Abstract
Angiography is a useful diagnostic tool in cases with massive gastrointestinal bleeding such as angiodysplasia and varicosis when endoscopy is not available. Angiodysplasia and varicosis have distinguishable characteristic features on angiography, such as the presence of a nidus, visible late-draining veins, and the typical vascular tuft. We recently treated a rare case of congenital angiodysplasia without the characteristic angiodysplasia features on angiography. Instead, the patient presented with a very rare case of idiopathic jejunal varicosis. A 42-year-old woman visited the emergency room with the chief complaint of melena for three days and a hemoglobin level of 5.9 g/dL. An abdominal CT angiogram showed varicosis at the jejunal mesentery. Angiography of the superior and inferior mesenteric arteries showed tortuous and dilated jejunal and ileal branches during the venous phase, suggesting a vascular malformation such as varicosis of the jejunum. Surgical exploration with intraoperative endoscopy revealed diffuse engorged veins and a 1.0-cm-diameter superficial ulcer covered with a blood clot that was 70 cm from the ligament of Treitz. A 100-cm segment of jejunum was resected. Histological examination revealed that the lesion was angiodysplasia, not varicosis. The final diagnosis was congenital angiodysplasia.
Keywords: Congenital angiodysplasia, Idiopathic varicosis, Jejunum, Angiography
INTRODUCTION
Angiodysplasia can be a major source of gastrointestinal bleeding, responsible for 6.0% of cases of lower gastrointestinal bleeding and 1.2-8.0% of cases of upper gastrointestinal bleeding. Small bowel angiodysplasia includes a large number of cases of gastrointestinal bleeding of unknown origin, in approximately 30-40% of patients.1 A small bowel varicosis is an uncommon cause of lower gastrointestinal bleeding. In addition, most cases of small bowel varicosis have cirrhotic causes and are usually located in the duodenum. Extrahepatic causes are result from occlusion of one of the major vessels.2
We present a rare case of congenital angiodysplasia of the jejunum that did not show the features characteristic of angiodysplasia angiographically but presented with idiopathic jejunal varicosis.
CASE REPORT
A 42-year old Korean woman visited emergency room with melena for three days. The patient complained of abdominal pain, dyspnea and dizziness and a 4 kg weight loss over one month. There was no significant medical history or family history. This was the first episode of melena for the patient.
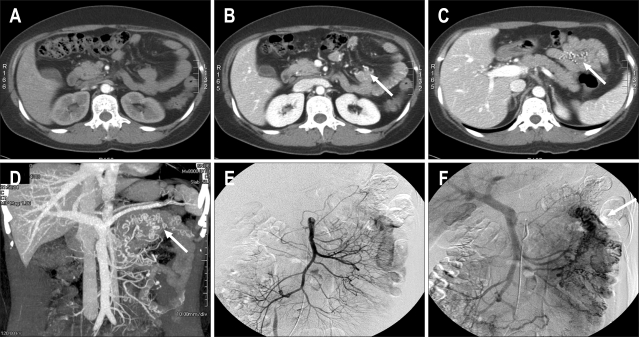
On physical examination, the conjunctiva was pale and the abdomen was soft but showed hyperactive bowel sound. The heart had normal beat sound and tachycardia (120 beats/min). The digital rectal exam showed old blood clots. The laboratory findings showed haemoglobi 5.9 g/dL, leukocytes 4,140/mm3 (68% neutrophils) and platelets 432,000/mm3. Assessment of anemia showed a low level of iron (11 ug/dL, normal value 50-130 ug/dL) but normal levels of TIBC (284 ug/dL, normal value 280-400 ug/dL) and low levels of ferritin (2.4 ng/mL, normal value 13-150 ng/mL). A transfusion of 540 mL of red blood cells was given, and the hemoglobin level reached 10.2 g/dL. Esophagogastroduodenoscopy and colonoscopy were within normal limits except for old blood clots in the ascending colon; however, there was no definite bleeding source identified. An abdominal CT angiography showed varicosis at the jejunal mesentery of unclear etiology; there was no sign of active bleeding (Figs. 1A, B, C and D). A superior mesenteric artery (SMA) and inferior mesenteric artery (IMA) arteriography showed tortuous and dilated jejunal and ileal branch in the venous phase (Figs. 1E, F), suggesting a vascular malformation, such as varicosis of the jejunum. However, there was no definite evidence of SMV obstruction or liver cirrhosis. Surgical exploration was recommended to prevent the recurrence of bleeding. However, the patient declined the exploration because the bleeding stopped and she was discharged.
Fig. 1.
Axial CT image of the arterial phase showing no abnormal vessels. (B) Axial CT image of the portal phase showing enhancing abnormal veins at the antimesenteric border of the small bowel (arrow). (C) Axial CT image of the portal phase showing enhanced abnormal veins of the mesentery at the small bowel loop (arrow). (D) Maximum-intensity projection reconstruction image of CT angiography showing tortuous abnormal veins in the left upper abdomen (arrow). (E) Superior mesenteric artery (SMA) arteriography of the arterial phase showing no abnormal vessels. (F) SMA arteriography of the portal phase showing enhancing abnormal veins at the mesentery of the small bowel loop (arrow).
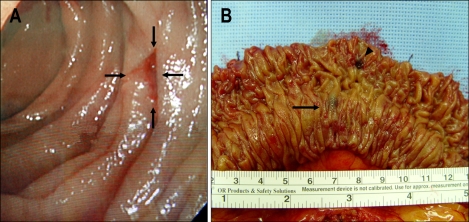
Two weeks later, the patient presented to the emergency room again with the same problem; presenting with melena for two days. This time the hemoglobin level was 8.6 g/dL, and surgery was performed immediately. On careful visual and manual examination of the small intestine, diffuse engorged veins were found 60 cm from the ligament of Treitz and 100 cm from the ileocecal (IC) valve. To determine the exact focus of bleeding, an intra-operative endoscopy was performed. Finally, a 1.0 cm sized superficial ulcer covered with a blood clot was found on 70 cm from the ligament of Treitz (Fig. 2A). A 100-cm segment of jejunum was resected and a termino- terminal anastomosis was performed. The gross examination of the resected specimen revealed a 0.9 cm sized ulceration of the mucosa as the bleeding focus and one blue-black lesion in the submucosal layer with venous distension (Fig. 2B). Histological examination revealed dilated and distorted vessels in the submucosa and mesentery and variable amounts of smooth muscle cells in the vascular walls. Some foci of thin-walled vessels were lined by a single layer of endothelium (Fig. 3). The final diagnosis was congenital angiodysplasia. The patient had an uneventful postoperative course and was discharged six days after surgery. After one year of follow up there has been no further episodes of bleeding.
Fig. 2.
(A) Intraoperative endoscopy showing a 1.0-cm-diameter superficial ulcer covered with a blood clot that was 70 cm from the ligament of Treitz. (B) Gross examination revealed a 0.9-cm ulceration in the mucosa as a bleeding focus (arrowhead) and one blue-black lesion in the submucosal layer, with venous distension (arrow).
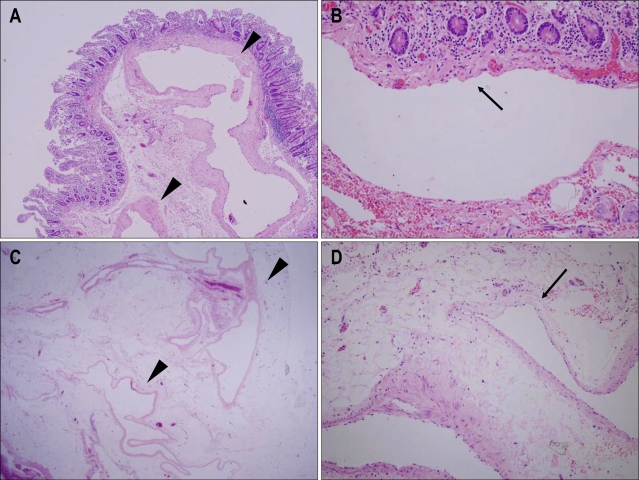
Fig. 3.
Microscopic images of the blue-black-colored polypoid submucosal lesion (A and B) and subserosal lesion (C and D). A and C: Ectatic thin-walled vessels with a few smooth muscle cells (arrowheads). B and D: Some foci of vascular walls were lined by only a single layer of endothelial cells (arrows) (H&E staining; A, ×40: B, ×200: C, ×12.5: D, ×200).
DISCUSSION
Angiodysplasias are vascular abnormalities frequently found in the gastrointestinal tract; they are a source of significant morbidity from bleeding, particularly in the elderly. The taxonomy of vascular abnormalities has been inconstant; there has been confusion regarding arteriovenous malformation, vascular ectasia, vascular malformation and telangiectasia.3,4 In cases with angiodysplasia and arteriovenous malformation, the two terms are frequently used interchangeably in the clinical literature because of equating angiographic with histologic documentation. However, in terms of pathological features, Eastman et al. stressed that they are distinct entities and not interchangeable. Angiodysplasias consists of thin tortuous veins that have no internal elastic layer. These thin-walled vessels may consist of only a single endothelial cell layer. Angiodysplasia may represent a degenerative condition among elderly patients, with abnormal vessels present in the mucosa and submucosa. By contrast, arteriovenous malformations demonstrate aberrant vessels with thickened, hypertrophic walls that vary in their thickness. An elastic segment can demonstrate areas with arterial features such as an internal elastic layer that interconnects with segments of veins with no internal elastic layer.5
The prevalence of angiodysplasia has been reported to be approximately ~6.0% in patients with lower gastrointestinal bleeding, and 1.2-8.0% of cases with hemorrhaging from the upper gastrointestinal tract. Small bowel angiodysplasia accounts for 30-40% of cases of gastrointestinal bleeding of unknown origin, and represents the most common cause for hemorrhage in this subset of patients. Lesions in the large bowel occur most frequently in the right colon.1
Small bowel varicosis is an uncommon cause of lower gastrointestinal bleeding. Few cases of jejunal varicoses have been described in the literature. Most cases of small bowel varices are located in duodenum, and they are usually associated with liver cirrhosis. In the absence of liver cirrhosis jejunal varicoses are associated with the occlusion of one of the major vessels in the area including the splenic, portal or superior mesenteric vein.2 However, in the present case, an abdominal CT showed normal liver parenchyma and the angiographic findings showed tortuous and dilated jejunal and ileal branches in the venous phase, similar to varicosis but without obstruction of major vessels.
Angiography and endoscopy are effective methods for the diagnosis of angiodysplasia. Angiography performed as an initial method of investigation can be used in patients with massive bleeding that might impair endoscopic inspection. However, in the present case, the characteristic angiography features of an angiodysplasia were absent.6,7 There was no visible late-draining vein. The typical vascular tuft was absent and there was no early filling vein visible. Moreover, the ecstatic jejunal vein was visible during the venous phase. Since the portal circulation was normal and no liver pathology was found, we were inclined to congenital angiodysplasia as a very rare case of idiopathic jejunal varicosis. Angiographic diagnosis was in disagreement with the histopathology diagnosis of angiodysplasia; this was probably because a congenital venous abnormality progresses to venous distension under the circumstance with portal hypertension.
Four theories have been proposed to explain the underlying pathogenesis of angiodysplasia. One theory is that angiodysplasias may develop in response to chronic low grade venous obstruction. This is consistent with the observation that angiodysplasias often occur in the right colon where the wall tension is highest.8 Another theory is that angiodysplasias may be a complication of chronic mucosal ischemia, which can occur during episodes of bowel obstruction or straining at stool.9 The third is that angiodysplasias can develop as a result of a complication of local ischemia associated with cardiac, vascular or pulmonary disease, such as end-stage renal disease, von Willebrand's disease, aortic stenosis, and liver cirrhosis.10 The last is that angiodysplasias may be congenital origin, which is probably more likely in young patients. In this case, the patient was 42-year old with no medical illness including constipation. Furthermore, there was no family history of gastrointestinal bleeding, and the lesion was located in the jejunum. So we suspected that the angiodysplasia was congenital. The bleeding focus was thought to be the congenital jejunal angiodyspalsia based on the following four reasons. First, the 1.0 cm superficial ulcer covered with a blood clot was found 70 cm from the ligament of Treitz. Second, no further bleeding occurred 1 year after the resection of this lesion, in spite of the two consecutive profuse episodes of melena. Third, no additional lesion was found in the colon and upper gastrointestinal tract. Fourth, no definite bleeding focus was found in the intraoperative small bowel endoscopy except for the 1.0 cm ulcer on a background of diffuse angiodysplasia.
In conclusion, we report a rare case of congenital angiodysplasia of the jejunum in a young woman, presenting with idiopathic jejunal varicosis on angiography but pathologically angiodysplasia. The patient was successfully treated by surgical resection. The current classification of intestinal angiodysplasia is subject to confusion and revision should be considered. Further studies are needed to better understand the biology of angiodysplasias.
References
- 1.Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807–818. [PubMed] [Google Scholar]
- 2.Cottam DR, Clark R, Hayn E, Shaftan G. Duodenal varices: a novel treatment and literature review. Am Surg. 2002;68:407–409. [PubMed] [Google Scholar]
- 3.Duray PH, Marcal JM, Jr, LiVolsi VA, Fisher R, Scholhamer C, Brand MH. Small intestinal angiodysplasia in the elderly. J Clin Gastroenterol. 1984;6:311–319. doi: 10.1097/00004836-198408000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Moore JD, Thompson NW, Appelman HD, Foley D. Arteriovenous malformations of the gastrointestinal tract. Arch Surg. 1976;111:381–389. doi: 10.1001/archsurg.1976.01360220077013. [DOI] [PubMed] [Google Scholar]
- 5.Eastman J, Nazek M, Mangels D. Localized arteriovenous malformation of the jejunum. Arch Pathol Lab Med. 1994;118:181–183. [PubMed] [Google Scholar]
- 6.Boley SJ, Sprayregen S, Sammartano RJ, Adams A, Kleinhaus S. The pathophysiologic basis for the angiographic signs of vascular ectasias of the colon. Radiology. 1977;125:615–621. doi: 10.1148/125.3.615. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JD, Max MH, Flint LM, Jr, Schweisinger W, Howard M, Aust JB. Bleeding vascular malformations of the intestine. Surgery. 1978;84:430–436. [PubMed] [Google Scholar]
- 8.Boley SJ, Brandt LJ. Vascular ectasias of the colon--1986. Dig Dis Sci. 1986;31:26S–42S. doi: 10.1007/BF01295988. [DOI] [PubMed] [Google Scholar]
- 9.Baum S, Athanasoulis CA, Waltman AC, et al. Angiodysplasia of the right colon: a cause of gastrointestinal bleeding. AJR Am J Roentgenol. 1977;129:789–794. doi: 10.2214/ajr.129.5.789. [DOI] [PubMed] [Google Scholar]
- 10.Rogers BH. Endoscopic diagnosis and therapy of mucosal vascular abnormalities of the gastrointestinal tract occurring in elderly patients and associated with cardiac, vascular, and pulmonary disease. Gastrointest Endosc. 1980;26:134–138. doi: 10.1016/s0016-5107(80)73303-5. [DOI] [PubMed] [Google Scholar]