Abstract
Background/Aims
Although endoscopic ultrasound guided fine needle aspiration (EUS-FNA) has been introduced and its use has been increasing in Korea, there have not been many reports about its performance. The aim of this study was to assess the utility of EUS-FNA without on-site cytopathologist in establishing the diagnosis of solid pancreatic and peripancreatic masses from a single institution in Korea.
Methods
Medical records of 139 patients who underwent EUS-FNA for pancreatic and peripancreatic solid mass in the year 2007, were retrospectively reviewed. By comparing cytopathologic diagnosis of FNA with final diagnosis, sensitivity, specificity, and accuracy were determined, and factors influencing the accuracy as well as complications were analyzed.
Results
One hundred twenty out of 139 cases had final diagnosis of malignancy. Sensitivity, specificity, and accuracy of EUS-FNA were 82%, 89%, and 83%, respectively, and positive and negative predictive values were 100% and 46%, respectively. As for factors influencing the accuracy of FNA, lesion size was marginally significant (p-value 0.08) by multivariate analysis.
Conclusions
EUS-FNA performed without on-site cytopathologist was found to be accurate and safe, and thus EUS-FNA should be a part of the standard management algorithm for pancreatic and peripancreatic mass.
Keywords: EUS-FNA, Pancreatic neoplasms, Peripancreatic mass, Diagnostic accuracy
INTRODUCTION
Pancreas cancer is a malignancy that has a very poor prognosis. Although its diagnosis can be suspected on noninvasive imaging modalities such as MDCT, MRI and PET, tissue diagnosis still remains as the confirmatory method to diagnose pancreatic cancer.
Tissue diagnosis was traditionally obtained through operation by exploratory laparatomy and less invasively by ultrasound guided transcutaneous fine needle aspiration (US-FNA) and CT guided transcutaneous fine needle aspiration (CT-FNA), but the latter methods require the passage of a needle through the skin and layers of various organs raising the possibility of needle tract seeding as well as other complications.1
For these reasons, it has been a common practice to undergo operation or chemotherapy based on presumed pancreatic cancer without a confirmative tissue diagnosis. However, such practice can not be considered ideal as this puts the patient at a risk from unnecessary surgery if presumed diagnosis of pancreatic cancer turns out to be a benign process or other pathologies.
Endoscopic ultrasound guided fine needle aspiration or biopsy (EUS-FNA/B) using linear EUS has several advantages over other modalities for obtaining a tissue diagnosis. With high resolution imaging, small lesion not detectable with other modalities can be discovered. Second, using a real time doppler imaging, surrounding vasculature can be avoided during tissue acquisition, and third, for the pancreas head lesion, needle tract of FNA is contained within the boundary of operative resection thus negating the risk of tumor spread by the procedure.2,3
Recently, EUS-FNA has also been introduced in Korea, and a few institutions have been active in its performance. A report of its use for evaluation of pancreatic lesions was reported in 2005, but the number of the patients was only 40.4
On the other hand, most studies from overseas have utilized EUS-FNA in the presence of on-site cytopathological assistance, but considering the limitation of such resources in domestic clinical setting, it is important to know the result of EUS-FNA performed without on-site cytopathologic evaluation. There have been only a few reports of such nature.5,6 Therefore, in this study, authors aimed to find the operating characteristics of EUS-FNA in the evaluation of solid pancreatic and peripancreatic lesions from a large number of cases encountered during one year at a tertiary referral center without the help of on-site cytopathologist and some factors influencing the results of EUS-FNA.
MATERIALS AND METHODS
1. Indications for EUS-FNA and patients
EUS-FNA was performed for evaluation of solid pancreatic and peripancreatic lesion excluding pancreatic cystic lesions. More specifically, EUS-FNA was first performed for tissue diagnosis prior to operation for suspected localized pancreatic cancer or other pancreatic neoplasms. Secondly, it was also performed for more advanced pancreatic cancer prior to chemotherapy and also to differentiate between pancreatic cancer and other diseases such as focal pancreatitis, metastatic tumor, and lymphoma. Thirdly, single or multiple intrabdominal mass lesions and adrenal gland lesion not easily approachable from other route were also indications for EUS-FNA. A total of 233 patients underwent EUS-FNA for pancreatic and peripancreatic lesions during the year 2007, and excluding 82 patients who had pancreatic cystic lesion and 12 patients who had inadequate follow up to determine the final nature of the lesion left 139 patients (male 75, female 64) for the analysis.
Medical records were reviewed to note the lesion location, size, FNA needle type used, result of cytopathologic report, final diagnosis, occurrence of complications, clinical features and operative findings.
2. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA)
EUS was performed in all cases under conscious sedation using midazolam and meperidine. All procedure was performed by two experienced endosographists (SSL and DWS). Linear EUS (GF-UM 2000, Olympus, Tokyo, Japan or GF-UCT 240, Olympus, Tokyo, Japan) was used for FNA/B. The type of the needle used in the procedure was determined by the operator at will, and they were 22G (Echotip-22ECHO 3-22®, Cook Endoscopy, Winston-Salem, NC, USA), 19G (Echotip-ECHO 19®, Cook Endoscopy, Winston-Salem, NC, USA), and TCB (Trucut needle Cook Quick-Core, Cook Endoscopy, Winston-Salem, NC, USA).
EUS-FNA was done in following steps. The tip of the scope was placed near the lesion of interest, and after confirming no intervening vessels were present using real time Doppler, the needle was inserted into the lesion. After removing the stylet, specimen was obtained by moving the needle back and forth inside the lesion while applying negative pressure with 10 cc syringe. Specimen released by reinsertion of stylet was fixed in formalin for cell block or core biopsy. Rest was released and smeared on the glass slide using a syringe and cytological analysis was performed.
Trucut biopsy was performed in a similar fashion but used a 19 G Trucut needle that has 18 mm specimen tray and an automated spring loaded biopsy system to obtain a core specimen large enough for histological examination. All procedures were terminated when the operator felt adequate specimen were obtained. No on-site cytopathologic evaluation was available during the procedure. When the procedure was finished, the patient was observed in the recovery room for one or two hours to monitor for symptoms and signs such as abdominal pain, nausea, vomiting, gastrointestinal bleeding and perforation. Next morning, complete blood count, liver function test along with serum amylase and lipase were checked.
3. Diagnosis
Final diagnosis of the patients were confirmed by collective findings of EUS-FNA result, surgical pathology, other pathologic result as from US guided biopsy, and by clinical and radiological follow up consistent with the diagnosis.
4. Data and statistical analysis
Result of EUS-FNA/B was classified as malignancy or benign, and as inadequate when aspirate was poorly cellular or not representative of the target organ. Comparing the EUS-FNA result with the final diagnosis as determined above, sensitivity, specificity, positive predictive value, negative predictive value and accuracy of EUS-FNA was determined. Inadequate result was considered inaccurate.
For the analysis of factors having influence on the result of EUS-FNA, univariate and multivariate logistic regression analysis was performed using SPSS 12.0 K (SPSS Inc., Chicago, IL, USA). For multivariate analysis, backward elimination method was used and p<0.05 was considered as statistically significant.
RESULTS
1. Baseline patient and lesion characteristics
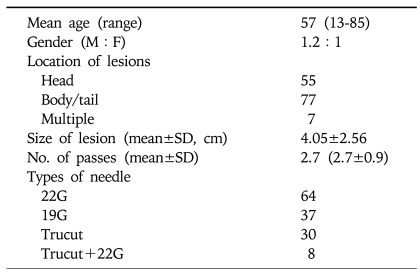
The baseline characteristics of the enrolled patients and lesion characteristics are summarized in Table 1. Mean age of the 139 patients included in the analysis was 57 years (range: 13-85 yr), and male to female ratio was 1.2:1. Average number of needle pass per procedure was 2.7 (2.7±0.9). Sixty four patients were performed with 22G fine needle, 30 with 19G Trucut needle, 8 with both 22G fine needle and 19G Trucut needle, and 37 patients with 19G fine needle.
Table 1.
Baseline Patient and Lesion Characteristics
Average size of the lesion was 4.05±2.56 (range 0.9-20.0) cm. Pancreatic lesions consisted of 103 cases and peripancreatic lesions 36 cases. When lesions were classified by location with reference to pancreas into pancreas head versus body and tail, 55 could be classified as head lesion, while 77 were classified as body/tail lesion, and 7 lesions were multiple.
The criteria for determining the final diagnosis was surgical pathology in 48 (35%) patients, other pathological result including US-FNA in 13 (9.4%) patients, confirmation of disease progression by clinical and radiological follow up in 78 (56%) patients. Average clinical and radiologic follow up duration was 144±124 days.
2. Result of EUS-FNA
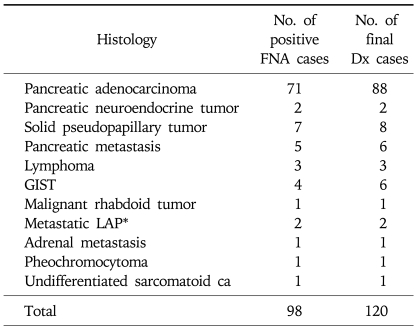
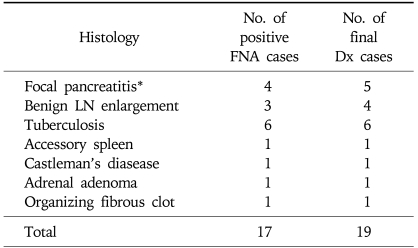
Based on the result of FNA, out of 139 total cases, 98 could be classified as malignant, 24 as benign, and 17 as inadequate. Diagnostic categories by FNA result was compared with the final diagnosis as determined by surgical or other pathology or clinicoradiological follow up (Tables 2 and 3). Most common malignancy was pancreatic adenocarcinoma.
Table 2.
Diagnostic Categories Compared between FNA and Final Diagnosis for Malignant Disease
FNA, fine needle aspiration.
*Stomach cancer (1), MUO (1).
Table 3.
Diagnostic Categories Compared between FNA and Final Diagnosis for Benign Disease
FNA, fine needle aspiration.
*Includes one case of autoimmune pancreatitis.
Retrospective review showed that of the 24 cases classified as benign by FNA result, 7 (29%) turned out to be malignant by final diagnosis and were therefore falsely negative. CT and EUS findings, however, were suggestive of pancreatic cancer in 6 patients, and the other patient had raised CA 19-9 level of 411 U/mL and hypermetabolic lesion (SUV 6.1) in the uncinate process of the pancreas strongly suggesting the presence of pancreatic cancer. These cases were all confirmed as pancreas cancer by additional biopsy (n=1), surgery (n=4), and by clinical follow up (n=2). Three (13%) other cases underwent surgery despite benign result on the FNA because neoplastic process could not be ruled out, and the result of surgical biopsy revealed accessory spleen, organizing fibrin clot, and Castleman's disease, respectively. In the rest of 14 (58%) cases, additional biopsy was not performed and clinical follow up showed benign course in all cases. Mean follow up duration for this latter group of patients was 9.4 (range: 3.0-17.5) months.
In 17 cases in which FNA was inadequate, 15 were determined to be malignant and two as benign. For the 15 cases, seven were diagnosed pathologically (6 surgical, 1 US-FNA) as pancreas cancer (n=2), solid pseudopapillary tumor (n=1), and GIST (n=2), and 8 were diagnosed through clinical follow up and by response to chemotherapy as pancreas cancer (n=7) and metastatic pancreas cancer from the breast cancer (n=1). Of the two benign cases, one was diagnosed through surgery as localized pancreatic abscess and the other clinically as benign lymph node enlargement associated with chronic pancreatitis.
Sensitivity, specificity, accuracy, positive and negative predictive value of EUS-FNA were analyzed and are presented in Table 4.
Table 4.
Utility of EUS-FNA in Establishing the Diagnosis of Solid Pancreatic and Peripancreatic Lesions
EUS-FNA, endoscopic ultrasound guided fine needle aspiration.
3. Complications associated with EUS-FNA
No one had evidence of clinical pancreatitis, but in 11 cases (8%, 11/139), serum amylase was raised threefold or more of normal value the next day. Five of the 11 patients underwent ERCP on the same day.
4. Factors having influence on the accuracy of EUS-FNA
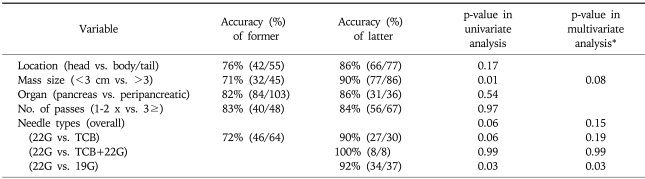
Factors having influence on the accuracy of EUS-FNA was analyzed (Table 5). Lesions smaller or greater than 3 cm showed accuracy of 71% (32/45) or 90% (77/86), respectively. And by the needle type, 22G needle showed accuracy of 71% while 19G and TCB needle showed accuracy of 92 and 90%, respectively. By univariate analysis, lesion size (<3 cm vs. ≥3 cm) was found as statistically significant factor with p-value of 0.01, and needle type showed statistical tendency with p-value of 0.06. Multivariate analysis with these factors revealed lesion size to have borderline significance with p-values 0.08, while needle type showed tendency but without statistical significance with p-value of 0.15.
Table 5.
Analysis of Factors Having Influence on the Accuracy of EUS-FNA
EUS-FNA, endoscopic ultrasound guided fine needle aspiration.
*Included variables were location, size, and needle type; n=126.
DISCUSSION
In this study, sensitivity, specificity, and accuracy of EUS-FNA in the diagnosis of pancreatic and peripancreatic mass lesion was 82%, 89%, and 83%, respectively. This is not substantially different from those of previous studies published abroad reporting sensitivity of 72-94% and specificity of 76-91%.3,7-10 However, inadequacy rate in our study was 12% which is higher than 1.5-4.0% inadequacy rate reported by previous studies. This difference arose because most previous studies unlike ours performed EUS-FNA with the help of on-site cytopathologic evaluation. Inadequacy rate in studies in which on-site cytopathologic evaluation was not available ranged from 9-20% which is similar to our results.5,6 In our study, negative predictive value was low at 46% but negative predictive value has been reported to be low in previous studies also. The low negative predictive value may be reflecting the fact that pancreas cancer comprised for most of lesions. Pancreas cancer when compared with other tumors is associated with more severe inflammation and fibrosis in the surrounding tissue, and this may cause malignant cells to be not detected even in cytologically adequate specimen.2,8 Therefore, one needs to be very cautious when interpreting a negative result of FNA in a pancreas lesion, and clinical, radiological, and EUS findings needs to be considered together before concluding the lesion is actually benign. If malignancy can not be ruled out, additional biopsy and careful clinical follow up is thought to be necessary.
In our study, when analyzed for factors influencing the accuracy of EUS-FNA, multivariate analysis showed lesion size to have borderline significance with p-value of 0.08. Influence of lesion size on the accuracy of EUS-FNA is somewhat controversial. Although some studies have found no difference in the accuracy of EUS-FNA for lesions greater or less than 3 cm in size,9,11 others including this study showed increasing size may be related to increased yield of FNA.12 Further studies will be needed to clarify the influence of lesion size on the accuracy of EUS-FNA.
Most studies regarding EUS-FNA have been performed with 22G fine needle, and more recently, 19G Trucut needle have been introduced. In this study, three different needle types including 19G fine needle was used, and their respective accuracies were compared. The result showed that needle type other than 22G fine needle was associated with higher accuracy although significance was reduced after multivariate analysis that included other factors such as lesion size and location. Trucut biopsy has the advantage of providing larger tissue specimen and is useful in diagnosing diseases where histological analysis is required.13-15 At the same time, however, Trucut biopsy is limited by not being able to sample pancreas head lesion due to curvature of scope tip when approaching from second portion of duodenum. On the other hand, sampling of pancreas head lesion was possible by 19G fine needle, and in this study, 10 out of 37 lesions were located in the head portion with success rate of 80%. There has been fewer report of 19G fine needle than regarding Trucut biopsy. In one report, 19G needle was found to be useful for diagnosing mediastianal and intrabdominal lymph node enlargement including lymphoma by providing core biopsy in addition to aspiration cytology.16 Previous studies have attempted to increase the yield of EUS-FNA by incorporating Trucut biopsy with 22G FNA, especially in the setting where on-site cytopathology is not available.6,13,17 Although limited in number (n=10), our study also showed cases in which Trucut biopsy and 22G fine needle was performed together, accuracy was 100%. Although not significant by multivariate analysis, result of our study suggests that appropriate use of different needles may be one means to improve sample adequacy especially in the clinical setting where on-site cytopathological evaluation is not available. We are currently performing a prospective study with the use of 19G needle.
Although vast majority of malignancy did consist of pancreas adenocarcinoma, there were a few cases in which final pathologic diagnosis was not pancreas adenocarcinoma despite initial imaging seeking differentiation with pancreatic cancer. In the present study, there were 5 cases of metastatic pancreatic tumor, and the primary focus was breast cancer (n=1), lung cancer (n=2; NSCLC1, SCLC1), renal cell cancer (n=1), leiomyosarcoma (n=1), and thymic cancer (n=1). In the past, pancreatic mass, with its difficult location for biopsy and concern about tumor seeding along the needle tract, was usually operated on without tissue confirmation when deemed resectable by imaging, and similarly, chemotherapy was not infrequently given based solely on imaging diagnosis for nonresectable disease. However, considering the accuracy and the safety of EUS-FNA, pathologic diagnosis of pancreatic lesion should be confirmed just like it is done for other gastrointestinal neoplasm.18,19
In summary, this is the largest Korean study thus far to report on the efficacy of EUS-FNA in the evaluation of pancreatic and peripancreatic lesions and factors influencing its success. This study is relevant to the Korean clinical setting where on-site cytopathologist is not available most of the time. Despite the absence of on-site cytopathologist, accuracy of EUS-FNA in our study was over 80%. Such good result along with its excellent safety makes us believe EUS-FNA should be considered as part of the algorithm for the diagnosis of pancreatic and peripancreatic mass lesions. It can provide a tissue diagnosis in most patients, including those in whom the diagnosis has proved difficult to establish, and thus guide decisions on their subsequent management.
In addition, our study showed despite the retrospective analysis that Trucut biopsy and 19G needle tended to have higher diagnostic rate, and this may be further explored as one of means to improve accuracy in the clinical setting where on-site cytopathologist is not available.
References
- 1.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253–258. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 2.Chang KJ. Maximizing the yield of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:S28–S34. doi: 10.1016/s0016-5107(02)70083-7. [DOI] [PubMed] [Google Scholar]
- 3.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 4.Cha JM, Seo DW, Lee SS, et al. The value of endoscopic ultrasound guided fine needle aspiration biopsy in the differential diagnosis of pancreatic mass. Korean J Gastrointest Endosc. 2005;31:147–154. [Google Scholar]
- 5.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 6.Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 8.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 9.Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–861. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 10.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VArdengh JC, Lopes CV, de Lima LF, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–3116. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savides TJ, Donohue M, Hunt G, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277–282. doi: 10.1016/j.gie.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 13.VAithal GP, Anagnostopoulos GK, Tam W, et al. EUS-guided tissue sampling: comparison of "dual sampling" (Trucut biopsy plus FNA) with "sequential sampling" (Trucut biopsy and then FNA as required) Endoscopy. 2007;39:725–730. doi: 10.1055/s-2007-966400. [DOI] [PubMed] [Google Scholar]
- 14.Ginès A, Wiersema MJ, Clain JE, Pochron NL, Rajan E, Levy MJ. Prospective study of a Trucut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest Endosc. 2005;62:597–601. doi: 10.1016/j.gie.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 15.Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005;62:417–426. doi: 10.1016/j.gie.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–924. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 17.Storch I, Jorda M, Thurer R, et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006;64:505–511. doi: 10.1016/j.gie.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 18.Boudghène FP, Deslandes PM, LeBlanche AF, Bigot JM. US and CT imaging features of intrapancreatic metastases. J Comput Assist Tomogr. 1994;18:905–910. doi: 10.1097/00004728-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, Bassi P. Pancreatic metastases: CT assessment. Eur Radiol. 1997;7:241–245. doi: 10.1007/s003300050144. [DOI] [PubMed] [Google Scholar]