Abstract
In most H. pylori-positive patients, gastric low-grade mucosa-associated lymphoid tissue (MALT) lymphomas regress both endoscopically and histopathologically after H. pylori eradication, but no factors that can be predictive of the response to the eradication have been definitively identified, and there is little information on how to determine the optimal observation period before additional treatment can be started. Here, clinical studies dealing with the diagnosis and treatment of gastric MALT lymphomas and H. pylori published during the last 5 years were systematically reviewed, and studies identifying the molecular approaches involved in the pathogenesis were summarized. Most of the clinical studies indicate a favorable effect of H. pylori eradication on the clinical outcome of gastric MALT lymphomas. Some studies suggest the necessity of additional treatment in nonresponders to H. pylori eradication, while others suggest the adoption of a watch-and-wait strategy. The molecular characteristics of MALT lymphomas could play an important role in prognostic prediction and the selection of further therapeutic intervention after the eradication. This updated review of gastric MALT lymphomas illustrates the potential efficacy of H. pylori eradication in tumor remission, but further molecular characterization is necessary to establish the most suitable therapeutic strategy for patients who do not respond to eradication.
Keywords: Helicobacter pylori, Eradication, API2-MALT1 fusion, BCL10, CD20
INTRODUCTION
Helicobacter pylori (H. pylori) is a gram-negative, microaerobic rod that lives in the gastric mucous layer, on the surface epithelial cells.1 H. pylori infection causes gastric mucosal inflammation, which could not only lead to chronic gastritis and peptic ulcer disease, but also to gastric adenocarcinoma or low-grade mucosa-associated lymphoid tissue (MALT) lymphoma.1 H. pylori eradication has become a widely accepted initial treatment strategy for stage I gastric marginal zone B cell lymphoma of MALT. Although in 60-80% of these cases, H. pylori-positive low-grade B-cell gastric MALT lymphoma regress both endoscopically and histopathologically after H. pylori eradication,2,3 both the factors that might be predictive of the response to the eradication therapy, as well as the optimal duration of observation needed prior to the potential start of additional second-line treatment in the non-responsive cases remain controversial.
LITERATURE REVIEW FOR GASTRIC MALT LYMPHOMA
A literature search was performed in PubMed using the keywords [gastric MALT lymphoma] and [H. pylori] for the publications during the last 5 years (2004-2008). After accumulating the list of included studies, publications not related to either gastric MALT lymphoma or H. pylori were excluded. In addition, the recent developments in the molecular mechanisms involved in the pathogenesis of gastric MALT lymphomas were reviewed.
The initial search yielded 21 manuscripts. Among these, 6 articles (1 review article, 3 articles dealing with non-gastric MALT lymphomas, and 2 articles not dealing with H. pylori) were excluded, while the remaining 15 were carefully reviewed. Among the 15 articles reviewed, 4 articles4-7 dealt with the diagnosis and the remaining 11 dealt with the treatment of gastric MALT lymphomas. Among the 11 articles dealing with the treatment, 9 articles8-16 reported on H. pylori eradication, 1 article17 reported reconsideration of surgical treatment after a median follow-up of 11.5 years after gastrectomy, and 1 article18 showed significant efficacy of rituximab.
1. Helicobacter pylori eradication
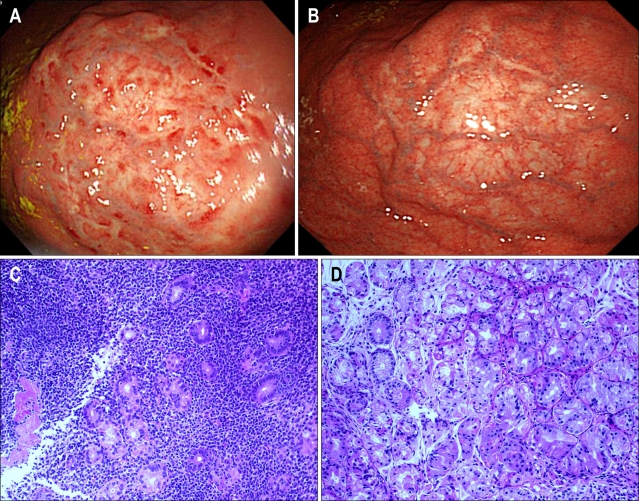
H. pylori eradication therapy was followed by complete remission (CR) of low-grade gastric MALT lymphoma in 46-94% of the patients (Table 1).6,8,9,12-16,18 Representative upper gastrointestinal (GI) endoscopic images of a 46-year-old female patient are shown in Fig. 1. Significant regression of the nodular and reddish colored low-grade MALT lymphoma lesion observed in the upper gastric corpus mucosa (Fig. 1A, one month after the H. pylori eradication) was noted at 22 months after the H. pylori eradication therapy (Fig. 1B). Histologically, characteristic lymphoepithelial lesion (LEL) identified in the upper gastric corpus one month after the eradication (Fig. 1C) disappeared 22 months after the H. pylori eradication (Fig. 1D).
Table 1.
Complete Remission (CR) Rates after H. pylori Eradication in Patients with Low-grade MALT Lymphomas
MALT, mucosa-associated lymphoid tissue.
Fig. 1.
(A) Endoscopy images of a low-grade MALT lymphoma lesion at the upper gastric corpus mucosa in a 46-year-old female patient obtained 1 month after H. pylori eradication, showing a nodular lesion whose surface contains patchy red spots on a discolored background. (B) Endoscopy image of the regressed low-grade MALT lymphoma lesion at the upper gastric corpus mucosa obtained 22 months after H. pylori eradication. (C) Histopathology image (H&E stain) of a low-grade MALT lymphoma lesion at the upper gastric corpus mucosa 1 month after H. pylori eradication. Small lymphoma cells infiltrated the gastric glands to form lymphoepithelial lesions (×20 objective lens). (D) Histopathology image (H&E stain) of the regressed low-grade MALT lymphoma lesion at the upper gastric corpus mucosa obtained 22 months after H. pylori eradication. Lymphoma cells had disappeared, with only partial mononuclear cell infiltrates remaining (×20 objective lens).
Gastric lymphomas are usually staged by endoscopic ultrasonography, according to the modified Ann Arbor staging system by Musshoff and Radaszkiewicz et al.19 Here, "E" means primary extranodal site. Stage E-I means the localized involvement of one or more GI sites on one side of the diaphragm without lymph node infiltration. Stage E-I is further characterized as E-I1 (lymphoma confined to mucosa and submucosa) or as E-I2 (lymphoma extending beyond submucosa). Stage E-II means the localized involvement of one or more GI sites on one side of the diaphragm with lymph node infiltration (any depth of lymphoma infiltration into the gut wall). Stage E-II is further characterized as E-II1 (Infiltration of regional lymph nodes) or as E-II2 (Infiltration of lymph nodes beyond regional area). Then, stage E-III means the localized involvement of the GI tract and/or lymph nodes on both sides of the diaphragm and stage E-IV means the localized GI bulk of lymphoma with or without infiltration of associated involvement of non-GI tract organs or tissues.
According to a prospective multicenter study conducted in Germany and Austria by Fischbach et al.16 on 95 patients with newly diagnosed MALT lymphoma, the long-term outcome (median follow-up; 45 months) after H. pylori eradication was characterized by CR in 56 patients (62%), minimal residual disease (MRD) in 17 patients (18%), and partial remission (PR) in 11 patients (12%). In this study, the regression rate was higher in patients with stage E-I1 disease as compared with that in those with stage E-I2 disease, as diagnosed by endoscopic ultrasonography (EUS).16 Wündisch et al.14 also reported the long-term outcomes of 120 patients with gastric MALT lymphoma (stage E-I) after H. pylori eradication; they reported a CR of 80% and a relapse rate of only 3%. In their study,14 both t(11;18)(q21;q21) translocation and ongoing B-cell monoclonality were associated with a significantly higher risk of no response or relapse, but they were also encountered in patients showing CR. In 2006, Hong et al.13 reported a high CR rate (94%) of gastric low-grade MALT lymphoma after H. pylori eradication, with a median interval to CR of 3 months.
Patients with minimally persisting lymphoma infiltrates after successful H. pylori eradication are considered as treatment failures and referred for further treatment. However, according to the report by Fischbach et al.10, most patients with minimal histological residuals after successful eradication had a favorable disease course without anti-cancer treatment, suggesting that a watch-and-wait strategy with regular follow up endoscopies and biopsies may be safe and preferred approach in the management of these patients.
In Taiwan,15 two multicenter prospective studies of H. pylori eradication for early-stage gastric lymphoma, one for low-grade MALT lymphoma, and one for high-grade transformed tumors (diffuse large B-cell lymphoma [DLBCL] with features of MALT), were directly compared. In this study, H. pylori was successfully eradicated in 97% of H. pylori-positive low-grade lymphoma patients and 92% of H. pylori-positive high-grade lymphoma patients, resulting in CR in 80% and 64% of the patients in the two groups, respectively. After a median follow-up of more than 5 years of the complete responders, tumor recurrence was observed in 13% of the patients with low-grade tumors, but none of the patients with the high-grade tumors, suggesting that anti-H. pylori therapy is considered as one of the treatment options even for early-stage H. pylori-positive gastric DLBCL.15
Todorovic et al.8 reported that in patients of primary gastric MALT lymphoma with a median follow-up duration of 5 years, the CR was 65% (22/34) and PR was 27% (9/34) after H. pylori eradication, and that the predictive factors for overall survival were the international prognostic index (IPI) score, blood hemoglobin level, erythrocyte sedimentation rate (ESR), and platelet count. Nakamura et al.9 reported that patients with gastric MALT lymphomas responsive to H. pylori eradication therapy had a good prognosis (CR rate: 65%, 56/86), and that in non-responders to the eradication, second-line treatments resulted in CR. However, especially in the case of non-responders to H. pylori eradication therapy who decline second-line treatment, careful follow-up is necessary to detect development of gastric adenocarcinoma and disease progression.
Helicobacter heilmannii is also known as another bacterial pathogen associated with the development of gastritis and gastric MALT lymphoma.20,21 According to the report by Joo et al.11, although H. heilmannii-associated gastritis is uncommon and milder than H. pylori-associated gastritis, the association may be worthy of note with respect to the development of MALT lymphoma.
In regard to H. pylori-negative cases, according to the report by Nakamura et al.12, although H. pylori-negative gastric MALT lymphoma is characterized by a high frequency of t(11;18)(q21;q21)22 and may require additional strategies other than antibiotics, 2 of 7 (29%) H. pylori-negative patients also responded to antibiotic treatment.
Gastric MALT lymphomas with the API2-MALT1 fusion is generally known as resistant to H. pylori eradication therapy. A recent study demonstrated the clinical activity of the monoclonal antibody to CD20, rituximab in gastric MALT lymphoma patients with the API2/MALT1 fusion.18 In their study,18 46% of the patients showed pathological and clinical CR, and 31% showed a partial response, with only two patients presenting with relapse at 26 and 14 months after weekly rituximab treatment, respectively. This finding suggests that the t(11;18)(q21;q21) translocation may not, after all, be a predictive marker of response or of subsequent relapse following rituximab therapy.18
On the other hand, some gastric MALT lymphomas that are negative for the API2-MALT1 fusion may show resistance to H. pylori eradication therapy. These tumors have not been well-characterized and the precise clinicopathological features are still unknown. Further studies regarding other chromosomal translocations and other bacterial infections that might be involved in the pathogenesis of gastric MALT lymphomas are necessary.
2. Chromosomal translocations in gastric MALT lymphoma (Table 2)
Table 2.
Molecular Alterations in Gastric MALT Lymphomas
MALT, mucosa-associated lymphoid tissue.
As mentioned above, gastric MALT lymphomas are characterized not only by regression in response to H. pylori eradication therapy,3 but also by chromosomal translocations.23 The API2-MALT1 fusion that results from chromosome translocation t(11;18)(q21;q21) has been reported to be a predictor of non-responsiveness to H. pylori eradication therapy.22 It has been suggested that since API2-MALT1 fusion transcripts might promote survival of the MALT lymphomas by producing inhibition of apoptosis, they help in the survival of MALT lymphomas.24,25
On the other hand, t(1;14)(p22;q32) occurs in 1-2% of MALT lymphomas and has been detected in the stomach, salivary glands, intestines, and lungs.26 The BCL10 gene was isolated from the breakpoint of the t(1;14) translocation, with deregulated expression of BCL10 in the MALT lymphomas.27,28 BCL10 activates nuclear factor kappa B (NF-kB), a transcription factor for several survival-related genes, via interaction with a MALT1 gene product.29 Moreover, recent studies have shown that the API2-MALT1 fusion protein activates NF-kB and is associated with nuclear BCL10 expression.29,30 Thus, some chromosomal translocations involving the API2-MALT1 fusion promoting tumor cell survival and deregulation of BCL10 activating several survival genes have been reported in MALT lymphomas.
3. Gene expression profiles of gastric MALT lymphomas (Table 2)
Recent studies using microarray analyses have revealed the gene expression profiles of gastric MALT lymphomas. Mueller et al.20 reported increased expression of genes previously to be associated with malignancies, including laminin receptor-1 and MDR-1, in LELs, and increased expression of calgranulin A/Mrp-8 in destructive LELs and malignant lymphomas. Thus, they suggest that the gene expression profiles only represent the histopathological stages of MALT lymphomas. Huynh et al.31 also reported the gene expression signatures in gastric MALT lymphomas. Several surface markers of hematopoietic cells, such as CD1c, CD40, CD44, CD53, CD83, CD86 and members of the HLA-D family, were found to be upregulated in MALT lymphomas. Since MALT lymphomas typically exhibit the following immunophenotype-CD19(+), CD20(+), CD21(+), CD79a(+), CD5(-), CD10(-), CD23(-), and cyclin D1(-), the gene expression profile does not simply reflect a higher B-cell component, but also the antigen-dependent survival of the lymphoma cells.
MicroRNAs (miRNAs) are small non-coding RNAs that can downregulate various target genes, including tumor-associated genes. Recent studies have demonstrated that aberrant expression of miRNAs plays critical roles in the development of human malignancies.32 Lu et al.33 have reported that miRNA expression profiles can be used to classify the developmental lineages and differentiation stages of tumors. Interestingly, miRNA expression profiles are more accurate for tumor classification than conventional mRNA profiles. We recently demonstrated overexpression of hematopoietic-specific miRNAs and oncogenic miRNAs in gastric MALT lymphomas with the API2-MALT1 fusion, and proposed that the miRNA expression profiles could be novel biomarkers and therapeutic targets for gastric MALT lymphomas.34,35
4. Aberrant DNA methylation in gastric MALT lymphomas (Table 2)
Since epigenetic alterations, such as DNA methylation, are known to be involved in the initiation and development of human malignancies, determination of the DNA methylation status might be a powerful tool for the diagnosis and treatment of malignancies.
Methylation of the p16/INK4a gene was observed in 60% of MALT lymphomas, however, the p16 gene methylation status was not correlated with the presence of API2-MALT1 fusion or any other clinicopathological factors, suggesting that aberrant methylation of the p16 gene might be an early event in MALT lymphomagenesis.36 Min et al.37 also demonstrated methylation of p16/INK4a and p57/KIP2 in 41.7 and 29.2%, respectively, of low-grade MALT lymphomas, respectively. Determination of the methylation profiles of 8 CpG islands, including p15, p16, p73, hMLH1, DAPK, MINT1, MINT2, and MINT31, revealed that H. pylori-dependent MALT lymphomas showed more than 4 methylated genes, while non H. pylori-dependent cases showed less than 2 methylated genes, indicating that H. pylori-dependent and non H. pylori-dependent gastric MALT lymphomas may have a different pathogenesis, including the aberrant DNA methylation pattern.38
Thus, aberrant DNA methylation plays critical roles in the pathogenesis of gastric MALT lymphomas and detection of the DNA methylation pattern may hold promise as a clinical tool for surveillance of gastric MALT lymphomas.
SUMMARY
Although numerous reports have suggested a good prognosis of gastric low-grade MALT lymphomas after H. pylori eradication, for cases not responsive to antibiotic therapy, further molecular characterization is necessary to establish the most suitable therapeutic strategy, including additional second-line treatment or a watch and wait policy. Molecular events associated with gastric MALT lymphomas such as chromosomal translocations, altered gene expressions and epigenetic modifications (Table 2) might provide predictive information for therapeutic responsiveness as well as for recurrence or relapse of the disease. Large-scale cohort studies with molecular examinations should be performed to establish the therapeutic strategies for gastric MALT lymphomas after the successful H. pylori eradication.
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid for Exploratory Research from the Japan Society for the Promotion of Science (JSPS) (No. 19659057 to H.S.) and a Keio Gijyuku Academic Development Fund.
References
- 1.Suzuki H, Hibi T, Marshall BJ. Helicobacter pylori: present status and future prospects in Japan. J Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolte M, Eidt S. Healing gastric MALT lymphomas by eradicating H. pylori? Lancet. 1993;342:568. doi: 10.1016/0140-6736(93)91404-a. [DOI] [PubMed] [Google Scholar]
- 3.Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 4.Yi ZH, Ouyang Q, Li GD, Chen DY. Combined histology and molecular biology for diagnosis of early stage gastric MALT lymphoma. Chin J Dig Dis. 2006;7:12–18. doi: 10.1111/j.1443-9573.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 5.Lo WY, Li JY, Chan YK, et al. Instability of clonality in gastric lymphoid infiltrates: a study with emphasis on serial biopsies. Am J Surg Pathol. 2005;29:1582–1592. doi: 10.1097/01.pas.0000188031.40836.00. [DOI] [PubMed] [Google Scholar]
- 6.de Mascarel A, Ruskone-Fourmestraux A, Lavergne-Slove A, Megraud F, Dubus P, Merlio JP. Clinical, histological and molecular follow-up of 60 patients with gastric marginal zone lymphoma of mucosa-associated lymphoid tissue. Virchows Arch. 2005;446:219–224. doi: 10.1007/s00428-005-1217-3. [DOI] [PubMed] [Google Scholar]
- 7.Fujimori K, Shimodaira S, Akamatsu T, Furihata K, Katsuyama T, Hosaka S. Effect of Helicobacter pylori eradication on ongoing mutation of immunoglobulin genes in gastric MALT lymphoma. Br J Cancer. 2005;92:312–319. doi: 10.1038/sj.bjc.6602262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todorovic M, Balint B, Jevtic M, et al. Primary gastric mucosa associated lymphoid tissue lymphoma: clinical data predicted treatment outcome. World J Gastroenterol. 2008;14:2388–2393. doi: 10.3748/wjg.14.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Seto M, Tajika M, et al. Clinical features and prognosis of gastric MALT lymphoma with special reference to responsiveness to H. pylori eradication and API2-MALT1 status. Am J Gastroenterol. 2008;103:62–70. doi: 10.1111/j.1572-0241.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685–1687. doi: 10.1136/gut.2006.096420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo M, Kwak JE, Chang SH, et al. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci. 2007;22:63–69. doi: 10.3346/jkms.2007.22.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S, Matsumoto T, Ye H, et al. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 2006;107:2770–2778. doi: 10.1002/cncr.22326. [DOI] [PubMed] [Google Scholar]
- 13.Hong SS, Jung HY, Choi KD, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter. 2006;11:569–573. doi: 10.1111/j.1523-5378.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 14.Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018–8024. doi: 10.1200/JCO.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 15.Chen LT, Lin JT, Tai JJ, et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97:1345–1353. doi: 10.1093/jnci/dji277. [DOI] [PubMed] [Google Scholar]
- 16.Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34–37. doi: 10.1136/gut.53.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo SH, Chen LT, Wu MS, et al. Long-term follow-up of gastrectomized patients with mucosa-associated lymphoid tissue lymphoma: need for a revisit of surgical treatment. Ann Surg. 2008;247:265–269. doi: 10.1097/SLA.0b013e3181582364. [DOI] [PubMed] [Google Scholar]
- 18.Martinelli G, Laszlo D, Ferreri AJ, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol. 2005;23:1979–1983. doi: 10.1200/JCO.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 19.Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992;102:1628–1638. doi: 10.1016/0016-5085(92)91723-h. [DOI] [PubMed] [Google Scholar]
- 20.Mueller A, O'Rourke J, Chu P, et al. Protective immunity against Helicobacter is characterized by a unique transcriptional signature. Proc Natl Acad Sci U S A. 2003;100:12289–12294. doi: 10.1073/pnas.1635231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Murayama SY, Serizawa H, et al. "Candidatus Helicobacter heilmannii" from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75:1214–1222. doi: 10.1128/IAI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Ye H, Ruskone-Fourmestraux A, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa M, Seto M, Hosokawa Y. Molecular pathogenesis of MALT lymphoma: two signaling pathways underlying the antiapoptotic effect of API2-MALT1 fusion protein. Leukemia. 2006;20:929–936. doi: 10.1038/sj.leu.2404192. [DOI] [PubMed] [Google Scholar]
- 24.Motegi M, Yonezumi M, Suzuki H, et al. API2-MALT1 chimeric transcripts involved in mucosa-associated lymphoid tissue type lymphoma predict heterogeneous products. Am J Pathol. 2000;156:807–812. doi: 10.1016/S0002-9440(10)64948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosokawa Y, Suzuki H, Suzuki Y, Takahashi R, Seto M. Antiapoptotic function of apoptosis inhibitor 2-MALT1 fusion protein involved in t(11;18)(q21;q21) mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2004;64:3452–3457. doi: 10.1158/0008-5472.CAN-03-3677. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki H. Mucosa-associated lymphoid tissue lymphoma: molecular pathogenesis and clinicopathological significance. Pathol Int. 2007;57:474–484. doi: 10.1111/j.1440-1827.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- 27.Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Siebert R, Yan M, et al. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14) (p22;q32) Nat Genet. 1999;22:63–68. doi: 10.1038/8767. [DOI] [PubMed] [Google Scholar]
- 29.Lucas PC, Yonezumi M, Inohara N, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem. 2001;276:19012–19019. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood. 2001;98:1182–1187. doi: 10.1182/blood.v98.4.1182. [DOI] [PubMed] [Google Scholar]
- 31.Huynh MQ, Wacker HH, Wündisch T, et al. Expression profiling reveals specific gene expression signatures in gastric MALT lymphomas. Leuk Lymphoma. 2008;49:974–983. doi: 10.1080/10428190802007734. [DOI] [PubMed] [Google Scholar]
- 32.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Saito Y, Nishizawa T, et al. Overexpression of microRNA-142 and microRNA-155 in AP12-MALT1-positive gastric low-grade MALT lymphoma. Gastroenterology. 2008;134:A61. (Abstract) [Google Scholar]
- 35.Saito Y, Suzuki H, Hibi T. The role of microRNAs in gastrointestinal cancers. J Gastroenterol. 2009;44(Suppl 19):18–22. doi: 10.1007/s00535-008-2285-3. [DOI] [PubMed] [Google Scholar]
- 36.Takino H, Okabe M, Li C, et al. p16/INK4a gene methylation is a frequent finding in pulmonary MALT lymphomas at diagnosis. Mod Pathol. 2005;18:1187–1192. doi: 10.1038/modpathol.3800400. [DOI] [PubMed] [Google Scholar]
- 37.Min KO, Seo EJ, Kwon HJ, et al. Methylation of p16 (INK4A) and p57(KIP2) are involved in the development and progression of gastric MALT lymphomas. Mod Pathol. 2006;19:141–148. doi: 10.1038/modpathol.3800505. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko Y, Sakurai S, Hironaka M, et al. Distinct methylated profiles in Helicobacter pylori dependent and independent gastric MALT lymphomas. Gut. 2003;52:641–646. doi: 10.1136/gut.52.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]