jbc.271.15.8675. [DOI] [PubMed] [Google Scholar]
- Gao G, Widmer J, Stapleton D, Teh T, Cox T, Kemp BE, Witters LA. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta. 1995;1266:73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, Tozzi MG. 5′-aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience. 2003;117:811–820. doi: 10.1016/s0306-4522(02)00836-9. [DOI] [PubMed] [Google Scholar]
- Ge S, Song L, Pachter JS. Where is the blood-brain barrier … really. J Neurosci Res. 2005;79:421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, Hennerici M, Macleod MJ, Sillesen H, Zweifler R, Welch KM. Statin treatment and stroke outcome in the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke. 2009;40:3526–3531. doi: 10.1161/STROKEAHA.109.557330. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABA(B) receptor. Neuron. 2007;53:159–162. doi: 10.1016/j.neuron.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 asthe major site at whichit phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Bolanos JP, Stewart VC, Brookes PS, Land JM, Clark JB. Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009;134:169–175. doi: 10.1016/j.ijcard.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents thatelevate cellular cAMP. J Biol Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Shiota M, Kusakabe H, Hikita Y, Nakao T, Nakamura Y, Muro T, Miura K, Yoshiyama M, Iwao H. Pravastatin accelerates ischemia-induced angiogenesis through AMP-activated protein kinase. Hypertens Res. 2009;32:675–679. doi: 10.1038/hr.2009.77. [DOI] [PubMed] [Google Scholar]
- Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94:1373–1379. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. Faseb J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Jung JE, Lee J, Ha J, Kim SS, Cho YH, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neurosci Lett. 2004;354:197–200. doi: 10.1016/j.neulet.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, Ha J. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–721. doi: 10.1093/carcin/bgn032. [DOI] [PubMed] [Google Scholar]
- Kleman AM, Yuan JY, Aja S, Ronnett GV, Landree LE. Physiological glucose is critical for optimized neuronal viability and AMPK responsiveness in vitro. J Neurosci Methods. 2008;167:292–301. doi: 10.1016/j.jneumeth.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou R, Sartoretto J, Michel T. Regulation of Rac1 by simvastatin in endothelial cells: differential roles of AMP-activated protein kinase and calmodulin-dependent kinase kinase-beta. J Biol Chem. 2009;284:14734–14743. doi: 10.1074/jbc.M808664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lee H, Cho JS, Lambacher N, Lee J, Lee SJ, Lee TH, Gartner A, Koo HS. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normalmotility and foraging behavior. J Biol Chem. 2008;283:14988–14993. doi: 10.1074/jbc.M709115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Jeon BT, Shin HJ, Lee DH, Han JY, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Temporal expression of AMP-activated protein kinase activation during the kainic acid-induced hippocampal cell death. J Neural Transm. 2009;116:33–40. doi: 10.1007/s00702-008-0158-9. [DOI] [PubMed] [Google Scholar]
- Li J, Hu X, Selvakumar P, Russell RR, III, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- Li J, Ronnett GV, Zeng Z, Guo H, McCullough LD.2006Inhibition of AMPK leads to sustained neuroprotection thatis mediated by neuronal NOSStroke International Conference 2006. Kissimmee, FL: American Heart Association
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, Zhu Y. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1789–1795. doi: 10.1161/ATVBAHA.108.172452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JM, Santidrian AF, Campas C, Gil J. 5-Aminoimidazole-4-carboxamide riboside induces apoptosis in Jurkat cells, but the AMP-activated protein kinase is not involved. Biochem J. 2003;370:1027–1032. doi: 10.1042/BJ20021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase asa possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J Neurosci. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act asa glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Hardie DG. AMP-activated protein kinase—a sensor of glycogen aswell asAMP and ATP. Acta Physiol (Oxf) 2009;196:99–113. doi: 10.1111/j.1748-1716.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Kotake Y, Hino A, Ohta S. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol Appl Pharmacol. 2008;230:358–363. doi: 10.1016/j.taap.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Neurath KM, Keough MP, Mikkelsen T, Claffey KP. AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia. 2006;53:733–743. doi: 10.1002/glia.20326. [DOI] [PubMed] [Google Scholar]
- Nishimoto-Hazuku A, Hirase T, Ide N, Ikeda Y, Node K. Simvastatin stimulates vascular endothelial growth factor production by hypoxia-inducible factor-1alpha upregulation in endothelial cells. J Cardiovasc Pharmacol. 2008;51:267–273. doi: 10.1097/FJC.0b013e3181624b44. [DOI] [PubMed] [Google Scholar]
- Ofir M, Hochhauser E, Vidne BA, Freimark D, Arad M.2007[AMP-activated protein kinase: how a mistake in energy gauge causes glycogen storage] Harefuah 146770–775.813–774 [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Yoshida J, Takayasu M.2009Modification of endothelial nitric oxide synthase through AMPK after experimental subarachnoid hemorrhage J Neurotraumae-pub ahead of print [DOI] [PubMed]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure. 2005;13:1453–1462. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase asa multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109 (Suppl 1:17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334 (Pt 1:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saposnik G, Young B, Silver B, Di Legge S, Webster F, Beletsky V, Jain V, Nilanont Y, Hachinski V. Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA. 2004;292:1839–1844. doi: 10.1001/jama.292.15.1839. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Mackiewicz M, Naidoo N, O'Callaghan JP, Pack AI. AMP-activated protein kinase phosphorylation in brain is dependent on method of killing and tissue preparation. J Neurochem. 2008;105:833–841. doi: 10.1111/j.1471-4159.2007.05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, Feldman L, Vassy J, Wilson R, Bass EB, Brancati FL. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168:2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–3302. doi: 10.1161/STROKEAHA.108.516450. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic MR, Callaerts P, Norga KK. Drosophila alicorn is a neuronal maintenance factor protecting against activity-induced retinal degeneration. J Neurosci. 2008;28:6419–6429. doi: 10.1523/JNEUROSCI.1646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Tuerk RD, Thali RF, Auchli Y, Rechsteiner H, Brunisholz RA, Schlattner U, Wallimann T, Neumann D. New candidate targets of AMP-activated protein kinase in murine brain revealed by a novel multidimensional substrate-screen for protein kinases. J Proteome Res. 2007;6:3266–3277. doi: 10.1021/pr070160a. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- Tzatsos A, Tsichlis PN. Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem. 2007;282:18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Uruno A, Sugawara A, Kudo M, Satoh F, Saito A, Ito S. Stimulatory effects of low-dose3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor fluvastatin on hepatocyte growth factor-induced angiogenesis: involvement of p38 mitogen-activated protein kinase. Hypertens Res. 2008;31:2085–2096. doi: 10.1291/hypres.31.2085. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Gibbs EM, Simpson IA. Glucose utilization and glucose transporter proteins GLUT-1 and GLUT-3 in brains of diabetic (db/db) mice. Am J Physiol. 1997;272:E267–E274. doi: 10.1152/ajpendo.1997.272.2.E267. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003a;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003b;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase asa novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196:55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Oh YT, Lee JY, Choi JH, Lee JH, Baik HH, Kim SS, Choe W, Yoon KS, Ha J, Kang I. Activation of AMP-activated protein kinase by kainic acid mediates brain-derived neurotrophic factor expression through a NF-kappaB dependent mechanism in C6 glioma cells. Biochem Biophys Res Commun. 2008;371:495–500. doi: 10.1016/j.bbrc.2008.04.102. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Shen J, Su H, Li B, Xing D, Du L. corticosterone injections induce a decrease of ATP levels and sustained activation of AMP-activated protein kinase in hippocampal tissues of malemice. Brain Res. 2008;1191:148–156. doi: 10.1016/j.brainres.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen B. Effects of Pravastatin on neuroprotection and neurogenesis after cerebral ischemia in rats. Neurosci Bull. 2007;23:189–197. [PubMed] [Google Scholar]
- Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- Zou MH, Wu Y. AMP-activated protein kinase activation asa strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008;35:535–545. doi: 10.1111/j.1440-1681.2007.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]