Abstract
Background/Aims
Although early recognition and treatment with effective antibiotics have lead to improvements in the prognosis of patients with spontaneous bacterial peritonitis (SBP), it remains to be a serious complication in cirrhotic patients. This study was designed to evaluate the clinical manifestations and prognosis of patients with liver cirrhosis and SBP in Korea.
Methods
This was a multicenter retrospective study examining 157 episodes of SBP in 145 patients with cirrhosis. SBP was diagnosed based on a polymorphonuclear cell count in ascitic fluid of >250 cells/mm3 in the absence of data compatible with secondary peritonitis.
Results
The mean age of the cohort was 56 years, and 121 (77%) of the 157 episodes of SBP occurred in men. Microorganisms were isolated in 66 episodes (42%): Gram-negative bacteria in 54 (81.8%), Gram-positive in 11 (16.7%), and Candida in 1. Isolated Gram-negative organisms were resistant to third-generation cephalosporin in 6 cases (17%), to ciprofloxacin in 11 (20.8%), and to penicillin in 33 (62.3%). The treatment failure and in-hospital mortality rates were 12.1% and 21%, respectively. A high Model of End-Stage Liver Disease (MELD) score, SBP caused by extended-spectrum β-lactamase-producing organisms, and hepatocellular carcinoma were independent prognostic factors of high in-hospital mortality.
Conclusions
SBP remains to be a serious complication with high in-hospital mortality, especially in patients with a high MELD score.
Keywords: Liver cirrhosis, Peritonitis, Prognosis, Resistance
INTRODUCTION
Patients with liver cirrhosis are vulnerable to bacterial infections due to defects in various host defense mechanism.1,2 Spontaneous bacterial peritonitis (SBP) is the most common infection in cirrhotic patients followed by urinary tract infections, pneumonia, and bacteremia.3 SBP occurs in 8-27% of hospitalized patients with cirrhosis and ascites.1,4 Although the precise pathogenesis of SBP has not been clearly defined, overgrowth and translocation of intestinal bacteria, especially aerobic Gram-negative bacilli, appears to be the most important step in the pathogenic process.5-8 In addition, porto-systemic shunt, a low level of serum complement, and decreased neutrophil chemotaxis may contribute to the development of bacteremia, and finally to the bacterial colonization of ascites.9-11 A high Child-Pugh score, a low protein level in ascites, and a high level of serum bilirubin have been previously identified to be risk factors for the development of SBP.5,12-14
When SBP was initially described in the English medical literature in the 1960s, its prognosis was extremely poor, with in-hospital mortality of 100%.15,16 However, recently, the outcome of SBP has been considerably improved due to early diagnosis and the introduction of effective antibiotics. Currently, the infection resolution rates are 77-91% and in-hospital mortalities 21-31%.17-21 In addition, the appropriate management of renal impairment in end-stage liver disease has been shown to further ameliorate the prognosis of SBP; a recent study concluded that the intravenous infusion of albumin in combination with cefotaxime reduced in-hospital mortality to 10%.22
Together with its grave short-term prognosis, recurrence rate after the resolution of an acute event may be as high as 70% during the subsequent year, and this remains as a challenging issue in the management of SBP.13 Thus, the oral administration of antibiotics such as quinolones and trimethoprim/sulfamethoxazole have been attempted to selectively achieve the intestinal decontamination of mainly aerobic Gram-negative bacilli, and such trials have significantly reduced the rate of recurrence and initial occurrence of SBP.23-26 However, prolonged antibiotic prophylaxis is accompanied by risks of altering bacterial species and of selecting antibiotic resistant organisms within the gut25,27 and may ultimately lead to modifications in causative microorganisms. Actually in a recent study, half of SBP cases despite the long-term norfloxacin prophylactic use were found to be caused by quinolone-resistant Gram-negative bacilli whereas only 16% of those not receiving prophylactic antibiotics were infected by the resistant organism.3
In addition, the misuse and overuse of broad spectrum antibiotics in general clinical practice has increased antibiotic-resistant microorganisms in many countries, which has raised concerns about potentially serious future clinical problems.28-30 In fact, one Korean study reported that the prevalences of microorganisms resistant to third-generation cephalosporins and quinolones were increasing in cirrhotic patients with SBP and that they exerted a negative influence on prognosis.31
In view of these expected changes regarding the cause and outcome of SBP, this multicenter retrospective study was performed to investigate the current status of SBP in Korea with specific regard to its microbiological features, treatment response and prognosis.
MATERIALS AND METHODS
1. Patients
All patients with liver cirrhosis hospitalized for SBP treatment to 9 tertiary medical centers between June 2005 and May 2006 were included in this retrospective study. A diagnosis of cirrhosis was established using histologic criteria or by clinical and ultrasonographic findings. SBP was diagnosed based on a polymorphonuclear (PMN) cell count in ascitic fluid of ≥250 cells/mm3 in the absence of clinical and radiological findings suggestive of secondary peritonitis.32 Secondary bacterial peritonitis was suspected when two or more organisms were isolated from ascites or when the ascitic PMN count was ≥250 cells/mm3 and at least two of the following three ascitic fluid findings were present: glucose <50 mg/dL; total protein >1 g/dL; and lactate dehydrogenase>the upper limit of normal for serum.33 SBP was considered as community acquired when it was present at admission, and as hospital-acquired when it was developed >48 hours after admission.34 Patients who had taken oral quinolone for more than 1 month before the episode of SBP were allocated to the previous prophylaxis group. Renal impairment at SBP diagnosis was defined as a serum creatinine >1.5 mg/dL.
2. Bacterial culture
Diagnostic paracentesis with ascitic fluid culture was performed in all patients with ascites who developed local symptoms or signs suggestive of peritoneal infection, systemic signs of infection such as fever or leukocytosis, or clinical deterioration without any obvious precipitating factor. When an ascitic PMN count was greater than or equal to 250 cells/mm3, blood culture was performed.
Ascitic fluid and peripheral blood were inoculated into aerobic and anaerobic blood-culture bottles (BACTEC NR 600; Becton Dickinson, Sparks, MD, USA) at bedside.35 At least 10 mL of ascitic fluid was inoculated into each bottle for aerobic and anaerobic cultures.35 All isolated organisms in culture were tested for antimicrobial susceptibility according to the diffusion method.36
3. Treatment response and prognosis
SBP resolution was considered when all signs of infection had disappeared and PMN count in ascitic fluid had reduced to <250 cells/mm3. Treatment failure was defined as a decrease of <25% in PMN count after 48 hours of treatment or a persistent PMN count of >250 cells/mm3 on the 7th day of treatment, a positive culture after 48 hours of treatment, failure to continue antibiotic treatment, or persistent clinical signs of infection. Patients who died before follow-up paracentesis for the assessment of treatment effect were also classified as having treatment failures. In-hospital mortality was defined as death from any cause during hospitalization.
4. Statistical analysis
Data are expressed as means±standard deviation (SD). Comparisons between groups were performed using the Student's t test for quantitative data and using the χ2 test or Fisher's Exact Test for categorical data. Cumulative mortality and cumulative SBP recurrence rate were calculated using Kaplan-Meier analysis.
Univariate analysis was used to identify factors predicting in-hospital mortality. Tested variables included age, sex, cause of cirrhosis, concomitant hepatocellular carcinoma (HCC), SBP, cirrhosis-related clinical and laboratory data obtained at the time of diagnosis of infection (including Child-Pugh and model for end-stage liver disease [MELD] scores37), other standard laboratory data, and source of SBP infection (community vs nosocomial). The MELD score was calculated using the following formula according to the method used by the United Network of Organ Sharing (http://www.unos.org): 9.57×loge (creatinine, mg/dL)+3.78×loge (bilirubin, mg/dL)+11.20×loge (INR)+ 6.43. Variables that had statistical significance (p<0.05) by univariate analyses were subjected to multivariate analysis using a step-wise binary logistic regression procedure to identify independent predictors of survival. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to determine significance by multivariate analysis. p-values of <0.05 were considered statistically significant. All analyses were performed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
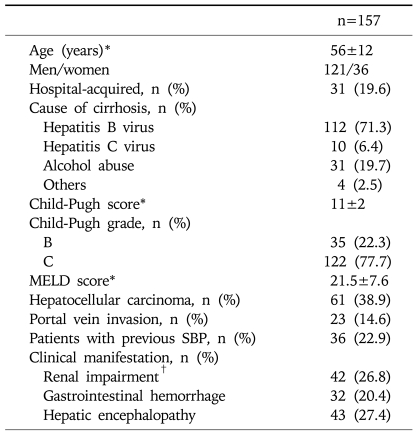
This study included 157 episodes of SBP which developed in 145 patients with liver cirrhosis. Baseline characteristics of the enrolled cases at SBP diagnosis are presented in Table 1. Mean patient age was 56±12 years and males predominated (77.1%). The most common cause of liver cirrhosis was chronic hepatitis B (71.3%), which was followed by alcohol abuse (19.7%) and chronic hepatitis C (6.4%). According to the Child-Pugh classification, 22.3% and 77.7% were grades B and C, respectively; no patients had Child-Pugh grade A. The mean MELD score was 21.5±7.6. At time of SBP diagnosis, 61 cases (38.9%) had HCC, and portal vein thrombosis was noted in 23 cases (14.6%). Thirty-six cases (22.9%) had a history of at least one episode of previous SBP and 10 (27.8%) had received prophylactic antibiotics to prevent SBP recurrence. Twenty-nine cases (18.5%) were nosocomial and 128 cases were community-acquired.
Table 1.
Baseline Characteristics of the Study Cohort
MELD, model for end-stage liver disease; SBP, spontaneous bacterial peritonitis.
*Mean±SD; †Defined as a serum creatinine >1.5 mg/dL.
2. Isolation of causative organisms and antibiotic-resistance
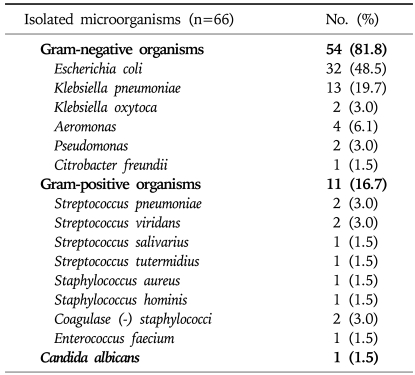
Causative organisms were isolated from ascitic fluid or peripheral blood in 66 cases (42%). Table 2 presents a profile of the organisms isolated. Gram-negative organisms accounted for 81.8% of all isolated organisms (54 of 66 cases). Escherichia coli was the most frequently isolated organism (32 of the 66 cases, 48.5%), followed by Klebsiella pneumoniae (13 cases, 19.7%) and Aeromonas species (4 cases, 6.1%). Gram-positive strains were isolated in 11 cases (16.7%).
Table 2.
Profiles of Isolated Microorganisms of the Enrolled Patients
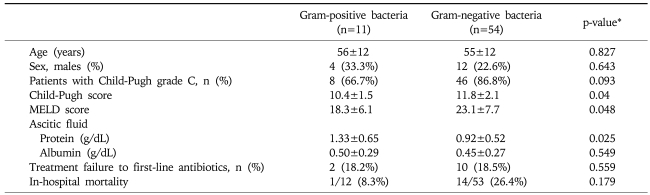
Distributions of patients by Child-Pugh grade were not significantly different between SBP patients with Gram-positive or Gram-negative bacteria (p=0.093). However, Child-Pugh and MELD scores were significantly lower in patients with SBP due to Gram-positive bacteria (Table 3).
Table 3.
Clinical and Laboratory Characteristics of the Patients, and Gram-stain Results
Data are presented as means±S.D. or n (%).
*p-values were determined using the Student's t-test or the chi-square test.
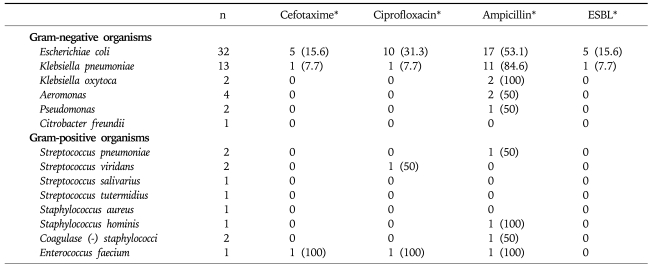
The antibiotic resistance profiles of the organisms isolated are shown in Table 4. Overall, 33 of 54 (61.1%) Gram-negative organisms isolated showed resistance to ampicillin and 11 of 54 (20.4%) to ciprofloxacin. Six cases (5 of 32 [15.6%] of Escherichia coli and 1 of 13 [7.7%] of Klebsiella pneumoniae) were resistant to cefotaxime and all of these 6 organisms also produced extended-spectrum β-lactamase (ESBL). However, all of these ESBL-producing organisms were sensitive to imipenem and meropenem.
Table 4.
Antibiotic Resistance Profiles of Isolated Organisms
ESBL, extended-spectrum β-lactamase.
*n (%).
3. Treatment outcome and prognosis
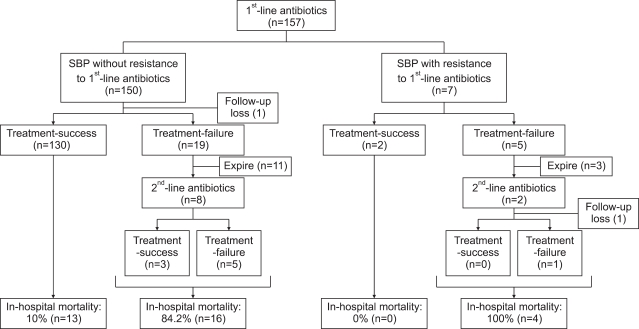
Treatment response and prognosis are presented in Fig. 1. Cefotaxime was the most frequently used antibiotics as a first-line treatment (150 cases, 95.5%), followed by cefoperazone (5 cases, 3.2%) and ciprofloxacin (2 cases, 1.3%). First-line antibiotics were used for 9±4 days and follow-up paracentesis was performed at 6±5 days after commencement of treatments. In 24 cases (15.3%), first-line treatment failed: 21 of 150 cases (14.0%) treated with cefotaxime, 2 cases treated with ciprofloxacin (100%) and one of 5 cases treated with cefoperazone (20%). Of these 24 cases, 14 patients (58.3%) died before commencement of second-line antibiotics (13 patients treated with cefotaxime and one patients treated with cefoperazone). In 2 patients treated with ciprofloxacin as a first-line treatment, cefotaxime was used as second-line antibiotic, and in 8 cases treated with cefotaxime as first-line treatment, meropenem (2 cases), imipenem (1), cefoperazone (1), ceftazidime (1), ceftriaxone (1), ciprofloxacin (1), and teicoplanin (1) were used as second-line antibiotics. Second-line treatment was successful only in 3 of 9 cases (33.3%). Multivariate analysis identified MELD score at admission (β, 0.123; OR, 1.131; 95% CI, 1.049-1.219; p=0.001) and SBP causing organism ESBL-producing organisms (β, 2.764; OR, 15.868; 95% CI, 2.335-107.843; p=0.005) as independent predictive factors of treatment failure to first-line antibiotics.
Fig. 1.
Treatment response and in-hospital mortality of enrolled cirrhotic patients with spontaneous bacterial peritonitis (SBP).
Thirty-three patients (21%) died during hospitalization: 30 of 150 patients treated with cefotaxime (20%), 1 of 2 patients treated with ciprofloxacin (50%), and 2 of 5 patients treated with ceftazidime (40%). The causes of in hospital mortality were hepatorenal syndrome (9 patients, 27.3%), gastrointestinal bleeding (3 patients, 9.1%) and infection (21 patients, 63.6%). In-hospital mortality was significantly greater for patients with SBP caused by ESBL-producing organisms (4 of 6 patients, 66.7%) than in the others (29 of 151 cases, 19.2%; p=0.005). Whereas 13 of 132 patients (9.8%) without treatment failure to first-line antibiotics died during hospitalization, 20 of 23 patients (87%) with treatment failure died (p<0.001).
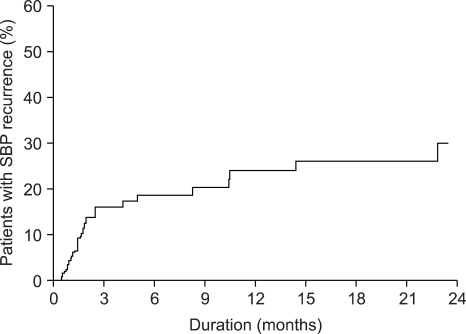
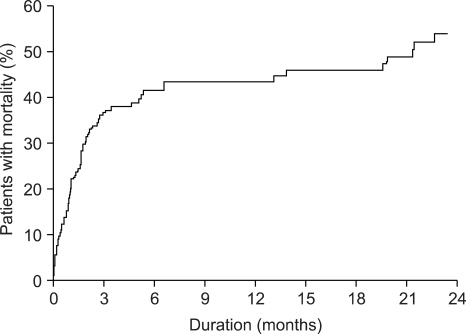
SBP recurred in 23 cases during 675±42 days of follow-up period in 124 survivors. The cumulative incidences of SBP recurrence at 3, 6, 9 and 12 months after discharge were 15.9%, 18.6%, 21.3% and 24%, respectively (Fig. 2). Thirty-five patients died during the follow-up period among 124 survivors due to hepatic failure (20 cases, 57.1%), gastrointestinal bleeding (7 cases, 20%), infection (6 cases, 17.1%), or an intracranial hemorrhage (1 case, 2.9%). Cumulative mortalities among survivors at 3, 6, 9 and 12 months after an episode of SBP were 17.8%, 24.5%, 27.1% and 27.1%, respectively, and overall mortality rates at 3, 6, 9 and 12 months were 36.3%, 41.5%, 43.5% and 43.5%, respectively (Fig. 3).
Fig. 2.
The cumulative incidence of recurrence of spontaneous bacterial peritonitis after resolution.
Fig. 3.
The cumulative incidence of mortality in all enrolled patients with spontaneous bacterial peritonitis.
4. Predictive factors of in-hospital mortality
By multivariate analysis, the followings were significant independent predictive factors of in-hospital mortality: MELD score at admission (β, 0.105; OR, 1.110; 95% CI, 1.049-1.175; p<0.001), SBP by an ESBL-producing organism (β, 2.568; OR, 13.043; 95% CI, 1.667-102.066; p=0.014), and HCC (β, 1.110; OR, 3.034; 95% CI, 1.247-7.387; p=0.014). When subgroup analysis was performed on 96 patients without HCC, MELD score at admission (β, 0.168; OR, 1.183; 95% CI, 1.079-1.297; p<0.001) and SBP by an ESBL-producing organism (β, 2.713; OR, 15.069; 95% CI, 1.193-190.280; p=0.036) retained their significances as independent predictive factors of in-hospital mortality.
DISCUSSION
This multicenter retrospective study was performed to evaluate the current clinical features and treatment outcomes of SBP in Korea. The major strength of this study was that enrollees were consecutive patients with cirrhosis and SBP who registered at nine medical centers distributed throughout Korea during 12 months period. Thus, our data is probably representative of the current clinical features of SBP in Korea.
According to our results, in-hospital mortality among cirrhotic patients with SBP was 21%, which is similar to that found by other recent studies.20,22,38 Although in-hospital mortality in this study was significantly lower than data published several decades ago,16,39,40 one in five of our patients with SBP could not survive during hospitalization. In the present study, MELD score at admission, the presence of HCC and SBP caused by ESBL-producing organisms were identified as independent predictive factors of in-hospital mortality. Of these factors, a high MELD score and the presence of hepatorenal carcinoma usually indicate the presence of more advanced liver disease.
A recent study suggested that culture-positive SBP in patients with cirrhosis is being caused more frequently by Gram-positive bacteria.31 According to this previous study, Gram-positive bacteria caused SBP in 25% of patients during 1998-1999 whereas in 59% during 2000-2002.31 However, the proportion of patients with SBP caused by Gram-positive strains was only 18.2%, and this finding concurs with a recently published single-center retrospective study of SBP in Korea.41 Therefore, it appears that profiles of causative organisms have remained substantially unchanged in Korea.
Another recent study suggested that SBP patients with Gram-positive bacteria are less frequently found in Child class C than SBP patients with Gram-negative bacteria, and that they have significantly higher ascitic fluid protein and albumin concentrations.42 In another study, it was suggested that community-acquired SBP develops in patients with less advanced liver disease and was frequently associated with Gram-positive bacteria.43 According to our results, although a smaller proportion of patients with SBP by Gram-positive bacteria was of Child-Pugh grade C, no statistical significance was observed. However, Child- Pugh score and MELD score were significantly lower in patients with SBP by Gram-positive bacteria in those with SBP by Gram-negative bacteria. In addition, ascitic fluid protein levels were significantly higher in patients with SBP by Gram-positive bacteria.
A recent study of Korean cirrhotic patients with SBP suggested that a significant increase in the prevalence of Gram-negative bacilli resistant to cefotaxime (from 7% to 28%) occurred between 1996 and 1999.31 However, in the present study, the prevalence of Gram-negative bacilli resistant to cefotaxime was only 17%. Furthermore, our data is consistent with recently published data on this issue.41 In addition, when we considered cases of SBP in which causative organisms were not isolated as SBP without resistance to cefotaxime, the cefotaxime resistance rate was only 3.8%. These findings indicate that cefotaxime should be considered an effective empiric treatment for SBP.
In the present study, the proportions of ESBL-producing Escherichiae coli (15.6%) and Klebsiella pneumoniae (7.7%) organisms were dissimilar from those found in a Korean study (33% and 25%, respectively, conducted in 1999).31 However, in-line with their results,31 the prognosis of patients with SBP by ESBL-producing organisms was dismal with a 66.7% in-hospital mortality rate. Since SBP by ESBL-producing organisms was an independent predictive factor of treatment failure and in-hospital mortality, rapid changes to appropriate antibiotics might be needed. ESBL-producing organisms are resistant to all antibiotics, except imipenem and meropenem. Therefore, when ESBL-producing organisms were to be identified cultured or when no clinical or laboratory improvement was to be noted on 3rd-generation cephalosporin, a prompt change of antibiotics to imipenem or meropenem should be considered.
The present study showed a high prevalence of Gram-negative organisms resistant to ciprofloxacin (20.8%), which was consistent with the findings of a recent study.31 Moreover, it has been suggested in several previous studies that the long-term administration of norfloxacin was highly effective in preventing SBP recurrence.23,44,45 Furthermore, a recently issued guideline recommends long- term prophylaxis with daily norfloxacin in patients who have survived an episode of SBP.46 However, norfloxacin prophylaxis could increase the frequency of SBP by quinolone-resistant bacteria, and some authors have reported the development of quinolone-resistant Gram-negative bacilli in the feces of cirrhotic patients on long-term norfloxacin prophylaxis.27,34 Quinolone is one of the most commonly used antibiotic in Korean out-patient clinics and therefore, the widespread use of quinolone might explain the high prevalence of quinolone-resistance observed in the present study, although only 8 cases had received norfloxacin prophylaxis according to a careful review of medical records.
We concluded that despite recent improvements in diagnosis and treatment, SBP was still associated with significant mortality in patients with cirrhosis, especially in patients with a high MELD score. In addition, the study indicated that cefotaxime was a good empiric treatment for SBP. However, in cases of treatment failure an antibiotic change to imipenem or meropenem should be considered.
ACKNOWLEDGEMENTS
This work was supported by a grant form Ministry for Health, Welfare and Family Affairs, Republic of Korea (No.A050021).
References
- 1.Runyon BA. Spontaneous bacterial peritonitis: an explosion of information. Hepatology. 1988;8:171–175. doi: 10.1002/hep.1840080131. [DOI] [PubMed] [Google Scholar]
- 2.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–674. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 4.Pinzello G, Simonetti RG, Craxi A, Di Piazza S, Spano C, Pagliaro L. Spontaneous bacterial peritonitis: a prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology. 1983;3:545–549. doi: 10.1002/hep.1840030411. [DOI] [PubMed] [Google Scholar]
- 5.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 6.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 7.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27–31. doi: 10.1097/00042737-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 9.Runyon BA. Early events in spontaneous bacterial peritonitis. Gut. 2004;53:782–784. doi: 10.1136/gut.2003.035311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Fernandez MA, Clark A, Triger DR. Neutrophil phagocytic and bactericidal function in primary biliary cirrhosis and other chronic liver diseases. Clin Exp Immunol. 1987;67:655–661. [PMC free article] [PubMed] [Google Scholar]
- 11.Fiuza C, Salcedo M, Clemente G, Tellado JM. Granulocyte colony-stimulating factor improves deficient in vitro neutrophil transendothelial migration in patients with advanced liver disease. Clin Diagn Lab Immunol. 2002;9:433–439. doi: 10.1128/CDLI.9.2.433-439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343–1346. doi: 10.1016/0016-5085(86)90185-x. [DOI] [PubMed] [Google Scholar]
- 13.Tito L, Rimola A, Gines P, Llach J, Arroyo V, Rodes J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27–31. doi: 10.1002/hep.1840080107. [DOI] [PubMed] [Google Scholar]
- 14.Guarner C, Sola R, Soriano G, et al. Risk of a first community-acquired spontaneous bacterial peritonitis in cirrhotics with low ascitic fluid protein levels. Gastroenterology. 1999;117:414–419. doi: 10.1053/gast.1999.0029900414. [DOI] [PubMed] [Google Scholar]
- 15.Kerr DN, Pearson DT, Read AE. Infection of ascitic fluid in patients with hepatic cirrhosis. Gut. 1963;4:394–398. doi: 10.1136/gut.4.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conn HO. Spontaneous peritonitis and bacteremia in Laennec's cirrhosis caused by enteric organisms: a relatively common but rarely recognized syndrome. Ann Intern Med. 1964;60:568–580. doi: 10.7326/0003-4819-60-4-568. [DOI] [PubMed] [Google Scholar]
- 17.Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457–462. doi: 10.1002/hep.1840050319. [DOI] [PubMed] [Google Scholar]
- 18.Toledo C, Salmeron JM, Rimola A, et al. Spontaneous bacterial peritonitis in cirrhosis: predictive factors of infection resolution and survival in patients treated with cefotaxime. Hepatology. 1993;17:251–257. [PubMed] [Google Scholar]
- 19.Llovet JM, Planas R, Morillas R, et al. Short-term prognosis of cirrhotics with spontaneous bacterial peritonitis: multivariate study. Am J Gastroenterol. 1993;88:388–392. [PubMed] [Google Scholar]
- 20.Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 21.Rimola A, Salmeron JM, Clemente G, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology. 1995;21:674–679. [PubMed] [Google Scholar]
- 22.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 23.Gines P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 24.Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology. 1995;22:1171–1174. doi: 10.1016/0270-9139(95)90626-6. [DOI] [PubMed] [Google Scholar]
- 25.Terg R, Llano K, Cobas SM, et al. Effects of oral ciprofloxacin on aerobic gram-negative fecal flora in patients with cirrhosis: results of short- and long-term administration, with daily and weekly dosages. J Hepatol. 1998;29:437–442. doi: 10.1016/s0168-8278(98)80062-7. [DOI] [PubMed] [Google Scholar]
- 26.Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med. 1995;122:595–598. doi: 10.7326/0003-4819-122-8-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Dupeyron C, Mangeney N, Sedrati L, Campillo B, Fouet P, Leluan G. Rapid emergence of quinolone resistance in cirrhotic patients treated with norfloxacin to prevent spontaneous bacterial peritonitis. Antimicrob Agents Chemother. 1994;38:340–344. doi: 10.1128/aac.38.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Mathai D, Biedenbach DJ, Lewis MT, Gales AC, Jones RN Japan Antimicrobial Resistance Study Group. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against over 2,000 clinical isolates from 22 medical centers in Japan. Diagn Microbiol Infect Dis. 1999;34:123–134. doi: 10.1016/s0732-8893(99)00019-x. [DOI] [PubMed] [Google Scholar]
- 29.Jones RN, Low DE, Pfaller MA. Epidemiologic trends in nosocomial and community-acquired infections due to antibiotic-resistant gram-positive bacteria: the role of streptogramins and other newer compounds. Diagn Microbiol Infect Dis. 1999;33:101–112. doi: 10.1016/s0732-8893(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 30.Goossens H. European status of resistance in nosocomial infections. Chemotherapy. 2005;51:177–181. doi: 10.1159/000086919. [DOI] [PubMed] [Google Scholar]
- 31.Park YH, Lee HC, Song HG, et al. Recent increase in antibiotic-resistant microorganisms in patients with spontaneous bacterial peritonitis adversely affects the clinical outcome in Korea. J Gastroenterol Hepatol. 2003;18:927–933. doi: 10.1046/j.1440-1746.2003.03086.x. [DOI] [PubMed] [Google Scholar]
- 32.Guarner C, Soriano G. Spontaneous bacterial peritonitis. Semin Liver Dis. 1997;17:203–217. doi: 10.1055/s-2007-1007198. [DOI] [PubMed] [Google Scholar]
- 33.Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127–133. doi: 10.1016/0016-5085(90)91300-u. [DOI] [PubMed] [Google Scholar]
- 34.Aparicio JR, Such J, Pascual S, et al. Development of quinolone-resistant strains of Escherichia coli in stools of patients with cirrhosis undergoing norfloxacin prophylaxis: clinical consequences. J Hepatol. 1999;31:277–283. doi: 10.1016/s0168-8278(99)80225-6. [DOI] [PubMed] [Google Scholar]
- 35.Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351–1355. doi: 10.1016/0016-5085(88)90372-1. [DOI] [PubMed] [Google Scholar]
- 36.National Commettee for Clinical Laboratory Standards. Performance standard for antimicrobial disk susceptibility test: approved standard M2-A6. Vallanova: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 37.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 38.Ricart E, Soriano G, Novella MT, et al. Amoxicillin-clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. J Hepatol. 2000;32:596–602. doi: 10.1016/s0168-8278(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 39.Correia JP, Conn HO. Spontaneous bacterial peritonitis in cirrhosis: endemic or epidemic? Med Clin North Am. 1975;59:963–981. doi: 10.1016/s0025-7125(16)31995-2. [DOI] [PubMed] [Google Scholar]
- 40.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984;4:1209–1211. doi: 10.1002/hep.1840040619. [DOI] [PubMed] [Google Scholar]
- 41.Cho JH, Park KH, Kim SH, et al. Bacteremia is a prognostic factor for poor outcome in spontaneous bacterial peritonitis. Scand J Infect Dis. 2007;39:697–702. doi: 10.1080/00365540701299582. [DOI] [PubMed] [Google Scholar]
- 42.Cholongitas E, Papatheodoridis GV, Lahanas A, Xanthaki A, Kontou-Kastellanou C, Archimandritis AJ. Increasing frequency of Gram-positive bacteria in spontaneous bacterial peritonitis. Liver Int. 2005;25:57–61. doi: 10.1111/j.1478-3231.2004.0985.x. [DOI] [PubMed] [Google Scholar]
- 43.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 44.Grange JD, Roulot D, Pelletier G, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: a double-blind randomized trial. J Hepatol. 1998;29:430–436. doi: 10.1016/s0168-8278(98)80061-5. [DOI] [PubMed] [Google Scholar]
- 45.Novella M, Sola R, Soriano G, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997;25:532–536. doi: 10.1002/hep.510250306. [DOI] [PubMed] [Google Scholar]
- 46.Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841–856. doi: 10.1002/hep.20066. [DOI] [PubMed] [Google Scholar]