Abstract
There are approximately one million new cases of colorectal cancer (CRC) per year worldwide, with substantial associated morbidity and mortality. The long natural history of colorectal neoplasia affords the opportunity to use preventive measures to improve survival in this disease. Currently screening for adenomatous polyps and early-stage cancers is the best methodology for improving survival. The increasing knowledge of CRC pathogenesis and its natural history is allowing the development of new tools to identify patients who will benefit most from colon cancer screening and the defining of appropriate surveillance intervals. The guidelines for screening for colorectal neoplasia have recently been substantially revised by several organizations based on developing technologies and a growing body of data on the efficacy of CRC screening.
Keywords: Colon cancer screening, Fecal occult blood testing, Colonoscopy, Computed tomographic colonography, Adenoma
INTRODUCTION
Cancers of the colon and rectum (CRC) are a major cause of cancer-associated morbidity and mortality world-wide. CRC is the fourth most common newly diagnosed internal cancer overall in the United States, after cancers of the lung, prostate, and breast, and currently constitutes 10% of new cancers in both men and women. In 2008, there were an estimated 149,000 new CRC cases in the United States and 51,000 related deaths (a rate second only to that of lung cancer).1 Globally, CRC is the fourth most common cancer in men and the third most common in women, accounting for approximately one million new cases per year.2 While there is at least a 25-fold variation in the occurrence of CRC worldwide, many countries where CRC mortality was previously low have reported substantial increases during the past decade.3 CRC has become one of the most common cancers in a number of Asian countries, for example. Despite evidence that 5-year survival is 90% when CRC is diagnosed at an early stage, less than half of cases are diagnosed when the cancer is still localized.
Rapid growth of knowledge about the molecular and biologic characteristics of epithelial cancers has provided useful insights into the pathogenesis of colonic neoplasia. New insights also have been gained in regard to primary prevention. Because CRC arises over long periods as the result of interactions between genetic predisposition and environmental insults, it has become possible to identify pre-neoplastic and early neoplastic lesions with the hope of improving survival rates. More complete knowledge of CRC pathogenesis and its natural history, especially in high-risk groups, is allowing the development of new tools to identify those who will benefit most from colon cancer screening and in defining proper surveillance intervals. During the past year guidelines for screening for colorectal neoplasia have been substantially revised by several organizations based on developing technologies, and a growing body of data regarding the efficacy of CRC screening. This review will focus on evolving concepts and an evidence-based approach to CRC screening.
PRINICIPLES OF SCREENING
Cancer prevention has been traditionally categorized as primary or secondary. Primary prevention refers to the identification of genetic, biologic, and environmental factors that are etiologic or pathogenetic and subsequent alteration of their effects on tumor development.4 Although several areas of study have been identified that may lead to primary prevention of CRC, available data do not yet provide a firm basis for the practical application of primary preventive measures in most cases. The goal of secondary prevention (which includes screening) is to identify existing preneoplastic and early neoplastic lesions and to treat them thoroughly and expeditiously. The assumption is that early detection improves prognosis. Screening an asymptomatic population for any disease is worthwhile if the disease represents a major health problem, effective therapy is available if the disease is found, a sensitive and specific screening test is available that is readily acceptable to patients and physicians, and the screening test is cost-effective.
The long natural history of colonic neoplasia, and mucosal progression through defined phenotypic changes (the adenoma to carcinoma pathway) associated with identifiable genetic alterations fundamental to this process, makes CRC feasible. The challenge has been to develop effective, easily administered, and cost-effective screening tests for the disease. Current evidence strongly suggests that screening for CRC reduces related mortality. Direct evidence for this is available from prospective trials of fecal occult blood testing (FOBT),5 indirectly for colonoscopy and polypectomy (versus historical controls) from the National Polyp Study6 and from a recent case-control trial.7 These findings have resulted in recommendation by numerous organizations, including the evidence-based United States Preventive Services Task Force (USPSTF), that screening for CRC should be performed in all persons aged 50 years to 75 years (see below).
Previous studies reviewed by the USPSTF indicated that CRC screening is likely to be cost effective (<$30,000 per additional year of life gained in the United States) regardless of the strategy chosen. A more recent decision analysis commissioned by the USPSTF used microsimulation models from the Cancer Intervention and Surveilance Modeling Network to assess life-years gained and colonoscopy requirements for screening strategies.8 This group concluded that their findings support colorectal cancer screening with colonoscopy every 10 years, annual screening with a sensitive FOBT, or flexible sigmoidoscopy every 5 years with a midinterval FOBT from age 50 to 75 years. This was part of the basis for recent modifications to the USPSTF guidelines.
The willingness of both patients and physicians to comply with recommendations for screening programs has a major impact on the effectiveness (and cost effectiveness) of CRC screening. Compliance by both the population at large and health care providers has historically has been poor, and interventions to increase screening adherence have been disappointing. The key questions of "who, how, and how often" to screen remain a source of debate.
GUIDELINES
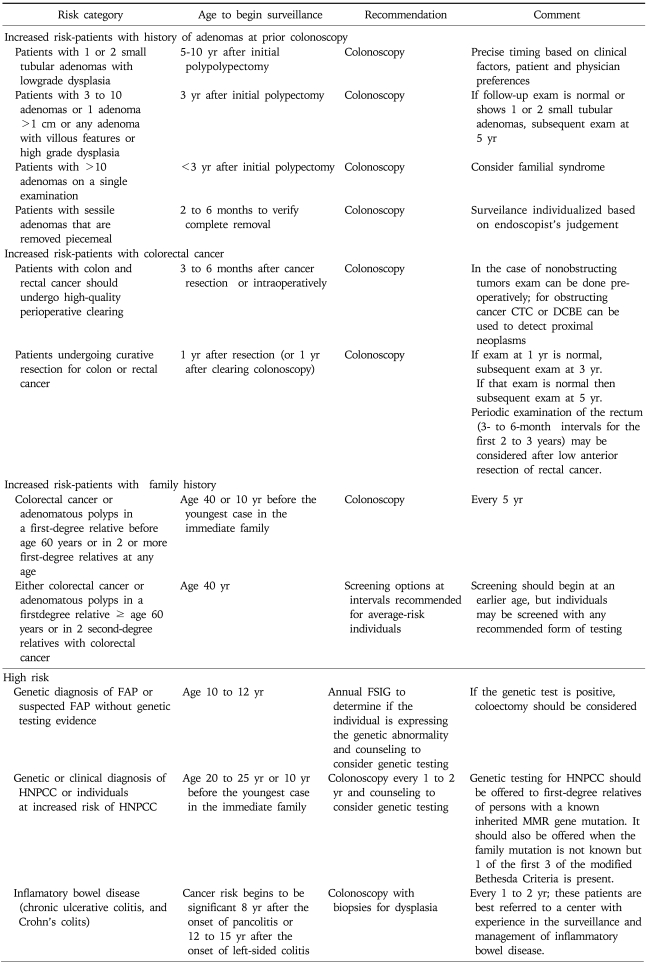
In 2008 new guidelines on screening and surveillance for early detection of colorectal cancer and adenomatous polyps was issued jointly by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology (Table 1).9 This update of previous guidelines is notable in that it grouped screening tests into those that primarily detect cancer (annual FOBT including those that are guaiac-based or immunochemical tests, and stool DNA tests interval not specified), and those that can detect early cancer and adenomatous polyps (flexible sigmoidoscopy every 5 years, colonoscopy every 10 years, double contrast barium enema every 5 years, or Computed tomography [CT] colonography every 5 years). In November 2008 the USPSTF also issued updated guidelines (Table 1).10 Based on a targeted evidence-based review and a decision analytic modeling analysis the USPSTF recommended screening of average risk individuals age 50 to 75 years with high sensitivity FOBT annually, sigmoidoscopy every 5 years plus FOBT every 3 years, or colonoscopy every 10 years. Notably the USPSTF indicated that while the benefits of screening outweigh the potential harms for 50- to 75-year-olds, the likelihood that detection and early intervention will yield a mortality benefit declines after age 75 because of the long average time between adenoma development and cancer diagnosis. Routine screening was therefore not recommended for adults age 76 to 85 years, and screening was not recommended at all in adults older than 85 years of age. These guidelines also indicated that for all populations there is insufficient evidence to assess the benefits and harms of screening with CT colonography or fecal DNA testing.
Table 1.
Guidelines for Screening Average Risk Individuals for Colorectal Cancer
FOBT, fecal occult blood test.
*U.S. Preventive Services Task Force. Screening for colorectal cancer: US Preventative Services Task Force Recommendation Statement. Ann Intern Med 2008;149:627-637. The US Preventative Services Task Force recommends screening for adults age 50 to 75 years. Screening for adults age 76 to 85 is not routinely recommended, and for adults older than 85 years screening is not recommended.
†Levin B, Lieberman DA, McFarland B, et al. Screening and surveilance for the early detection of colorectal cancer and adenomatous polyps 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130-160. Testing options are divided into those that detect adenomatous polyps and cancer (flexible sigmoidoscopy, colonoscopy, double-contrast barium enema, computed tomographic colonography), and those that primarily detect cancer (FOBT, stool DNA testing).
In 2008 an Asia Pacific Working Group on Colorectal Cancer published consensus recommendations for colorectal cancer screening.11 This group concluded that the incidence, anatomical distribution and mortality of CRC among Asian populations are not different compared with Western countries. They concluded that screening for colorectal cancer should be started at age 50 years. FOBT (guaiac-based and immunochemical), flexible sigmoidoscopy and colonoscopy were recommended for CRC screening. Double contrast barium enema and CT colonography are not preferred. In resource-limited countries FOBT is the first choice for CRC screening.
In a recent survey conducted by the International Digestive cancer alliance (IDEC) across Europe, 21 of 39 countries have reported national screening guidelines promoted by medical and professional organizations.12
Although each of the options for CRC screening has inherent characteristics related to accuracy, prevention, potential costs, and risks, the concept has long been that any one of the tests is better than no test at all. Multiple options can be confusing, however, to both patients and physicians. Furthermore, the test options are not of equal efficacy, and such guidelines may lead to coverage of suboptimal testing by insurance payers or health care agencies.
SCREENING TECHNIQUES
1. Fecal occult blood testing
Qualitative chromogen tests, which rely on the oxidative conversion of a colorless compound to a colored one in the presence of the pseudoperoxidase activity of hemoglobin, have been standardized employing guaiac-impregnated paper and developing solutions (hydrogen peroxide in denatured alcohol) and have been widely studied and utilized clinically (e.g., Hemoccult, Hemoccult II). These tests are available commercially, convenient, and inexpensive, however, their effectiveness in detecting occult blood in the stool depends on the degree of fecal hydration (increases sensitivity), amount of hemoglobin degradation during storage or by fecal flora (decreases sensitivity), and the absence of interfering substances that can either enhance or inhibit oxidation of the indicator dye. Dietary components may also lead to false positive tests.
Mortality data are available from the Minnesota Study, a randomized, controlled trial that has provided the best evidence for the effectiveness of screening with FOBT.13,14
After 13 years of follow-up, data indicate a 33% reduction in CRC-associated mortality with annual screening but an insignificant reduction of approximately 5% with biannual screening. Approximately 80% of samples were rehydrated, yielding a high positivity rate of 9.8% (compared with 2.4% for nonhydrated slides). This resulted in a 38% rate of colonoscopy, leading some to suggest that a substantial portion of the mortality reduction resulted from chance detection through colonoscopy of non-bleeding cancers. This challenge has been refuted by the investigators, who find that only 6% to 11% of the mortality reduction was explained by chance detection. Cumulative 18-year CRC mortality remained 33% lower in the group screened annually with FOBT than in the control group. A group tested with biennial screening now demonstrated a 21% lower CRC mortality than did the control group. Other randomized studies reported similar results.5 Data from Funen, Denmark, suggested an 18% decrease in CRC mortality associated with FOBT, and data from Nottingham, UK, also indicate a 15% reduction in mortality during follow-up. Data from a New York study suggested a 43% reduction in mortality in the screened group at 10 years. A randomized French trial also demonstrated a reduction in CRC mortality with biennial FOBT screening compared with a control population (mortality ratio, 0.84; 95% confidence interval [CI], 0.71-0.99) in 11 years of follow-up; reduction is mortality was more pronounced in compliant individuals (mortality ratio, 0.67; 95% confidence interval, 0.56-0.81). Updated screening data for CRC using FOBT (Hemoccult) has recently been reviewed by the Cochrane Collaboration.5 Data is presented for 11.7 years follow up from Nottingham, 17 years from Funen, 15.5 years fro Goteborg and 18 years from Minnesota. Combined results from these four randomized controlled trials showed that participants allocated to screening had a 16% reduction in the relative risk of CRC-related mortality (RR 0.85, CI 0.78-0.90). In the 3 studies that used biennial screening, there was a 15% reduction (RR 0.85, CI 0.78-0.92) in CRC mortality. When adjusted for screening attendance in the individual studies, there was a 25% relative risk reduction in mortality (RR 0.75, CI 0.66-0.84) for those attending at least one round of screening using FOBT.
Methods that may decrease the false-positive FOBT rates while maintaining or increasing sensitivity currently are being refined and compared for efficiency with Hemoccult-type slide tests. Fecal immunochemical tests (FITs) are designed to detect human globin and are not affected by diet or drugs. One FIT, HemeSelect, showed good performance characteristics compared with standard heme-based FOBT tests in early studies and was used in a combination test to confirm positive Hemoccult Sensa (a sensitive guaiac-based test similar to Hemoccult) in a large managed care setting. More recently, a FIT using a brush-based sampling technique and an immunogold membrane, which uses a dual antibody system specific for human hemoblobin, has undergone initial evaluation. Strategies that use an immunochemical-based FOBT have been shown to be cost-effective when used for colorectal cancer screening in Japan. Quantitative immunochemical FOBT has been shown to have good sensitivity and specificity for detection of clinically significant neoplasia in recent studies of asymptomatic and symptomatic individuals,15,16 but test performance in prospective screening programs has been less well studied. Fecal immunochemical tests have, however, been included in recent screening guidelines as an alternative to guaiac-based tests (see above).
2. Proctosigmoidoscopy
The benefit of proctosigmoidoscopy in screening programs for CRC was suggested by several uncontrolled studies that used rigid proctosigmoidoscopy. Those studies suggested that proctosigmoidoscopy in asymptomatic average-risk persons might detect early-stage cancers and that detection and removal of adenomas could result in a lower than expected frequency of rectosigmoid cancers in the screened population.
Two case-control studies provided strong evidence that sigmoidoscopy can reduce CRC mortality. A study from the Kaiser-Permanente Medical Care Program17 compared 261 members who died of cancer of the rectum or distal colon with 868 age- and sex-matched control subjects. Only 8.8% of case subjects had undergone screening by rigid sigmoidoscopy, compared with 24.2% of controls. Rigid sigmoidoscopy had no effect on mortality in another group whose lesions were beyond the reach of the sigmoidoscope. Furthermore, the beneficial effect of sigmoidoscopy extended 10 years. This and a second case-control study indicate that sigmoidoscopy can result in a 70% to 80% reduction in mortality from cancers within reach of the sigmoidoscope. Because approximately 50% of all CRCs can be detected using the 60-cm flexible sigmoidoscope these data suggest that periodic sigmoidoscopic screening could reduce overall CRC-related mortality by about one third. Because the flexible sigmoidoscope is superior to rigid instruments in detecting lesions, the flexible sigmoidoscope has replaced the rigid sigmoidoscope for CRC screening. A case-control study using FS and polypectomy demonstrated a 60% reduction in colon cancer incidence associated with this procedure. Randomized, controlled trials are now underway to measure the effect of screening with FS on CRC mortality.18,19 The Prostate, Lung, Colon and Ovarian cancer Screening Trial (PLCO) sponsored by the US National Cancer Institutes has enrolled 155,000 individuals in a recent prospective, randomized trial that compares FS to a usual-care control group.18,20 Follow-up is planned through 2,015 with cancer-related mortality as the major end point. The UK Flexible Sigmoidoscopy Screening Trial,21 is a randomized trial examining the hypothesis that a single flexible sigmoidoscopy screening offered at approximately age 60 years can lower the incidence and mortality of colorectal cancer.
3. Colonoscopy, barium enema, and CT colonography
Colonoscopy may well be the most effective tool for CRC screening, but data from prospective, randomized trials are lacking. The National Polyp Study of polypectomy and surveillance strongly suggested a reduction in CRC mortality as the result of removing adenomatous polyps compared to historic reference populations.6 A recent Canadian population based study compared the risk of developing colorectal cancer after a negative colonoscopy in all Ontario residents with a history of a complete negative colonoscopy with controls consisting of the Ontario population without a history of colonoscopy.22 In the negative colonoscopy cohort, the relative risk of distal colorectal cancer was significantly lower than the control group in each of the 14 years of follow up, while the relative risk for proximal colorectal cancer was significantly lower mainly during the last 7 years of follow up. A second Canadian case-control study demonstrated that complete colonoscopy was also associated with fewer deaths from left-sided colorectal cancer, but not from right-sided cancer.7 These recent findings are of interest in light of arguments that colonoscopy is preferable to sigmoidoscopy, because there may be a substantial incidence of proximal colonic cancers and advanced adenomas beyond the reach of the sigmoidoscope. Some of these individuals may not have distal findings on sigmoidoscopy that would trigger a subsequent colonoscopy. Two trials23,24 suggested that approximately 50% of individuals with advanced proximal neoplasms (adenoma >1 cm; adenoma with villous features or dysplasia; cancer) have no distal neoplasms. Less than 2% of those who did not have distal neoplasms, however, had an advanced proximal lesion.23 Given the need for colonoscopic follow-up, should FOBT or sigmoidoscopy be positive, colonoscopy may also be cost-effective.25
A decision analysis commissioned by the USPSTF supports colonoscopy every 10 years as a screening option measured in life-years gained (see above).
High contrast endoscopy using dye or stain solutions combined with colonoscopy (chromoendoscopy), or high resolution optical methods (e.g., narrow band imaging, lasar confocal endoscopy) has been suggested as a means of identifying lesions in high risk groups or as an adjunct to colonosocopy where flat lesions (so called "flat adenomas") are suspected. Recent evidence suggests that flat or depressed neoplasms are more common than previously appreciated, and carry a high relative risk of containing in situ or invasive carcinoma.26
Air contrast barium enema (ACBE) has been included as an option in a variety of screening guidelines. No studies, however, have directly addressed the effectiveness of barium enema for colon cancer screening. Several studies have indicated that the sensitivity of ACBE is less than that of colonoscopy,27 especially for detecting lesions less than 1 cm. A recent population based study28 suggested that if a cancer is present, there is approximately a one in five chance that it will be missed by ACBE.
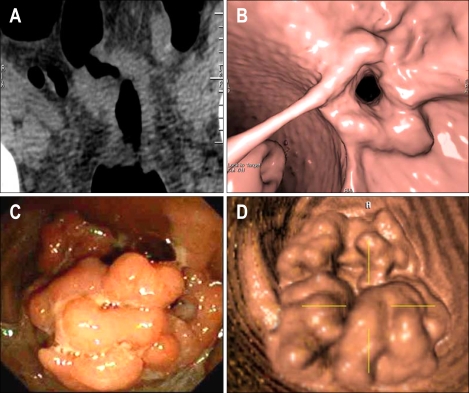
CT colonography, or "virtual" colonoscopy, involves the use of helical CT to generate high-resolution, two-dimensional images of the abdomen and pelvis. Three-dimensional images of the colon can be reconstructed by computer generation off-line (Fig. 1). CT colonography has the potential advantage of being a rapid and safe method of providing full structural evaluation of the entire colon. Low sensitivity and specificity and the need for rapid high-resolution helical CT scanners originally precluded its wide application for routine CRC screening, but software and techniques designed to improve the speed, accuracy, and reproducibility of results are now available. The accuracy and potential of CT colonography as a screening tool for colorectal neoplasia has been debated because initial studies yielded a wide range of sensitivity.29,30 Two recently published trials provide evidence that CT colonography may be a valid alternative for primary colon cancer screening. The National CT Colonography Trial31 directed by the American College of Radiology Imaging Network (ACRIN) was a multicenter study that employed CT colonography and same day colonoscopy using a standard matching protocol in 2,600 asymptomatic individuals. Per patient sensitivity of CT colonography for adenomas greater than 10 mm was 90% with a negative predictive value of 99%. A second trial32 compared CT colonography and optical colonoscopy in parallel screening cohorts and demonstrated similar rates of detection of advanced neoplasia in both groups. Several key issues need to be addressed as the use of CT colonography becomes more widespread, principal among which is determination of the acceptable size cut-off of a lesion detected by CT colonography that will necessitate a follow-up colonoscopy. Other issues include the need for bowel preparation, the logistics of same-day colonoscopy, the ability to detect flat lesions, the significance of extracolonic lesions detected by CT, and the impact on compliance, and cost-effectiveness. Methodologies that employ CT colonography without cathartic preparation and with "fecal tagging" may make this a more attractive option for screening. CT colonography may also aid in detecting lesions located behind folds or near the anal verge.
Fig. 1.
CT colonography ("virtual colonoscopy"). (A) Two-dimensional CT image of an annular CRC. (B) Three-dimensional reconstruction of image in A. (C) Large sessile colon polyp evident in colonoscopy. (D) Three-dimensional image of lesion in C obtained by CT colonography.
4. Fecal DNA testing
A great deal of knowledge has been accumulated recently about genetic alterations that occur during colon carcinogenesis, but specific tests are not currently available for most patients at risk for developing sporadic CRC. A molecular approach to CRC screening is attractive since it targets biologic changes that are fundamental to the neoplastic process. The feasibility of detecting altered DNA in stool has been demonstrated using a multitarget assay panel of molecular markers.33 One multicenter study compared fecal DNA testing using such a panel with the FOBT and colonoscopy.34 The fecal DNA panel consisted of 21 mutations: 3 in the K-ras gene, 10 in the APC gene, 8 in the p53 gene; the MSI marker BAT-26; and a marker of long DNA thought to reflect disordered apoptosis of cancer cells sloughed into the colonic lumen. Although most of the lesions identified by colonoscopy were not detected by either noninvasive test, multitargeted fecal DNA testing detected a higher proportion of important lesions compared with Hemoccult. A second recently published study35 compared stool DNA and FOBT for detection of "screen-relevant neoplasia" (curable stage cancer, high grade dysplasia or adenomas >1 cm). This blinded, multicenter cross sectional study used two different methodologies for detecting alterations in stool DNA, a 23 marker panel and a new test targeting 3 broadly informative markers (point mutations on K-ras, a scanned mutator cluster region of APC, and methylated vimentin. While the multipanel test provided no improvement over FOBT (HemoccultSensa) for detection of screen relevant neoplasms, the new test showed promise by detecting significantly more neoplasms than FOBT. A recently developed digital melting curve assay designed to quantify low-abundance mutations in stool samples has demonstrated a high sensitivity for detecting individuals with colorectal neoplasms in preliminary studies.36 The recent joint guidelines published by American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology include fecal DNA testing as an option for detecting colorectal cancers.
5. Genetic testing in high risk groups
Genetic testing is now a reality for families with familial adenomatous polyposis (FAP).37 Testing for altered products of the APC gene allows for early and accurate identification of family members at risk who require intensive surveillance (Table 2). Proper genetic counseling, however, must be incorporated into the screening process. Genetic testing for mutations in the hMSH2 and hMLH1 genes is appropriate when hereditary nonpolyposis colorectal cancer (Lynch Syndrome or HNPCC) is suspected but presents more difficulty than screening for FAP, because not all the genes involved have been identified, and the preferred method by which families should be screened has yet to be determined.38 A generally accepted approach in individuals suspected of having HNPCC based on clinical criteria is first to perform microsatellite instability (MSI) testing on the affected individual's tumor using a panel of microsatellite markers. Germline mutation testing for hMLH1 and hMSH2 is performed if the tumor is MSI-high (suggesting a mutation in a mismatch repair gene). In cases in which HNPCC is strongly suspected based on clinical criteria, or when a mutation is established in a family member, germline testing is performed as a first step. If testing for hMLH1 and hMSH2 is negative, but HNPCC is strongly suspected, germline testing for hMSH6 can be performed. An allele of APC designated I1307K39 is relatively infrequent in the general population but common in the Jewish population of Ashkenazi descent. There is a modest increase in the relative risk for CRC in those with this allele, but the penetrance for CRC is low compared with carrier frequencies, and genetic testing for I1307K is not recommended.
Table 2.
Guidelines for the Surveillance of Cancer in People at Increased or High Risk
CTC, computed tomographic colonography; DCBE, double contrast barium enema; FAP, familial adenomatous polyposis; HNPCC, hereditary nonpolyposis colorectal cancer; FSIG, flexible sigmoidoscopy; MMR, mismatch repair.
Derived from Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps 2008: a joint guideline from the American Cancer Society, the Multi-Society Task Force on colorectal cancer and the American College of Radiology. CA Cancer J Clin 2008;58:130-160.
APPROACH TO SCREENING
1. Average-risk individuals
Patients registered in a health care system should be categorized according to risk, so that appropriate screening can be added to other variables of medical evaluation. Relative risk should be assessed by family history and by personal history using questionnaires. A variety of options are available for screening average-risk individuals (≥50 years of age with no personal or family history of colorectal adenoma or carcinoma, and no personal history of inflammatory bowel disease). Current guidelines for screening average risk individuals have already been discussed and the joint guidelines recently set forth by the American Cancer Society, US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology as well as recently published guidelines from the USPSTF are presented in Table 1.
National and regional colorectal cancer screening programs have recently been adopted by several countries including Canada (Ontario 2007), countries in the European Union, and in some Asian countries. In the Rupublic of Korea, for example, CRC screening has been part of a national cancer screening program since 2004. Annual FOBT (immunochemical or Hemoquant) is offered to individuals ages 50 and higher. Double contrast barium enema and/or colonoscopy are used to follow up positive FOBTs. The total target is 4.4 million, and the screening rate was about 16% in 2006. The screening program is free of charge. An inventory of colorectal cancer screening activities in 25 countries comprising the International Cancer Screening Network (sponsored by the US national Cancer Institute) was published in May 2008.40 The primary modality for screening varies between countries, and depends on health care resources and availability of manpower and expertise for various methods.
2. Individuals at increased risk for colorectal cancer
1) Personal or family history of colorectal adenoma
Table 2 lists the updated (2008) ACS/Multi-Society/ACR guidelines for screening, surveillance, and early detection of colorectal adenomas and cancer for individuals at increased risk or at high risk of disease These guidelines suggests that those whose index lesion consists of 1 or 2 small tubular adenomas with low grade dysplasia should have a follow-up colonoscopy 5 to 10 years after the initial polypectomy. The precise timing within this interval should be based on clinical factors (prior findings, family history, patient and physician preferences). One recent study examined the relative risk for advanced neoplasia within 5.5 years of a baseline colonoscopy.41 There was a strong association between the results of baseline screening colonoscopy and the rate of serious incident lesions during surveillance. This study confirmed that patients with 1 or 2 small tubular adenomas represent a low risk group compared with other patients with colorectal neoplasia. In those with a large (>1 cm) adenoma, multiple (3 to 10) adenomas, or adenomas with high-grade dysplasia or villous change, colonoscopy should be repeated within 3 years after the initial polypectomy. Although the risk for recurrence of advanced adenomas at this follow up interval is greater in patients with high risk adenomas than those with low risk adenomas, the incremental risk is small.42 If the exam is normal or shows only 1 or 2 small tubular adenoms with low grade dysplasia, then the interval for the subsequent exam should be 5 years. Patients with >10 adenomas on a single examination should have a follow up colonoscopy less than 3 years after the initial polypectomy and the presence of an underlying familial syndrome should be considered. Patients with sessile adenomas that are removed piecemeal should have follow up colonoscopy in 2 to 6 months to verify complete removal.
2) Individuals with a personal history of colorectal cancer
Patients with colon or rectal cancer should have high quality perioperative clearing. Colonoscopy should be performed preoperatively, by intraoperative colonoscopy, or within 3 to 6 months after cancer resection. Those who have had a colon cancer resected should have colonoscopy performed one year after surgery or the original clearing colonoscopy. If the examination performed at one year is normal, then the interval before the next colonoscopy should be 3 years, then (if normal) at 5 years (Table 2). Periodic examination of the rectum for the purposes of identifying local recurrence is usually performed at 3- to 6-month intervals for the first 2 or 3 years after low anterior resection for rectal cancer. Serum CEA levels should be measured at regular intervals because postoperative CEA determinations may be cost-effective for detecting recurrent cancers. How long an asymptomatic patient who has had multiple negative examinations should be tested by various modalities is at present unclear. It should be noted that these recommendations are to some extent "educated guesses", and not all are based on prospective, randomized trials.
3. High-risk groups
1) Familial adenomatous polyposis and familial cancer
Screening should include genetic testing to detect abnormal (truncated) APC gene products if a diagnosis can be made by this method in one family member. Those who test positive should have annual or biannual FS, beginning at age 10-12 years, to assess for emergence of adenomas and to plan appropriate timing for colectomy. If genetic testing is unavailable, annual FS should begin at age 10 to 12 years. Genetic testing should always be combined with education and counseling of the individual as well as family members. Colorectal surveillance (sigmoidoscopy) should begin at age 18 to 20 in individuals with attenuated FAP (AFAP) and in individuals with MUTYH mutations (here colonoscopy is recommended).
Patients with a family history of HNPCC must be examined colonoscopically, beginning at age 20 to 25 years, or at an age 10 years younger than that of the index case, because one cannot rely only on the FOBT in these very-high-risk patients. A reasonable approach is to perform colonoscopy every 2 years. The search is primarily for the scattered adenomas that antedate carcinomas in these syndromes. Genetic testing for HNPCC should be offered to first-degree relatives of those with a known mismatch repair gene mutation, or in those who meet the modified Bethesda criteria for HNPCC.38 Genetic testing should be accompanied by counseling of the individual and the family members. The benefits of colonoscopic surveillance in patients with HNPCC mutations are suggested by screening trials.43
The approach to patients with a suggestive family history (e.g., one first-degree relative with colon cancer) is not firmly established, but existing data suggest that these patients should be monitored more rigorously than average-risk individuals. The joint guidelines from the ACS and US Multi-Society Task Force on Colorectal Cancer recommend that if CRC or adenomatous polyps occurred in any first-degree relative before age 60 years, or in two or more first-degree relatives at any age, then colonoscopy should be performed every 5 years, beginning at age 40 years, or 10 years before the youngest case in the immediate family. If either colorectal cancer or adenomatous polyps occurred in a first degree relative 60 years of age or older, or colorectal cancer occuureed in 2 second degree relatives, then screening should begin at age 40 using screening options recommended for average-risk individuals. In those with more than two affected first-degree relatives, special care should be taken to exclude the diagnosis of HNPCC, and periodic colonoscopy is advised.
2) Inflammatory bowel disease
Colorectal cancer arising in the setting of inflammatory bowel disease (ulcerative colitis, Crohn's disease) was recently reviewed in this journal.44 The appropriate surveillance schedule for patients with IBD has not been determined in long-term prospective trials. Colonoscopy combined with mucosal biopsy may be effective in detecting preneoplastic and early neoplastic lesions in patients with UC. The current recommendation is for colonoscopy every 1 or 2 years for patients who have had universal colitis for 8 years or left-sided UC for 12 to 15 years. Biopsies should be taken throughout the colon at 10-cm intervals, with special attention to areas that suggest a dysplasia-associated mass lesion (DALM). Although this biopsy procedure enables histology of only a small area of the colon, the short-term risk of carcinoma for patients with a negative biopsy is low. If dysplasia is high-grade or associated with a macroscopic lesion or mass, colectomy is recommended. A histologic diagnosis of low-grade dysplasia merits endoscopic follow-up at short intervals (e.g., at 3 to 6 months) as does an "indeterminate" reading resulting from active inflammation. Colectomy has been advocated by some for confirmed low-grade dysplasia. Patients with Crohn's disease of the colon should be evaluated endoscopically as dictated by symptoms, and special attention should be paid to strictured areas. Studies suggest a role for surveillance colonoscopy.45
QUALITY ASSURANCE
Colonoscopy is now the most common endoscopic procedure performed in the United States. Guidelines and screening programs in many countries recommend colonoscopy as a follow-up to positive screening FOBTs. As the number of colonoscopies (and colonoscopists) increases, quality assurance measures will need to be adopted. One measure of quality assurance relates to adequate visualization of the colonic mucosa. One study from a community based practice in the US46 suggests that detection of overall and advanced neoplasia may be related to withdrawal time during colonoscopy. After implementing a protocol of inspection during a minimum withdrawal time of 8 minutes, greater rates of detection were observed. Others, while agreeing that adequate visualization of the mucosa is an important quality assurance parameter, have suggested that adequate exams relate to the experience and quality of the endosocopist and not withdrawal time per se. A "Physician Performance Measurement Set" for endoscopy and surveillance has been proposed in a joint document by the American Society for Gastrointestinal Endoscopy, the American Gastroenterological Association, the Physician Consortium for Performance Improvement, and the National Committee for Quality Assurance.
1. Future directions
While considerable progress has been made in understanding the molecular biology and natural history of colorectal cancer and how this may impact preventive measures, implementation of patient friendly and cost-effective CRC screening efforts remains a global problem. CRC is curable when detected in its early stages, yet at best one third of cancers when diagnosed are considered early cancers. Far less than one half of individuals who meet established guidelines for screening actually undergo CRC screening. The cost-effectiveness of any screening program is highly dependent on adequate compliance. Compliance with screening recommendations and optimal utilization of resources, both financial and with respect to manpower, are issues which need to be resolved.
Efforts at primary prevention of CRC through chemoprevention have been somewhat successful,4 but not established for clinical practice except in high-risk groups. While it is unlikely that chemoprevention will make screening unnecessary, successful efforts at primary prevention may lead to fewer and safer screening exams. Current guidelines for screening and surveillance for colorectal neoplasia have recognized the importance of risk stratification in determining proper screening intervals for both average risk individuals and those at higher risk. Proper risk stratification and compliance with guidelines based on these efforts will be key to developing cost-effective models for CRC screening. Development of sensitive and specific noninvasive screening tests for CRC is a high priority for population-based screening programs. Radiographic techniques such as CT colonography may prove effective for screening average-risk individuals, but proper and cost-effective follow-up of those in whom lesions are found need to be adequately defined. Colonoscopy, while sensitive and specific, is impractical for population screening in many countries.11,12,40 Screening methods based on the biology of cancers such as stool DNA testing or serum-based methods for specific tumor-associated markers are actively being developed and are eagerly awaited.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. In: Verma M, editor. Methods in molecular biology, cancer epidemiology. Vol. 471. New Jersey: Humana Press; 2009. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–3697. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 5.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007:CD001216. doi: 10.1002/14651858.CD001216.pub2. Available from http://www.thecochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 8.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Preventative Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 11.Sung JJ, Lau JY, Young GP, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 12.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 13.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 14.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 15.Levi Z, Rozen P, Hazazi R, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146:244–255. doi: 10.7326/0003-4819-146-4-200702200-00003. [DOI] [PubMed] [Google Scholar]
- 16.Guittet L, Bouvier V, Mariotte N, et al. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2009;56:210–214. doi: 10.1136/gut.2006.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 18.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 19.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247–1256. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Schoen RE, Pinsky PF, Weissfeld JL, et al. Results of repeat sigmoidoscopy 3 years after a negative examination. JAMA. 2003;290:41–48. doi: 10.1001/jama.290.16.2123-b. [DOI] [PubMed] [Google Scholar]
- 21.UK Flexible Sigmoidoscopy Screening Trial Investigators. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomized trial. Lancet. 2002;359:1291–1300. doi: 10.1016/S0140-6736(02)08268-5. [DOI] [PubMed] [Google Scholar]
- 22.Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117–1121. doi: 10.1016/j.cgh.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–584. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 26.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 27.Winawer SJ, Stewart ET, Zauber AG, et al. National Polyp Study Work Group. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. N Engl J Med. 2000;342:1766–1772. doi: 10.1056/NEJM200006153422401. [DOI] [PubMed] [Google Scholar]
- 28.Toma J, Paszat LF, Gunraj N, Rabeneck L. Rates of new or missed colorectal cancer after barium enema and their risk factors: a population-based study. Am J Gastroenterol. 2008;103:3149–3151. doi: 10.1111/j.1572-0241.2008.02199.x. [DOI] [PubMed] [Google Scholar]
- 29.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 30.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713–1719. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 31.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 33.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 34.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 35.Ahlquist DA, Sargent DJ, Loprinzi CL, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441–450. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou H, Taylor WR, Harrington JJ, et al. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009;136:459–470. doi: 10.1053/j.gastro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 38.Half EE, Bresalier RS. Clinical management of hereditary colorectal cancer syndromes. Curr Opin Gastroenterol. 2004;20:32–42. doi: 10.1097/00001574-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Stern HS, Viertelhausen S, Hunter AG, et al. APC I1307K increases risk of transition from polyp to colorectal carcinoma in Ashkenazi Jews. Gastroenterology. 2001;120:392–400. doi: 10.1053/gast.2001.21170. [DOI] [PubMed] [Google Scholar]
- 40.International Cancer Screening Network. Bethesda: International Cancer Screening Network; c2009. Inventory of colorectal cancer screening activities in ICSN countries, May 2008 [Internet] [last modified 2009 Feb 9]. Available from: http://appliedresearch.cancer.gov/icsn/colorectal/screening.html. [Google Scholar]
- 41.Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–426. doi: 10.7326/0003-4819-148-6-200803180-00004. [DOI] [PubMed] [Google Scholar]
- 43.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 44.Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut and Liver. 2008;2:61–73. doi: 10.5009/gnl.2008.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology. 2001;120:820–826. doi: 10.1053/gast.2001.22449. [DOI] [PubMed] [Google Scholar]
- 46.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]