Abstract
Minimal hepatic dysfunction can be common in acute Q fever, but severe acute cholestatic hepatitis is rarely reported. We report on a 55-year-old male with acute Q fever and severe acute cholestatic hepatitis. He complained of fever, jaundice, ascites, and restlessness on admission. A liver biopsy revealed the presence of compact fibrin-ring granulomas. Serologic titers for C. burnetii IgM and IgG were 2048:1 and 1024:1, respectively. C. burnetii DNA was detected by a nested polymerase chain reaction on the liver tissue.
Keywords: Acute Q fever, Fibrin-ring granuloma, Severe cholestatic hepatitis
INTRODUCTION
Q fever is a worldwide zoonosis caused by Coxiella burnetii, a strictly intracellular gram-negative bacterium. The common reservoirs for C. burnetii are cattle, sheep, and goats. Humans are infected by the inhalation of contaminated aerosol and dusts derived from infected animal products such as placenta, feces, and urines. The incubation period of the disease is 2-3 weeks after exposure. The clinical presentation of Q fever polymorphic is nonspecific and may be divided into acute and chronic Q fever.1 The former most commonly presents itself as a self-limited febrile illness and subclinical hepatitis. Elevated levels of the hepatic enzyme range from twice to four-fold the highest reference value. Severe clinical forms of acute Q fever are meningitis, meningoencephalitis, myocarditis and pericarditis.2 Organs are not usually seriously affected, and the overall prognosis is excellent except for those with myocarditis or meningoencephalitis.3,4 Hepatic complication of acute Q fever, such as a severe acute cholestatic hepatitis has until now never been reported in Korea. Therefore, we report a case of acute Q fever with a severe acute cholestatic hepatitis.
CASE REPORT
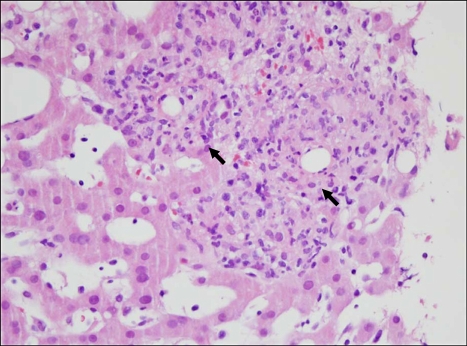
A 55-year-old male who was previously healthy was referred to a gastroenterologist for evaluation and treatment of unknown hepatitis with marked hyperbilirubinemia. He complained of striking fever, chills and myalgia that had started two weeks earlier. And his illness such as dark-colored urine, swelling of the leg, abdominal distension, anxiety, insomnia and restlessness had commenced five days before hospitalization. He had no significant medical history. He did not smoke, drink, or take any herbal medicine. He did not suffer any trauma, either. Upon physical examination, his body temperature was 40.2℃, his pulse rate was 102/min, his respiratory rate was 24/min and his blood pressure was 140/79 mmHg. Upon neurologic examination, there were no focal neurologic and diffuse meningeal irritation signs. Both scleras were markedly icteric. A systolic murmur (Grade II) was heard in the anterior chest, but the breathing sound was clear. Diffuse abdominal tenderness but no rebounding tenderness was noted in the abdomen. Pitting edema was present in bilateral pretibial regions. Laboratory tests revealed that the white blood cell count was 12,700/mm3 (neutrophils of 83%, lymphocyte of 9.3%), hemoglobin was 11.5 g/dL and platelets were 241,000/mm3. Liver function test showed alanine transaminase (ALT) of 102 IU/L, aspartic acid transaminase (AST) of 131 IU/L, alkaline phosphatase of 265 IU/L, total bilirubin of 22.5 mg/dL, direct bilirubin of 20.2 mg/dL, albumin of 2.3 g/dL and creatinine of 1.1 mg/dL. Prothrombin time (PT INR) was 1.28 (control 0.8-1.2) and activated partial thrombin time (aPTT) was 53.6 s (control, 29-45). C-reactive protein was 14.91 mg/dL. A serology test for hepatitis A, B, C and E was negative, and a test for Epstein-Barr virus and Cytomegalovirus indicated previous infections. Antibodies for human immunodeficiency virus type 1 (HIV-1) and VDRL were negative. A test for antimitochondrial antibodies, antinuclear antibodies and antismooth muscle antibody was negative. A chest X-ray on the first day of hospitalization was normal. Abdominal computed tomography (CT) revealed a small amount of ascites and mild hepatomegaly. Magnetic resonance cholangiography (MRCP) was performed and exhibited no stone and no evidence of biliary tract obstruction. There was no growth in the blood cultures. An electrocardiogram was normal, and echocardiography showed no vegetation. Under the impression of the severe acute cholestatic hepatitis associated with a fever of unknown origin, a transjugular liver biopsy was done. Microscopic examination revealed numerous compact fibrin-ring granulomas (Fig. 1), characterized by a central, round, clear space surrounded by an eosinophilic fibrin ring and infiltrated by histiocytes, lymphocytes, and neutrophils. Considering his history carefully, one month ago he had eaten uncooked goat meat and ingested its blood. Therefore, an empirical trial was done by administering doxycycline 200 mg daily for 2 weeks. A concurrent serologic test for C. burnetii immunoglobulin M (IgM) and G (IgG) was done. Symptoms of cholestatic hepatitis had subsided for three days after taking doxycycline. A significant rise in the titers of IgM and IgG antibodies against C. burnetii were 2048:1 and 1024:1, respectively. A polymerase chain reaction (PCR) was done on the liver tissue specimen and identified the presence of C. burnetii DNA. After discharge, he had visit at out-patient department one month later, the liver function tests were normal and he had completely recovered.
Fig. 1.
Microscopic findings. The liver core biopsy sample demonstrates distinctive fibrin-ring granulomas (arrows), showing central lipid cores, eosinophilic ring of fibrin, and mixed infiltrate of histiocytes, lymphocytes, and neutrophils (H&E, ×400).
DISCUSSION
Common manifestations of acute Q fever usually present an influenza-like illness with a varying degree of pneumonia and hepatitis. The most frequent clinical presentation of acute Q fever may vary from one area to another. Indeed, acute Q fever hepatitis has been reported in 9.0% to 67% in the endemic area, such as the south of Spain, Ontario, and France. Clinical and laboratory findings of acute Q fever hepatitis are a mild elevation of liver enzymes, hepatomegaly, and/or splenomegaly, which have been reported in 55-67%, 16-54% and 14-23% respectively. Jaundice, in 3-5%, is a rare clinical feature of acute Q fever hepatitis.5,6 In this case, acute Q fever presented a severe hepatitis with a marked hyperbilirubinemia (Total bilirubin >20 mg/dL) and ascites. These characteristic findings were different from previous reports. In southern Taiwan, Chang et al. have reported that total serum bilirubin ranged from 2 to 18.7 mg/dL. The total bilirubin of the two patients from the above paper was above 10 mg/dL. In one patient, acute cholecystitis with empyema formatiom was suspected initially. An exploratory laparotomy was performed and a T tube was inserted in the gallbladder.
The other was initially diagnosed with cholelithiasis with acute cholangitis. Endoscopic retrograde cholangiopancreatography with endoscopic nasobiliary drainage was done.7 In France, seven cases of acute acalculous cholecystitis associated with Q fever have been reported. According to the French report, three patients have symptoms of jaundice, and a cholecystectomy was performed in five of the patients within two or three days of their admission.8 In this case, the biliary tract and gallbladder were relatively normal in appearance. Consequently, marked hyperbilirubinemia and ascites result from severe hepatic dysfunction and not from obstructive causes. Although the correlation between the bilirubin level and the severity of acute Q fever hepatitis is unclear, it may prolong days of hospitalization, febrile duration and recovery.7 However, since neurologic symptoms in acute Q fever have been reported less frequently, a clinical suspicion of neurologic involvement is very important in order to rule out meningoencephalitis. Symptoms that arouse suspicion are severe headaches that are strong enough to prompt a lumbar puncture to rule out meningitis and progressive focal neurologic signs.9 In this case, he did not complain of headaches. There was no meningeal irritation or progressive focal neurologic signs. Although behavioral abnormalities were common in the meningoencephalitis, they were nonspecific. Therefore, it is more likely that symptoms such as anxiety, insomnia, and restlessness result from severe acute cholestatic hepatitis and not from meningoencephalitis. It is true that the pathogenic mechanisms and interaction of C. burnetii with the host's immune systems are still poorly understood, but hematogenous spread of C. burnetii results in infection of the liver, spleen, bone marrow and reproductive tract, causing the formation of granulomatous lesions.10 There are three types of granulomatous lesions: clear central space with fibrinoid ring type, containing fibrin without a ring configuration type and nonspecific type.11 The granulomas of Q fever are histologically characterized by clear central space with fibrinoid ring type. These granulomas are thought to be characteristic of Q fever hepatitis.12 Also, clear central space with fibrinoidring type graulomas have also been described in patients with leishmaniasis, infectious mononucleosis, giant cell arteritis, and allopurinol hypersensitivity.13-16 Recently, it has been noted in chronic hepatitis C and acute hepatitis A.17,18 In this case, the biopsy specimen revealed that typical granuloma consists of fibrin-ring with clear central space. There is no evidence of neoplastic cell infiltrations or vasculitis. Although hepatic fibrin-ring granulomas are characteristic, they are not pathognomic. In fact, it is difficult to isolate C. burnetii, and infection risk is very high in cultures of C. burnetii. Therefore, a definitive test of infection is serologically diagnosed in practice.19 This test detects specific antibodies to C. burnetii by employing the complement fixation (CF) method or indirect immunoflorescence (IFA) method.20,21 In general, serologic tests using the IFA method are currently used because they are eight times more sensitive than the CF method.22 In the acute phase of infection, within a few days after the onset of clinical symptoms, specific IgM globulins could be detected, rising to peak levels in the fourth and eighth week and then falling in the twelfth week. This was accompanied by a rise in IgG in the second and third week. Therefore, high IgM values indicate acute infection.23 In this case, a significant rise in the titers of IgM and IgG antibodies against C. burnetii were 2048:1 and 1024:1. Polymerase chain reaction (PCR)-based methods have been used as an additional confirmative test because isolation of C. burnetii is time-consuming.24 Macrolides are commonly used for the empirical treatment of richettsial disease. According to the study regarding the effectiveness of macrolides in acute Q fever, the mean times until defervescence of doxycycline were 2.9 days.25-27 In this case, an empirical therapeutic trial was done by using doxycycline at 200 mg daily for 2 weeks. Symptoms of cholestatic hepatitis had subsided rapidly after taking doxycycline.
Therefore, we report a case of acute Q fever with a severe acute cholestatic hepatitis.
References
- 1.Bolaños M, Santana OE, Pérez-Arellano JL, et al. Q fever in Gran Canaria: 40 new cases. Enferm Infecc Microbiol Clin. 2003;21:20–23. doi: 10.1016/s0213-005x(03)72869-9. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon A, Villanueva JL, Viciana P, et al. Q fever: epidemiology, clinical features and prognosis. A study from 1983 to 1999 in the South of Spain. J Infect. 2003;47:110–116. doi: 10.1016/s0163-4453(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 4.Luksić B, Punda-Polić V, Ivić I, Bradarić I, Bradarić N. Clinical and epidemiological features of hospitalized acute Q fever cases from Split-Dalmatia County (Croatia), 1985-2002. Med Sci Monit. 2006;12:CR126–CR131. [PubMed] [Google Scholar]
- 5.Tissot Dupont H, Raoult D, Brouqui P, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 6.Ergas D, Keysari A, Edelstein V, Sthoeger ZM. Acute Q fever in Israel: clinical and laboratory study of 100 hospitalized patients. Isr Med Assoc J. 2006;8:337–341. [PubMed] [Google Scholar]
- 7.Chang K, Yan JJ, Lee HC, Liu KH, Lee NY, Ko WC. Acute hepatitis with or without jaundice: a predominant presentation of acute Q fever in southern Taiwan. J Microbiol Immumol Infect. 2004;37:103–108. [PubMed] [Google Scholar]
- 8.Rolain JM, Lepidi H, Harlé JR, et al. Acute acalculous cholecystitis associated with Q fever: report of seven cases and review of the literature. Eur J Clin Microbiol Infect Dis. 2003;22:222–227. doi: 10.1007/s10096-003-0899-1. [DOI] [PubMed] [Google Scholar]
- 9.Bernit E, Pouget J, Janbon F, et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700. doi: 10.1001/archinte.162.6.693. [DOI] [PubMed] [Google Scholar]
- 10.Woldehiwet Z. Q fever (coxiellosis): epidemiology and pathogenesis. Res Vet Sci. 2004;77:93–100. doi: 10.1016/j.rvsc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Wu CS, Chang KY, Lee CS, Chen TJ. Acute Q fever hepatitis in Taiwan. J Gastroenterol Hepatol. 1995;10:112–115. doi: 10.1111/j.1440-1746.1995.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Cone LA, Curry N, Shaver P, Brooks D, DeForge J, Potts BE. Q fever in the Southern California desert: epidemiology, clinical presentation and treatment. Am J Trop Med Hyg. 2006;75:29–32. doi: 10.4269/ajtmh.2006.75.1.0750029. [DOI] [PubMed] [Google Scholar]
- 13.Moreno A, Marazuela M, Yebra M, et al. Hepatic fibrin-ring granulomas in visceral leishmaniasis. Gastroenterology. 1988;95:1123–1126. doi: 10.1016/0016-5085(88)90192-8. [DOI] [PubMed] [Google Scholar]
- 14.Nenert M, Mavier P, Dubuc N, Deforges L, Zafrani ES. Epstein-Barr virus infection and hepatic fibrin-ring granulomas. Hum Pathol. 1988;19:608–610. doi: 10.1016/s0046-8177(88)80215-6. [DOI] [PubMed] [Google Scholar]
- 15.de Bayser L, Roblot P, Ramassamy A, Silvain C, Levillain P, Becq-Giraudon B. Hepatic fibrin-ring granulomas in giant cell arteritis. Gastroenterology. 1993;105:272–273. doi: 10.1016/0016-5085(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 16.Vanderstigel M, Zafrani ES, Lejonc JL, Schaeffer A, Portos JL. Allopurinol hypersensitivity syndrome as a cause of hepatic fibrin-ring granulomas. Gastroenterology. 1986;90:188–190. doi: 10.1016/0016-5085(86)90092-2. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Ishii M, Nagura H, et al. Transient hepatic fibrin-ring granulomas in a patient with acute hepatitis A. Liver. 1995;15:276–279. doi: 10.1111/j.1600-0676.1995.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 18.Glazer E, Ejaz A, Coley CJ, 2nd, Bednarek K, Theise ND. Fibrin ring granuloma in chronic hepatitis C: virus-related vasculitis and/or immune complex disease? Semin Liver Dis. 2007;27:227–230. doi: 10.1055/s-2007-979473. [DOI] [PubMed] [Google Scholar]
- 19.Beslagić E, Hamzić S, Zvizdić S, Bajrović T, Velić R. Laboratory diagnosis of Q-fever with the indirect immunofluorescence test. Med Arh. 2002;56:89–92. [PubMed] [Google Scholar]
- 20.Dupuis G, Péter O, Peacock M, Burgdorfer W, Haller E. Immunoglobulin responses in acute Q fever. J Clin Microbiol. 1985;22:484–487. doi: 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devine P, Doyle C, Lambkin G. Combined determination of Coxiella burnetii-specific immunoglobulin M (IgM) and IgA improves specificity in the diagnosis of acute Q fever. Clin Diagn Lab Immunol. 1997;4:384–386. doi: 10.1128/cdli.4.3.384-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavanet P, Pechinot A, Lobjoy B, Portier H. Comparative value of complement fixation and indirect immunofluorescence reactions for the detection of Q fever. Pathol Biol (Paris) 1984;32:53–55. [PubMed] [Google Scholar]
- 23.Schmeer N, Krauss H, Wilske B. Serodiagnosis of human Q-fever: demonstration of non-complement binding IgM antibodies in the enzyme-linked immunosorbent assay (ELISA) Immun Infekt. 1984;12:245–251. [PubMed] [Google Scholar]
- 24.Ogawa M, Setiyono A, Sato K, Cai Y, Shiga S, Kishimoto T. Evaluation of PCR and nested PCR assays currently used for detection of Coxiella burnetii in Japan. Southeast Asian J Trop Med Public Health. 2004;35:852–855. [PubMed] [Google Scholar]
- 25.Pérez-del-Molino A, Aguado JM, Riancho JA, Sampedro I, Matorras P, Gonzalez-Macias J. Eythromycin and the treatment of Coxiella burnetti pneumonia. J Antimicrob Chemother. 1991;28:455–459. doi: 10.1093/jac/28.3.455. [DOI] [PubMed] [Google Scholar]
- 26.Keysary A, Itzhaki A, Rubinstein E, Oron C, Keren G. The in-vitro anti-rickettsial activity of macrolides. J Antimicrob Chemother. 1996;38:727–731. doi: 10.1093/jac/38.4.727. [DOI] [PubMed] [Google Scholar]
- 27.Gikas A, Kofteridis DP, Manios A, Pediaditis J, Tselentis Y. Newer macrolides as empiric treatment for acute Q fever infection. Antimicrob Agents Chemother. 2001;45:3644–3646. doi: 10.1128/AAC.45.12.3644-3646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]