Abstract
The role of metastasectomy for recurrent disease in patients with adenoid cystic carcinoma (ACC) is not defined clearly yet. A 52-year-old woman found two hepatic metastatic nodules 3 years after the completion of treatment for primary ACC of the trachea. After confirming the absence of other lesions, metastasectomy was performed on the two metastatic nodules. Regular follow-up for more than 24 months showed no evidence of recurrent disease after the hepatic metastasectomy. Therefore, we suggest metastasectomy as an option for certain cases of metastatic ACC.
Keywords: Adenoid cystic carcinoma, Trachea, Metastasectomy
INTRODUCTION
Adenoid cystic carcinomas (ACCs) are rare tumors, and mostly arise from salivary gland, especially minor salivary glands.1 They can also arise in various sites of the head and neck including the tracheobronchial tree and even in sites outside the head and neck, such as breast, uterine cervix, prostate, and the skin.2 They have "paradoxical" behaviors. They grow slowly but have relentless and progressive clinical course. They are usually feasible for surgical treatment at presentation but recur inevitably even after many years after complete resection. They seldom metastasize to regional lymph node but often metastasize to distant sites such as lung and bone. Therefore, 5-year survival rates are high but 10- to 20-year survival rates are dismally low.3 If metastasis occurs, watchful waiting, radiation therapy, palliative surgery, and systemic chemotherapy would be treatment options, but no standard palliative treatment modalities are exist.4 Despite the role of metastasectomy to control metastatic tumors in patients with different primary tumors of ACCs is uncertain and controversial, there are some series or anecdotal experiences of promising results of metastasectomy of pulmonary metastatic tumors or of hepatic metastatic tumors.5-9 We here present one case of metastasectomy of hepatic metastatic tumors of ACC of the trachea, in which the patient lives for more than 24 months without the evidence of progressive disease or further recurrence.
CASE REPORT
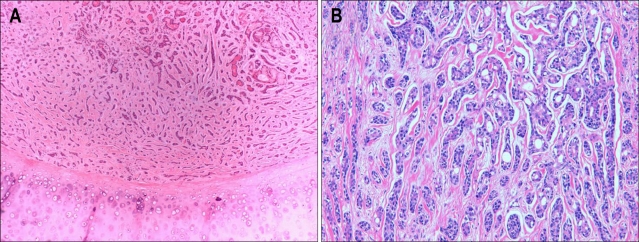
A 52-year-old woman came to Oncology Clinic to consult treatment of hepatic metastatic tumors. She had undergone laryngotrachectomy and thyoridectomy with mediastinal tracheostomy for ACC of the trachea followed by radiotherapy for residual tumors at surgical resection margin 3 years before the consultation. The pathology of the resected tumors, measuring 7×4×3 cm from the just below level of vocal cord to tracheal ring, had been reported to be ACC with predominantly tubular histology, extending to subglottic laryngeal wall and thyroid gland without lymphovascular tumor emboli. The tumor cells had uniform round-to-angulated basophilic nuclei, and did not demonstate notable pleomorphism or mitotic activity. They formed tubular structures with microcysts in the cord, with surrounding hyalinized stroma (Fig. 1). Although she had to undergo the radiotherapy for residual tumors, no distant metastasis had been identified on whole body positron emission tomography (PET) and bone scan at the time of the initial operation.
Fig. 1.
Pathology of the primary tumor. The tumor cells had uniform round-to-angulated basophilic nuclei, and did not demonstrate notable pleomorphism or mitotic activity. They appeared as tubular structures with microcysts in the cord, with surrounding hyalinized stroma. The tumor showed a mixed (A) but predominantly tubular (B) pattern (A, H&E stain, ×40; B, H&E stain, ×200).
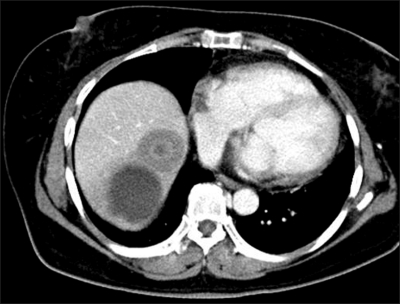
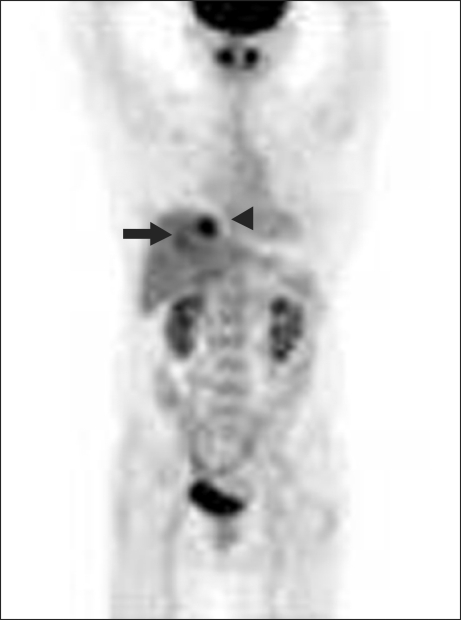
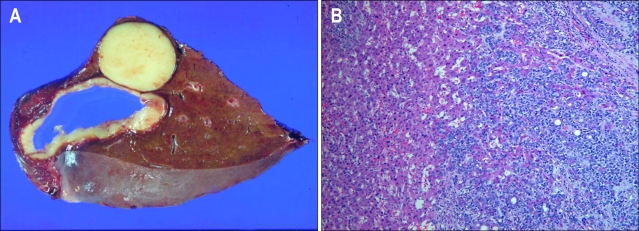
Her chest CT showed two nodules in the segment VII and VIII of liver (Fig. 2) while her abdomen/pelvis CT and whole body PET-CT showed no other metastatic lesions except the above two metastatic nodules (Fig. 3). Her performance status was good with ECOG performance status of 0 and her liver function test as well as other organ function tests was normal. She underwent the right lobectomy of the liver as metastasectomy. The final pathology confirmed that there were the two tumors only, measuring 5×4×4 cm in the segment VII and 3×3×2 cm in the segment VIII, respectively and there was no remnant tumor. The pathology of hepatic tumors was the same to that of the prior tumor of the trachea. No lymphovascular invasion and perineural invasion was identified in the metastatic tumors as well (Fig. 4). To date, she has been followed up regularly without evidence of progressive disease or further recurrence for more than 24 months.
Fig. 2.
CT scan of the upper abdomen. There were two masses in liver segments VII and VIII. Segment VIII contained a poorly enhanced, round, 3-cm mass, and segment VII contained a peripherally enhanced, round, 4.5-cm cystic mass.
Fig. 3.
Whole PET scan. Two hypermetabolic lesions were present in the liver (solid arrow and arrowhead).
Fig. 4.
Metastatic adenoid cystic carcinoma (ACC) in the liver. (A) Gross findings showing two lesions. (B) Microscopic findings showing a metastatic ACC (H&E stain, ×100).
DISCUSSION
The role of metastasectomy in ACCs is still debatable, but sometimes suggestive in a selected subset of patients. Usually, metastasectomy is often undergone when all metastatic tumors are potentially completely resectable under the control of the primary site and patients have an adequate performance status and a good cardiopulmonary function. Of interest, low tumor burden or less than 6 metastatic tumors and favorable disease-free interval of more than 3 years were sometimes suggested to be selection criteria for pulmonary metastasectomy of ACC.7 The lack of systemic therapeutic alternatives is also suggested one of important selection criteria. In the current case, she had disease-free interval of 3 years, potentially resectable 2 metastatic nodules, and good performance status and residual liver function as well as cardiopulmonary function.
Some argue that as tumor growth of metastatic tumors of ACCs is slow, watchful waiting can be more appropriate, especially when there are no or few symptoms. However, although metastatic tumors grow optimistically slowly, they have relentless and progressive disease course, and long-term outcomes are dismal. Whereas, the response rates for cytotoxic chemotherapy are extremely variable and inconsistent, and response duration was generally short.10 In addition, disease stabilization is more often seen after cytotoxic chemotherapy than objective response but it should be interpreted very cautiously because the ACC, as mentioned above, usually grow slowly. Therefore, systemic cytotoxic chemotherapy should be reserved for those who have symptomatic and rapidly progressive disease. If recurrent or metastatic tumors are totally resectable with conserving organ functions, metastasectomy could be considered as one of the treatment options, such as cytotoxic chemotherapy or watchful waiting.
In conclusion, metastasectomy in patients with ACCs can be resorted to in a very highly selected subset of patients, who have potentially completely resectable metastatic tumors under the control of primary tumors and adequate performance and organ functions.
References
- 1.Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127–132. doi: 10.1097/00020840-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623–628. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]
- 3.Westra WH. The surgical pathology of salivary gland neoplasms. Otolaryngol Clin North Am. 1999;32:919–943. doi: 10.1016/s0030-6665(05)70182-1. [DOI] [PubMed] [Google Scholar]
- 4.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 5.Bobbio A, Copelli C, Ampollini L, et al. Lung metastasis resection of adenoid cystic carcinoma of salivary glands. Eur J Cardiothorac Surg. 2008;33:790–793. doi: 10.1016/j.ejcts.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Labow DM, Dang N, et al. Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol. 1999;6:572–578. doi: 10.1007/s10434-999-0572-8. [DOI] [PubMed] [Google Scholar]
- 7.Locati LD, Guzzo M, Bossi P, et al. Lung metastasectomy in adenoid cystic carcinoma (ACC) of salivary gland. Oral Oncol. 2005;41:890–894. doi: 10.1016/j.oraloncology.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi SS, Nadkarni MS, Shrikhande SV, et al. Hepatic resection for metastasis from adenoid cystic carcinoma of parotid gland. Indian J Gastroenterol. 2005;24:29–30. [PubMed] [Google Scholar]
- 9.Zeidan BA, Hilal MA, Al-Gholmy M, El-Mahallawi H, Pearce NW, Primrose JN. Adenoid cystic carcinoma of the lacrimal gland metastasising to the liver: report of a case. World J Surg Oncol. 2006;4:66. doi: 10.1186/1477-7819-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–769. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]