Abstract
Background/Aims
Chronic hepatitis C virus (HCV) infection is associated with a higher risk of the development of insulin resistance. If HCV is a causal factor for insulin resistance, then clearance of HCV might decrease insulin resistance. The aim of this study was to elucidate the effects of clearance of HCV on insulin resistance.
Methods
We analyzed 28 patients with HCV infection who received combination treatment of 180 µg of pegylated interferon α-2a and ribavirin at our institution from May 2004 to November 2006. Insulin resistance was calculated according to the homeostasis model assessment of insulin resistance (HOMA-IR) method.
Results
Twenty-two patients (78.6%) achieved sustained virologic response (SVR), where the fasting plasma glucose level significantly decreased after antiviral treatment. Fasting serum insulin and HOMA-IR also significantly decreased after antiviral treatment, whereas the BMI value was not significantly affected. For the nonresponders (n=6), no significant changes were evident in BMI, fasting plasma glucose, fasting serum insulin, and HOMA-IR at 6 months after the end of antiviral treatment. Logistic regression analysis indicated that the only independent factor contributing to the reduction of insulin resistance was the complete disappearance of HCV RNA at 6 months after the end of antiviral treatment (SVR).
Conclusions
The clearance of HCV by the combination therapy of pegylated interferon α-2a and ribavirin improves insulin resistance by reducing fasting serum insulin and glucose levels.
Keywords: Chronic hepatitis C, Antiviral treatment, Insulin resistance, Homeostasis model assessment, Pegylated interferon
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is associated with a greater risk for the development of insulin resistance.1 Greater insulin resistance is more prevalent among patients with HCV infection compared with those with other liver diseases and with the general population.2 In patients with HCV infection, insulin resistance is involved in progression of hepatic fibrosis,3 the development of hepatocellular carcinoma,4,5 extrahepatic manifestations,6 and prognosis.7 Thus, insulin resistance plays a crucial role in patients with HCV infection.
The relationship between the severity of insulin resistance and the HCV replicative level has not been cleared delineated. A recent work seems to suggest the possible relationship,8 but it is still not clear whether HCV replication directly increases insulin resistance, or whether hyperinsulinemia stimulates viral replication, as suggested by in vitro data.9 This lack of correlation may be due to the fact that the global level of insulin resistance might depend on the contribution from the adipose tissue and the muscle, two extrahepatic compartments that are not infected by HCV.
The investigation considering the effect of antiviral therapy is another classical way to prove or disprove an association between infection and pathology. Romero-Gómez et al.10 have shown that insulin sensitivity might improve in patients who achieve HCV RNA clearance, while it did not improve in non-responders, despite the decrease in body mass index (BMI). An improved glucose tolerance has been reported to follow successful antiviral treatment in at least two other studies.11,12 However, further, independent confirmation of these observations might be useful, especially, for the patients with chronic HCV infection who received pegylated interferon plus ribavirin combination treatment. The ability of this antiviral treatment to improve glucose metabolism would support the notion that HCV causes insulin resistance in patients with HCV infection. Accordingly, we studied the effects of clearance of HCV by combination treatment of pegylated interferon plus ribavirin on insulin resistance.
MATERIALS AND METHODS
1. Subjects
This was a prospective cohort study analyzing 28 patients with HCV infection who underwent the combination treatment of 180 µg of pegylated interferon α-2a (Pegasys®, Roche, Seoul, Korea) and ribavirin (800-1,000 mg per day, according to the genotype of HCV) at our institution from May 2004 to November 2006. The diagnosis was based on elevated serum aminotransferase level, histological examination, consistent detection of anti-HCV and HCV-RNA. Patients who coincided with other causes of liver disease such as chronic hepatitis B, autoimmune hepatitis, or alcoholic liver disease (greater than 80 g alcohol per day for at least 1-month duration prior to the onset of illness) were excluded, as were those who had been taking corticosteroids or those with a history of, or evidence of, pancreatitis or pancreatic tumor. Clinical data was collected before antiviral treatment included age, sex, and alcohol consumption. BMI was calculated as body weight in kilograms divided by the square of height in meter (kg/m2). Informed consent for participation in the study was obtained from each subject. The study protocol conformed to the ethical guidelines of 1975 Declaration of Helsinki.
2. Laboratory determination
Venous blood samples were taken in the morning after 12-h overnight fast. Plasma glucose, serum alanine aminotransferase, aspartate aminotransferase, albumin, and total bilirubin levels were measured by using standard clinical methods (Department of Clinical Laboratory, Kangbuk Samsung Hospital, Seoul, Korea). Serum insulin levels were measured by electrochemi-luminescence immunoassay by using an autoanalyzer (Elecsys 1010/2010; Elecsys Modular Analytics E170; Roche, Basil, Switzerland). Serum C-peptide levels were measured by a commercially available kit (Immulite, Diagnostic Products, Los Angeles, CA, USA). All patients had positive anti-HCVAb, as measured by EIA3 (Abbott Laboratories, Chicago, IL, USA). HCV-RNA was measured by Amplicor-HCV-Monitor 1.0 (Roche Diagnostics GmbH, Mannheim, Germany) at 12 weeks after the initiation of antiviral treatment, at the end of antiviral treatment and 6 months after the end of antiviral treatment. HCV genotype was determined by polymerase chain reaction with type-specific primers and genotypes were classified according to Simmonds's classification system.13
Insulin resistance (IR) is defined as the condition in which normal amounts of insulin are inadequate to produce a normal insulin response from fat, muscle and liver cells. The most common type of insulin resistance is associated with a disease state known as metabolic syndrome. IR can progress to full type 2 diabetes. Insulin resistance was calculated on the basis of fasting levels of plasma glucose and serum insulin, according to the homeostasis model assessment (HOMA) method. The formula for the HOMA model is as follows: insulin resistance (HOMA-IR) = fasting glucose (mg/dL) × fasting insulin (µIU/mL)/405.
3. Treatment and follow-up
All patients were treated with 180 µg of pegylated interferon α-2a (Pegasys®, Roche, Seoul, Korea) by subcutaneous injection once a week plus ribavirin (Viramid®, Ilsung Pharmaceuticals Co., Ltd., Seoul, Korea, 800 mg/day for HCV genotype 2a and 1,000 mg/day for HCV genotype 1b) for either 6 months (for genotype 2a) or 1 year (for genotype 1b). Patients were followed up until 6 months after the conclusion of antiviral treatment and classified into two groups: sustained responder (n=22), who had undetectable HCV-RNA by Amplicor-HCV-Monitor 1.0 at 6 months after the conclusion of antiviral treatment; nonresponder (n=6) who had detectable HCV-RNA by Amplicor-HCV-Monitor 1.0 at 6 months after the conclusion of antiviral treatment.
4. Statistical analysis
All data are expressed as mean±SD (standard deviation). The Wilcoxon signed rank test was employed for analysis of paired samples. Statistical comparisons between the patients group were performed by Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables. Statistical correlation between HCV-RNA level and HOMA-IR was performed by Spearman's correlation. A multivariate logistic regression model was used to determine the independent factors associated with the delta HOMA-IR value. Data were analyzed using SPSS package (SPSS 11.5 version, SPSS Inc., Chicago, IL, USA). p values less than 0.05 were considered significant.
RESULTS
1. Characteristics of patients
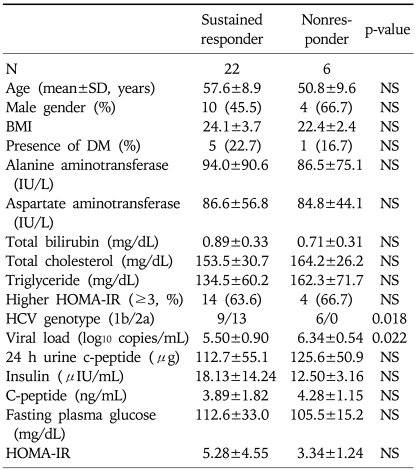
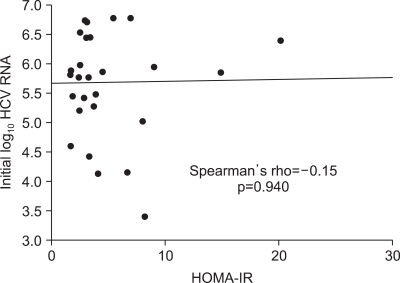
Baseline characteristics of the patients are summarized in Table 1. There was no significant difference in age, gender distribution, BMI, presence or absence of DM, the levels of serum aminotransferases, total bilirubin, total cholesterol, triglyceride, fasting plasma glucose, insulin, c-peptide and HOMA-IR between patients group of sustained responder and nonresponder. In our study, the rate of sustained virologic response (SVR) in patients with higher HOMA-IR (>3) was not significantly different from that of patients with lower HOMA-IR (≤3) (14/18 [77.8%] for higher HOMA-IR group vs. 8/10 [80.0%] for lower HOMA-IR group, p>0.05). HCV viral load determined before the initiation of antiviral treatment (expressed as log10 copies/mL) was significantly higher in nonresponder group compared with that of responder group (p=0.022). In nonresponder group, the infection rate of genotype 1b (100%) was significantly higher than that of responder group (40.9%) by Fisher's exact test (p=0.018). There was no significant correlation between the insulin resistance (HOMA-IR) and the status of viral replication (HCV-RNA) before the initiation of antiviral treatment (Fig. 1).
Table 1.
Characteristics of the Patients
N, number; SD, standard deviation; NS, not significant; BMI, body mass index; DM, diabetes mellitus; HCV, hepatitis C virus; HOMA-IR, homeostasis model assessment-insulin resistance.
Fig. 1.
Correlation between insulin resistance (HOMA-IR) and the status of viral replication (HCV RNA) before antiviral treatment.
2. Changes in BMI, fasting plasma glucose, insulin, c-peptide and HOMA-IR after antiviral treatment
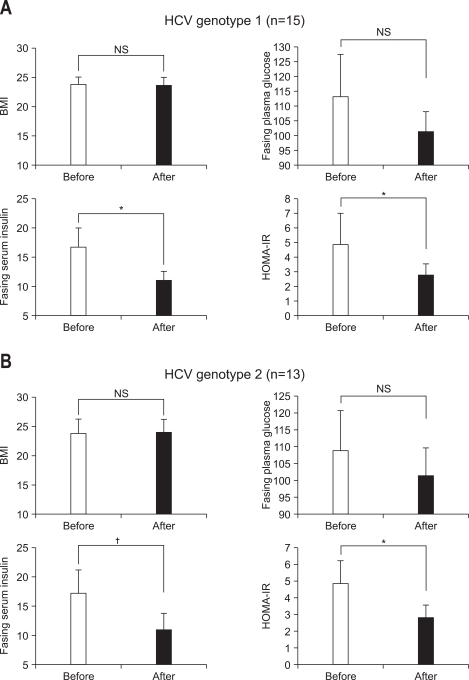
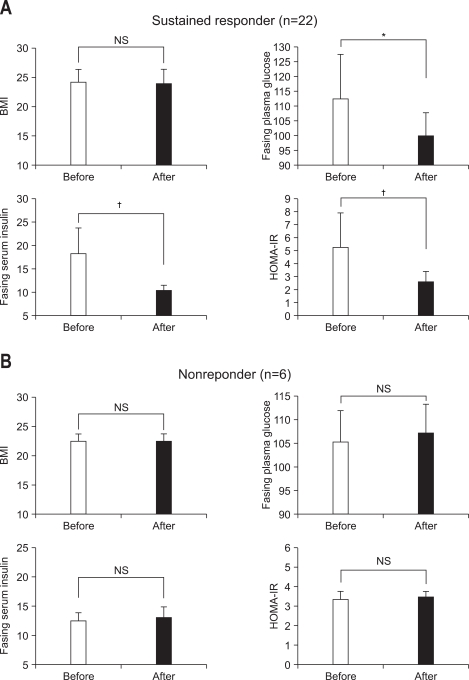
Changes in BMI, fasting plasma glucose, insulin and HOMA-IR, classified with the presence or absence of SVR after antiviral treatment are summarized in Fig. 2. For sustained responders (n=22), fasting plasma glucose level significantly decreased to 99.9±14.9 mg/dL from 112.6±33.0 mg/dL (p=0.035 by Wilcoxon signed rank test) at 6 months after the end of antiviral treatment. Especially, fasting serum insulin and HOMA-IR significantly decreased to 10.40±3.12 µIU/mL (p<0.01 by Wilcoxon signed rank test) and 2.62±1.07 (p<0.01 by Wilcoxon signed rank test) from 18.13±14.24 µIU/mL and 5.28±4.55 at 6 months after the end of antiviral treatment, respectively. However, there was no significant change in BMI value at 6 months after the end of antiviral treatment compared with that before antiviral treatment (from 23.8±3.5 to 23.7±3.5 kg/m2, p=0.087). For nonresponders (n=6), no significant differences were seen in BMI (from 22.4±2.4 to 22.4±2.4 kg/m2, p=0.564), fasting plasma glucose (from 105.5±15.2 to 107.0±14.6 mg/dL, p=0.833), fasting serum insulin (from 12.50±3.15 to 13.06±4.02 µIU/mL, p=0.345) and HOMA-IR (from 3.34±1.24 to 3.45±1.28, p=0.500) at 6 months after the end of antiviral treatment compared with those before antiviral treatment.
Fig. 2.
BMI, fasting plasma glucose, fasting serum insulin, and HOMA-IR before and after antiviral treatment in sustained responders (A, n=22) and nonresponders (B, n=6). Data were obtained before and at 6 months after the end of antiviral treatment. Bar and vertical line is mean and SD value, respectively.
*p<0.05, †p<0.01.
BMI, body mass index; NS, not significant.
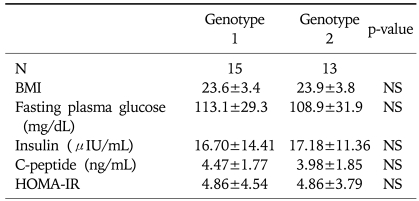
No significant differences of BMI, fasting plasma glucose, serum insulin, serum c-peptide and HOMA-IR were noticed between the patients group of genotype 1 and 2 HCV infection (Table 2). Changes in BMI, fasting plasma glucose, insulin and HOMA-IR, classified by the genotype of HCV after antiviral treatment are summarized in Fig. 3. The levels of BMI were not significantly altered after antiviral treatment compared with those before antiviral treatment in patients with both the genotype 1 and 2 HCV infection (for genotype 1, 23.6±3.4 to 23.5±3.2, p=0.184 by Wilcoxon signed rank test: for genotype 2, 23.9±3.8 to 24.0±3.8, p=0.833 by Wilcoxon signed rank test). Fasting plasma glucose levels were not significantly altered at 6 months after the end of antiviral treatment compared with those before the treatment for patients with both the genotype 1 and 2 HCV infection (for genotype 1, 113.1±29.3 mg/dL to 101.4±10.5 mg/dL, p=0.280 by Wilcoxon signed rank test: for genotype 2, 108.9±31.9 mg/dL to 101.5±19.2 mg/dL, p=0.184 by Wilcoxon signed rank test). For both patients with genotype 1 and 2 HCV infection, fasting serum insulin significantly decreased at the 6 months after the end of antiviral treatment (for genotype 1, 16.70±14.41 µIU/mL to 11.02±3.61 µIU/mL, p=0.047 by Wilcoxon signed rank test; for genotype 2, 17.18±11.36 µIU/mL to 10.92±3.37 µIU/mL, p=0.003 by Wilcoxon signed rank test). In addition the values of HOMA-IR significantly decreased at 6 months after the end of antiviral treatment for both patients with genotype 1 and 2 HCV infection (for genotype 1, 4.86±4.54 to 2.79±1.10, p=0.018 by Wilcoxon signed rank test; for genotype 2, 4.86±3.79 to 2.81±1.25, p=0.003 by Wilcoxon signed rank test).
Table 2.
Comparisons of BMI, Fasting Plasma Glucose, Serum Insulin, C-peptide and HOMA-IR Levels between the Patients Group Infected with Genotype 1 and 2 HCV
HCV, hepatitis C virus; N, number; SD, standard deviation; NS, not significant; BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance.
Fig. 3.
BMI, fasting plasma glucose, fasting serum insulin, and HOMA-IR before and after antiviral treatment in patients with genotype 1 HCV infection (A, n=15) and genotype 2 HCV infection (B, n=13). Data were obtained before and at 6 months after the end of antiviral treatment. Data are mean and SD values.
*p<0.05, †p<0.01.
BMI, body mass index; NS, not significant.
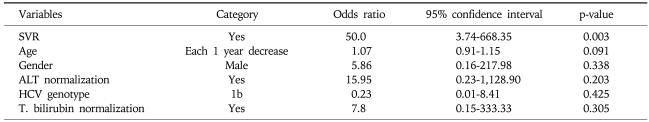
We have categorized the patients group into the minus (decreasing) delta HOMA-IR group and plus (increasing) delta HOMA-IR group, respectively. We than performed multivariate logistic regression analysis for the independent contributing factors to the minus (decreasing) delta-HOMA-IR group, and identified that SVR (complete disappearance of HCV RNA at 6 months after the end of antiviral treatment) was the only independent contributing factor to minus (decreasing) delta-HOMA-IR group (Table 3).
Table 3.
Multivariate Logistic Regression Analyses for the Independent Contributing Factors to the Minus (Decreasing) Delta HOMA-IR Group
SVR, sustained virologic response; ALT, alanine aminotransferase; T. bilirubin, total bilirubin.
DISCUSSION
The present study demonstrated that clearance of HCV by combination treatment of pegylated interferon α-2a plus ribavirin improved HOMA-IR due to the decrease in fasting serum insulin and plasma glucose level. These findings indicate that HCV itself might be involved in the development of insulin resistance.
Insulin resistance can be caused by many factors. Obesity is a common factor for the development of insulin resistance.14 Although, greater insulin resistance was noticed in our study patients, mean values of BMI were within normal limit at the enrollment and no significant change of BMI was noticed after the antiviral treatment.
In an epidemiologic study, Bahtiyar et al.15 reported that obesity was not associated with the development of insulin resistance in patients with HCV infection. In addition, the development of insulin resistance was noticed by 1 month of age in the absence of either overt liver injury or excessive body weight gain in HCV core transgenic mice16 and serum HCV core protein levels were associated with HOMA-IR values in patients with chronic hepatitis C.17 Moreover, a significant increase in the incidence of diabetes was seen in subjects with higher titers of HCV core compared with subjects with low titers of HCV core or anti-HCV negative subjects at the population level during 7 years of follow-up.18 Taken together, these findings suggest that HCV itself may cause insulin resistance.
In our study, insulin resistance (HOMA-IR) was improved only in sustained responder group who had undetectable HCV-RNA at 6 months after the end of antiviral treatment and insulin resistance was not improved in nonresponder group who had not achieved HCV clearance at 6 months after the end of antiviral treatment. These results of our study might add another piece of a puzzle for the causal relationship of HCV itself to insulin resistance. However, there are some difficulties in establishing a definite relationship between HCV infection and diabetes on the basis of our results only. Practically, there are other numerous factors in patients, perturbing the verification of the definite relationship, such as advanced liver injuries, obesity and aging. Additionally, before the initiation of antiviral treatment, significant correlation between the status of HCV replication and insulin resistance was not noticed in patients group of our study (Fig. 1). However, previous study16 showed that the HCV core gene transgenic mice exhibited insulin resistance as early as 1-month old, despite an apparent absence of glucose intolerance. Furthermore, development of insulin resistance without any liver injury or excessive body weight gain, clearly indicates that infection of HCV per se is a cause of the development of insulin resistance. Additionally, multivariate logistic regression analysis performed in our study showed that only SVR (complete complete disappearance of HCV RNA at 6 months after the end of antiviral treatment) was the independent contributing factor to the minus (decreasing) delta HOMA-IR group and other clinical parameters, such as age, gender, HCV genotype, the temporal change of BMI, and ALT (indicator of liver inflammation) were not significant contributing factors to minus (decreasing) delta HOMA-IR group. These contradictory findings of previous and our studies suggest that it is still not clear whether HCV replication itself directly increases insulin resistance. We speculated that mechanisms other than replication numbers of HCV, such as various pathologic changes induced by HCV infection might influence the development of insulin resistance.
Interferon induces insulin resistance immediately after administration in healthy controls.19 In chronic hepatitis C patients treated for 2 weeks, interferon induces a decrease in glucose uptake by peripheral tissue and the liver.20 However, this effect disappears when analyzed after 3 months of treatment21 or during the follow up at the end of treatment.22 In our study, the clearance of the HCV induced an improvement in insulin resistance, despite a potential increase in insulin resistance associated with the interferon treatment. Even in nonresponders, interferon did not worsen insulin resistance and these data also support a link between active HCV replication and insulin resistance.
Previous study by Romero-Gómez et al.10 has shown that insulin resistance strongly influences the rate of SVR in patients infected by genotype 1. However, in our study, the rate of SVR in patients with higher HOMA-IR (>3) was not significantly different from that of patients with lower HOMA-IR (≤3). This finding could be attributed to the relatively small number of nonresponders (n=6) included in our study.
Previous report23 has shown that HCV genotype 1 and 4 are independently associated with increased HOMA-IR and insulin resistance. Another report24 has shown that HCV genotype 3 has a negative independent association with insulin resistance. In our study, no significant differences of BMI, fasting plasma glucose, serum insulin and HOMA-IR levels were noticed between the patients group infected with genotype 1 and 2 HCV (Table 2). To our best knowledge, our study is the first report comparing the genotype 1 and 2 HCV infection for the association with several metabolic parameters, such as BMI, fasting plasma glucose level, serum insulin and c-peptide, and insulin resistance. However, further comparisons among the variable genotypes of HCV more than genotype 1 and 2 (such as HCV genotype 3 and 4) will delineate the true impact of each HCV genotype on the metabolic parameters. Significant reduction of insulin and HOMA-IR were noticed in both patients group of genotype 1 and 2 HCV infection at 6 months after the end of antiviral treatment (Fig. 3). In contrast, fasting plasma glucose level was not significantly changed in both HCV genotype 1 and 2 patients group at 6 months after the end of antiviral treatment. However, in sustained responders, significant reduction of fasting plasma glucose level was noticed after the end of antiviral treatment, so the difference of the results of the temporal change of fasting plasma glucose level according to the presence or absence of SVR or HCV genotype might be attributed to the alteration of number of each patients group between the two statistical comparisons.
The drawback of our study was insufficient number of enrolled patients, especially for the nonresponders, to elucidate the causal relationship between the successful HCV eradication by the combination treatment of pegylated interferon plus ribavirin and the improvement of insulin resistance.
In conclusion, our study found that the clearance of HCV by the combination therapy of pegylated interferon α-2a and ribavirin improved insulin resistance by the reduction of fasting serum insulin and glucose level.
ACKNOWLEDGEMENTS
This study was financially supported by grant from the IN-SUNG Foundation for Medical Research.
References
- 1.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 2.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 3.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Adami HO, Chow WH, Nyrén O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 6.Nagao Y, Kawaguchi T, Tanaka K, Kumashiro R, Sata M. Extrahepatic manifestations and insulin resistance in an HCV hyperendemic area. Int J Mol Med. 2005;16:291–296. [PubMed] [Google Scholar]
- 7.Sumie S, Kawaguchi T, Komuta M, et al. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–552. [PubMed] [Google Scholar]
- 8.Harrison SA. Correlation between insulin resistance and hepatitis C viral load. Hepatology. 2006;43:1168. doi: 10.1002/hep.21125. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Chand N, Comar K, Mirshahi F. Hyperinsulinemia blocks the inhibition of hepatitis C virus (HCV) replication by interferon: a potential mechanism for failure of interferon therapy in subjects with HCV and non-alcoholic fatty liver disease. Hepatology. 2004;40:179A. [Google Scholar]
- 10.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Konrad T, Zeuzem S, Vicini P, et al. Evaluation of factors controlling glucose tolerance in patients with HCV infection before and after 4 months therapy with interferon-alpha. Eur J Clin Invest. 2000;30:111–121. doi: 10.1046/j.1365-2362.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 13.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74:2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 14.Chiles R, Tzagournis M. Excessive serum insulin response to oral glucose in obesity and mild diabetes. Study of 501 patients. Diabetes. 1970;19:458–464. doi: 10.2337/diab.19.6.458. [DOI] [PubMed] [Google Scholar]
- 15.Bahtiyar G, Shin JJ, Aytaman A, Sowers JR, McFarlane SI. Association of diabetes and hepatitis C infection: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2004;4:194–198. doi: 10.1007/s11892-004-0023-7. [DOI] [PubMed] [Google Scholar]
- 16.Shintani Y, Fujie H, Miyoshi H, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Nagao Y, Tanaka K, et al. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109–114. [PubMed] [Google Scholar]
- 19.Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes. 1989;38:641–647. doi: 10.2337/diab.38.5.641. [DOI] [PubMed] [Google Scholar]
- 20.Imano E, Kanda T, Ishigami Y, et al. Interferon induces insulin resistance in patients with chronic active hepatitis C. J Hepatol. 1998;28:189–193. doi: 10.1016/0168-8278(88)80004-7. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Takeda N, Ishimori M, Akai A, Miura K, Yasuda K. Effects of long-term interferon-alpha treatment on glucose tolerance in patients with chronic hepatitis C. J Hepatol. 1999;31:215–220. doi: 10.1016/s0168-8278(99)80216-5. [DOI] [PubMed] [Google Scholar]
- 22.Tai TY, Lu JY, Chen CL, et al. Interferon-alpha reduces insulin resistance and beta-cell secretion in responders among patients with chronic hepatitis B and C. J Endocrinol. 2003;178:457–465. doi: 10.1677/joe.0.1780457. [DOI] [PubMed] [Google Scholar]
- 23.Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]