Abstract
Background/Aims
The protease inhibitors, nafamostat and gabexate, have been used to prevent pancreatitis related to endoscopic retrograde cholangiopancreatography (ERCP). In vitro, nafamostat inhibits the pancreatic protease activities 10-100 times more potently than gabexate. We evaluated the efficacy of nafamostat for prophylaxis against post-ERCP pancreatitis in comparison with gabexate.
Methods
Five hundred patients (208 patients in the nafamostat-treated group and 292 in the gabexate-treated group) were analyzed retrospectively after selective exclusion. The incidences of pancreatitis and hyperamylasemia after the ERCP were compared between the nafamostat and gabexate groups.
Results
The incidences of acute pancreatitis and hyperamylasemia were 9.1% and 40.9%, respectively, in the nafamostat-treated group, and 8.6% and 39.4% in the gabexate-treated group. The frequencies of post-ERCP pancreatitis and hyperamylasemia did not differ significantly between the two groups, Post-ERCP pancreatitis in two group did not vary according to the different ERCP procedures. The mean serum amylase level at 6 h after ERCP was significantly lower in the nafamostat-treated group than in the gabexate-treated group (p=0.020). However, the difference in serum amylase level did not persist at 18 h and 36 h post-ERCP.
Conclusions
Administration of nafamostat before ERCP was not inferior to gabexate in protecting against the development of pancreatitis.
Keywords: Gabexate, Nafamostat, Endoscopic retrograde cholangiopancreatography, Pancreatitis, Hyperamylasemia
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is an important procedure for the diagnosis and treatment of biliary and pancreatic conditions. Prospective studies show that acute pancreatitis occurs in 3-17% of cases following the ERCP and that the post-ERCP pancreatitis (PEP) is associated with substantial morbidity, and even with mortality.1-3 Although PEP in most cases are mild, 10% of cases progress to severe pancreatitis, resulting in prolonged hospital stays and life-threatening consequences.3
Several attempts have been made to minimize the occurrence and severity of PEP; identifying high-risk patients, developing less traumatic endoscopic interventions to limit pancreatic injury, and finding effective pharmacologic agents to administer prophylactically before ERCP. The efficacy of such agents (somatostatin, octreotide, diclofenac, indomethacin, and gabexate mesylate) to reduce the risk of PEP had been studied extensively.4-7
Although the pathophysiologic precipitant of acute pancreatitis remains unclear, it has been demonstrated in an animal model which stimulation of exocrine pancreatic secretion leads to further deterioration of acute pancreatitis.8 Premature intracellular activation of proteolytic enzymes results in autodigestion, impaired acinar secretion, and local inflammatory responses.9
Nafamostat (FUT-175; 6-amidino-2-naphthyl p-guanidino-benzoate dimethane-sulfonate) is a low molecular weight protease inhibitor that inhibits serine proteases (such as trypsin), kallikrein, C1r and C1s, complement activation, thrombin, and plasmin.10 Nafamostat had been shown to be 10-100 times more potent than gabexate in in vitro experiments,11 and it was more effective than gabexate in the treatment of necrotizing pancreatitis in a rat model.12 Nafamostat has been used for the treatment of severe pancreatitis or prophylaxis against pancreatitis after endoscopic procedures, and no serious side effects were reported. However, nafamostat has scarcely been studied for the prophylaxis against post-ERCP pancreatitis.
The present study was designed to evaluate retrospectively the efficacy of intravenous nafamostat in preventing PEP and hyperamylasemia in comparison with gabexate.
MATERIALS AND METHODS
1. Patients
Between 2005 and 2007, 1,074 patients undergoing ERCP in the gastrointestinal endoscopy unit of Kangnam St. Mary's Hospital (a tertiary referral center) were consecutively enrolled and analyzed retrospectively. Nafamostat or gabexate was administered intravenously to 500 patients to prevent post-ERCP pancreatitis. Patients with naïve ampulla of Vater were included. Exclusion criteria were as follows: age<18 years, previous surgery (Billroth II gastrectomy, Roux-en-Y anastomosis, and choledochojejunostomy), previous sphincterotomy or biliary stenting, stenting into pancreatic duct, acute pancreatitis before ERCP, and combined use of octreotide or somatostatin.
This study was approved by the Institutional Review Board of our hospital. Patient's anonymity was preserved and the study protocol confirmed to the Declaration of Helsinki as revised in Edinburg in 2000.
2. Administration of nafamostat or gabexate and follow-up
Six hundred mg of gabexate (Foy®; Dong-A Pharm, Seoul, Korea) or 50 mg of nafamostat (Futhan®; SK Chemical Life Science, Seoul, Korea) was dissolved in 5% glucose solution and administered by continuous intravenous infusion beginning 30 minutes before the endoscopy session and continuing for 12 hours afterwards. Therapy with antibiotics, analgesics, and sedatives was permitted, whereas concomitant therapy with somatostatin or octreotide was a basis for exclusion. Benzodiazepines, anti-spasmodic agents, and non-narcotic analgesics, alone or in combination, were also allowed. Ioxitalamic acid (Telebrix®, Guerbet, Roissy CdG Cedex, France), a water-soluble, monomeric, ionic contrast medium was used during the endoscopic maneuvers. One experienced senior endoscopist, with a career experience of over 1,000 ERCPs and an annual ERCP caseload of over 300, directly performed or supervised all the procedures. If the cannulation or a therapeutic procedure by a fellow-in-training was unsuccessful, the supervisor assumed the procedure. After endoscopy, patients were to in fasting state for at least 18 hours. Serum amylase was measured before endoscopy and 6, 18, and 36 hours afterward. The presence of abdominal pain attributable to the pancreas and the use and type of analgesic therapy at those times were evaluated.
3. Definition
The definition of pancreatitis was based on the consensus criteria.13 Post-ERCP pancreatitis was defined as the followings: a newly developed or increased abdominal pain within 24 hours after ERCP requiring analgesic agents, and the elevation of serum amylase level at least three times of normal upper limit around 18 hours after the procedure (the next morning). The severity was graded mild when hospitalization lasted 2 to 3 days, moderate when 4 to 10 days, and severe when hospitalization was prolonged for more than 10 days or any of the following occurred: hemorrhagic pancreatitis, pancreatic necrosis, pancreatic pseudocyst, or a need for percutaneous drainage or surgery. Hyperamylasemia was defined as an elevation of serum amylase level in the morning after ERCP above the upper limit of normal if basal enzyme level was normal or as any further elevation in the enzyme if basal enzyme level exceeded the upper limit of normal. Visualization of the entire pancreatic duct by contrast injection was regarded as pancreatic duct injection. Precut was performed at periampullary area and infundibulotomy was not performed.
4. Statistical analysis
The chi-square test was used for comparisons of categorical data and student t-test was used for comparisons of continuous data. Serum amylase data after ERCP were subjected to student t-test at each time after ERCP and to analysis of variance with repeated measures (repeated measures ANOVA) through the follow-up duration. The statistical analyses were performed using SPSS, version 14.0 (SPSS Inc., Chicago, IL, USA). p-values<0.05 were considered significant.
RESULTS
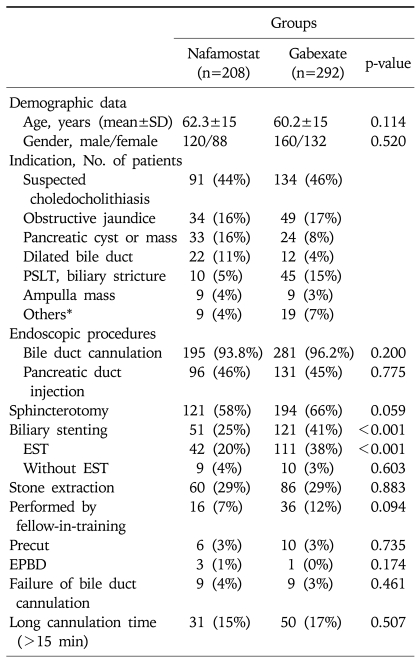
Five hundred patients were enrolled in the study after exclusion criteria were applied; 208 patients were in the nafamostat group and 292 patients in the gabexate group. The mean age was 61.1±15.0 years and 220 (44%) patients were females (Table 1). The most common indication for ERCP was suspected calculi in the common bile duct (45%) or obstructive jaundice (17%). Pancreatic cyst or mass was the reason of ERCP in 11% of the patients, biliary stricture of post-transplantation liver in 11%, and dilated biliary tree seen on the imaging study in 7%. The nafamostat and gabexate groups were similar in respect to patient demographics and the common distribution of indications for the procedure. In details of endoscopic procedures, two groups showed no difference except biliary stenting (p<0.001).
Table 1.
Baseline Characteristics of the Patients
SD, standard deviation; PSLT, post-liver transplantation; EST, endoscopic sphincterotomy; EPBD, endoscopic papillary balloon dilatation.
*Intrahepatic lithiasis (n=10), bile leakage (n=8), chronic pancreatitis (n=6), acute cholangitis (n=3), and suspected sphincter of Oddi dysfunction (n=1).
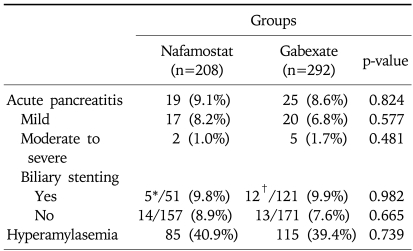
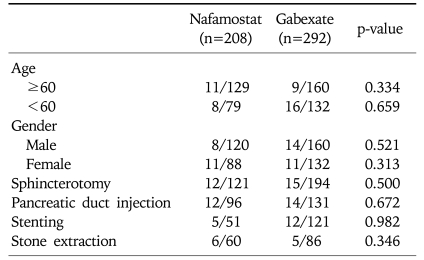
Table 2 shows the incidence of acute pancreatitis after ERCP. The overall incidence of acute pancreatitis was 8.8% (9.1% in the nafamostat group and 8.6% in the gabexate group). Although acute pancreatitis after ERCP occurred slightly more often in the nafamostat group than the gabexate group, it was not statistically significant. The overall incidence of hyperamylasemia was 40.0% (40.9% in the nafamostat group and 39.4% in the gabexate group), and the incidence also did not show significant difference in two groups. Among 44 patients in whom developed pancreatitis, four patients in the gabexate group and one patient in the nafamostat group had moderate pancreatitis, and one patient in each group had severe pancreatitis. Moderate to severe pancreatitis occurred slightly more in the gabexate group, however it had no significance. Because the gabexate group underwent more biliary stenting than the nafamostat group, the incidences of PEP were compared in the subgroups to eliminate the inclusion bias. There were no difference in the occurrence of PEP between the nafamostat group and the gabexate group according to biliary stenting. The different types of endoscopic procedures performed and their associated rates of incidence of pancreatitis are shown in Table 3. The incidence of pancreatitis was not significantly different in each subgroup according to endoscopic procedures.
Table 2.
Incidence of Acute Pancreatitis and Hyperamylasemia after ERCP
ERCP, endoscopic retrograde cholangiopancreatography.
*All five patients underwent endoscopic sphincterotomy; †Ten patients underwent endoscopic sphincterotomy.
Table 3.
Incidence of Pancreatitis in the Two Subgroups
Data are number of patients with post-endoscopic retrograde cholangiopancreatography pancreatitis/total number of patients in the subgroups.
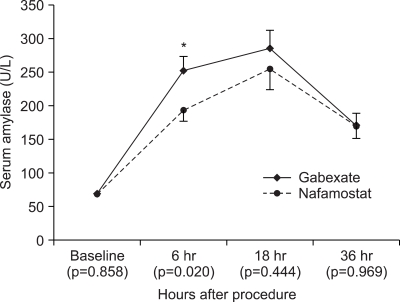
The mean amylase levels at baseline were 68.2 U/L in the nafamostat group and 68.9 U/L in the gabexate group (Fig. 1). After ERCP, the mean amylase levels at 6 hours in patients in the nafamostat and the gabexate groups were 192.5 U/L and 252.1 U/L. The amylase level in the nafamostat group was significantly lower than that in the gabexate group (p=0.020). The mean amylase level at 18 hours (the next morning) in the nafamostat group was also lower than that in the gabexate group (254.0 U/L and 285.7 U/L, respectively), but it was not statistically significant. Thirty six hours after ERCP the mean amylase level of two groups became very close, and the difference in the amylase level was only 1.0 U/L. The overall comparison of serum amylase values showed no difference through 36 hours after ERCP in two groups (repeated measures ANOVA, p=0.296).
Fig. 1.
Serial changes in serum amylase (mean and SE values) before and after ERCP in the nafamostat- and gabexate-treated groups. The mean 6-h post-ERCP serum amylase level of the gabexate-treated group was significantly higher than that of the nafamostat-treated group; however, the mean post-ERCP amylase level did not differ significantly between the two groups thereafter. The overall post-ERCP amylase levels in the two groups did not differ up to 36 h after ERCP (repeated-measures analysis of variance, p=0.296).
ERCP, endoscopic retrograde cholangiopancreatography.
*p=0.020.
Before ERCP, 42 patients had baseline serum amylase concentrations greater than the upper limit of normal, but less than 3 times the normal limit (13 patients in the nafamostat group and 29 patients in the gabexate group). Twelve patients had a mild increase in the amylase level and five patients (11.9%) had acute pancreatitis after ERCP. It was not different from the incidence of PEP in normal baseline amylase group.
Nine of PEP patients had radiologic evaluation of the pancreas 1-6 days after the ERCP. Seven patients had normal findings of pancreas. One patient showed peripancreatic infiltration with pancreas swelling on abdominal computerized tomography four days after ERCP. The remaining one patient showed a radiologic finding of severe pancreatitis on abdominal computerized tomography one day after ERCP; diffusely enlarged pancreas, large amount of peripancreatic fluid, and ascites at the perihepatic, pericholecystic space, and right paracolic gutter. Follow-up abdominal computerized tomography was taken 8 days after ERCP in this patient, and it revealed interval partial regression of pancreatic swelling and peripancreatic fluid collection. The time elapsed until amylase normalized after ERCP in PEP patients was 2-17 days (mean±SD, 3.4±2.4 days) except for one patient. One patient with chronic pancreatitis had mildly elevated serum amylase level until six months after ERCP. No patients died secondary to acute pancreatitis.
DISCUSSION
In the present study, the nafamostat group showed low amylase level in the early phase after ERCP. However, the incidence of post-ERCP pancreatitis was not different between the nafamostat and gabexate group. The incidence of pancreatitis was also not different in subgroups according to different endoscopic procedures.
Every endoscopist performing ERCP wish to avoid PEP, especially severe PEP. There are two approaches to avoid or reduce PEP. The first approach is the preventive administration of agents to decrease pancreatic protease activity. The second approach is to determine the risk factors for PEP, including patient-related and procedure-related factors. Many studies have focused on these issues. Prevention of PEP with gabexate has been the subject of several studies. Some have demonstrated that gabexate is effective in reducing the incidence of post-ERCP pancreatitis,1,14-16 yet others did not find any benefit in administering the drug.17,18 In the present study, the incidences of hyperamylasemia and acute pancreatitis in the gabexate group were 39.4% and 8.6%, respectively, which are similar or a little higher than other prospective studies.1,14,17,18 Patient selection, indications for ERCP, therapeutic maneuvers performed, and different modalities of administering the drug are likely to explain the different results.
The autodigestion of the pancreatic parenchyma by aberrantly activated proteases with subsequent inflammation and tissue destruction are thought to be crucial in the development of acute pancreatitis. These early events in the course of this disease had been found by previous experimental studies in which the activation of trypsin resulting from the fusion of zymogen granules and lysosomes in acinar cells, and the premature activation of trypsinogen in the extracellular matrix was proven to be the most important events.19,20 As a protease inhibitor and a highly diffusible molecule, nafamostat may be protective against intracellular trypsin activation. Once trypsin is activated, nafamostat may also play a protective role because it inhibits the activation of other proteases. Nafamostat is a very safe medication and the adverse events are few like gabexate. If any, they are mild and self-limiting conditions such as mild nausea, vomiting, and diarrhea. In present study, there were no life-threatening or serious adverse events which required treatment following the administration of nafamostat.
The incidence of PEP in the gabexate group was slightly lower (0.5%) than in the nafamostat group, and it was not statistically significant. When α is 0.05, the lower one-sided 95% confidence interval for difference of pancreatitis incidence was calculated as -3% in the present study.21 If the specific limit for the least relevant difference of PEP incidence is ±5%, the calculated lower confidence interval (-3%) lies above the specific limit for the least relevant difference. It indicates that the effect of nafamostat is not inferior to the effect of gabexate for the prevention of PEP. In addition, type 2 error risk overlooking the effect of the least relevant difference (5%) was estimated at 0.041 (the power was 95.9%).21 Therefore, our result shows that the effect of nafamostat was not inferior to gabexate with a sufficient power.
Among 42 patients with slightly elevated serum amylase concentrations at baseline without abdominal pain, 12 patients (28.6%) showed a mild increase of amylase level after ERCP and PEP occurred in 5 patients (11.9%). The incidence was not significantly different from that of the patients with normal amylase concentration at baseline. This finding agrees with another study involving patients with elevated serum enzyme levels at baseline.14
The limitation of this study is that it was not a prospective study and there was no comparison with placebo group. Some of recent some meta-analysis showed prophylactic use of gabexate is ineffective in reducing pancreatitis.3,7,22 Because our results indicate that nafamostat was not inferior to gabexate in preventing PEP, the efficacy of nafamostat for prophylaxis pancreatitis may not be clearly confirmed without comparison with placebo. Although the patients with no preventive treatment existed in our study population, the number of patients was too small to be compared with other groups. One study which was performed in the same country as our study and had similar including population showed that the incidence of PEP in the placebo group was 9.9% being a little higher than our result (8.8%).23 However, it could not be said that this difference had significance. Therefore, the further study would be needed in Asian countries in which gabexate and nafamostat was frequently used.
In conclusion, administration of nafamostat before ERCP was not inferior to gabexate in protecting against the development of pancreatitis. Further prospective randomized study would be performed to compare with placebo in the future.
References
- 1.Manes G, Ardizzone S, Lombardi G, Uomo G, Pieramico O, Porro GB. Efficacy of postprocedure administration of gabexate mesylate in the prevention of post-ERCP pancreatitis: a randomized, controlled, multicenter study. Gastrointest Endosc. 2007;65:982–987. doi: 10.1016/j.gie.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 2.Cheon YK, Cho KB, Watkins JL, et al. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007;66:1126–1132. doi: 10.1016/j.gie.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Zheng M, Chen Y, Yang X, Li J, Zhang Y, Zeng Q. Gabexate in the prophylaxis of post-ERCP pancreatitis: a metaanalysis of randomized controlled trials. BMC Gastroenterol. 2007;7:6. doi: 10.1186/1471-230X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–1791. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 5.Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 6.Andriulli A, Caruso N, Quitadamo M, et al. Antisecretory vs. antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. JOP. 2003;4:41–48. [PubMed] [Google Scholar]
- 7.Andriulli A, Leandro G, Federici T, et al. Prophylactic administration of somatostatin or gabexate does not prevent pancreatitis after ERCP: an updated meta-analysis. Gastrointest Endosc. 2007;65:624–632. doi: 10.1016/j.gie.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Pezzilli R, Romboli E, Campana D, Corinaldesi R. Mechanisms involved in the onset of post-ERCP pancreatitis. JOP. 2002;3:162–168. [PubMed] [Google Scholar]
- 9.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845–864. doi: 10.1016/s0016-5107(04)00353-0. [DOI] [PubMed] [Google Scholar]
- 10.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981;661:342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki M, Ino Y, Motoyoshi A, et al. Pharmacological studies of FUT-175, nafamostat mesilate: V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J Pharmacol. 1986;41:155–162. doi: 10.1254/jjp.41.155. [DOI] [PubMed] [Google Scholar]
- 12.Keck T, Balcom JH, Antoniu BA, et al. Regional effects of nafamostat, a novel potent protease and complement inhibitor, on severe necrotizing pancreatitis. Surgery. 2001;130:175–181. doi: 10.1067/msy.2001.115827. [DOI] [PubMed] [Google Scholar]
- 13.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 14.Cavallini G, Tittobello A, Frulloni L, Masci E, Mariana A, Di Francesco V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography: Gabexate in digestive endoscopy--Italian Group. N Engl J Med. 1996;335:919–923. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- 15.Masci E, Cavallini G, Mariani A, et al. Comparison of two dosing regimens of gabexate in the prophylaxis of post-ERCP pancreatitis. Am J Gastroenterol. 2003;98:2182–2186. doi: 10.1111/j.1572-0241.2003.07698.x. [DOI] [PubMed] [Google Scholar]
- 16.Testoni PA, Mariani A, Masci E, Curioni S. Frequency of post-ERCP pancreatitis in a single tertiary referral centre without and with routine prophylaxis with gabexate: a 6-year survey and cost-effectiveness analysis. Dig Liver Dis. 2006;38:588–595. doi: 10.1016/j.dld.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Andriulli A, Clemente R, Solmi L, et al. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002;56:488–495. doi: 10.1067/mge.2002.128130. [DOI] [PubMed] [Google Scholar]
- 18.Andriulli A, Solmi L, Loperfido S, et al. Prophylaxis of ERCP-related pancreatitis: a randomized, controlled trial of somatostatin and gabexate mesylate. Clin Gastroenterol Hepatol. 2004;2:713–718. doi: 10.1016/s1542-3565(04)00295-2. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig W, Jimenez RE, Werner J, et al. Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology. 1999;117:717–725. doi: 10.1016/s0016-5085(99)70466-x. [DOI] [PubMed] [Google Scholar]
- 20.Steer ML. Frank Brooks memorial lecture: the early intraacinar cell events which occur during acute pancreatitis. Pancreas. 1998;17:31–37. doi: 10.1159/000026152. [DOI] [PubMed] [Google Scholar]
- 21.Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol. 2007;46:947–954. doi: 10.1016/j.jhep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Rudin D, Kiss A, Wetz RV, Sottile VM. Somatostatin and gabexate for post-endoscopic retrograde cholangiopancreatography pancreatitis prevention: meta-analysis of randomized placebo-controlled trials. J Gastroenterol Hepatol. 2007;22:977–983. doi: 10.1111/j.1440-1746.2007.04928.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee KT, Lee DH, Yoo BM. The prophylactic effect of somatostatin on post-therapeutic endoscopic retrograde cholangiopancreatography pancreatitis: a randomized, multicenter controlled trial. Pancreas. 2008;37:445–448. doi: 10.1097/MPA.0b013e3181733721. [DOI] [PubMed] [Google Scholar]