Abstract
Liver transplantation has become a lifesaving procedure for patients who have chronic end-stage liver disease and acute liver failure. The satisfactory outcome of liver transplantation has led to insufficient supplies of deceased donor organs, particularly in East Asia. Hence, East Asian surgeons are concentrating on developing and performing living-donor liver transplantation (LDLT). This review article describes an update on the present status of liver transplantation, mainly in adults, and highlights some recent developments on indications for transplantation, patient selection, donor and recipient operation between LDLT and deceased-donor liver transplantation (DDLT), immunosuppression, and long-term management of liver transplant recipients. Currently, the same indication criteria that exist for DDLT are applied to LDLT, with technical refinements for LDLT. In highly experienced centers, LDLT for high-scoring (>30 points) Model of End-Stage Liver Disease (MELD) patients and acute-on-chronic liver-failure patients yields comparably good outcomes to DDLT, because timely liver transplantation with good-quality grafting is possible. With increasing numbers of liver transplantations and long-term survivors, specialized attention should be paid to complications that develop in the long term, such as chronic renal failure, hypertension, diabetes mellitus, dyslipidemia, obesity, bone or neurological complications, and development of de novo tumors, which are highly related to the immunosuppressive treatment.
Keywords: Liver transplantation, Deceased donor liver transplantation, Living donor liver transplantation
INTRODUCTION - HISTORICAL PERSPECTIVES
Today, liver transplantation is a lifesaving procedure for patients with chronic end-stage liver disease and acute liver failure (ALF) when there are no available medical and surgical treatment options.1-3 Thomas Starzl performed the first three human liver transplantation at the University of Colorado in 1963, but did not achieve 1-year survival until 1967. Over the next 15 years, relatively few liver transplantation were performed, and the 1-year survival rate was only 30% until the late 1970s and early 1980s when the implementation of cyclosporine-based immunosuppression led to doubling of the 1-year survival rate.1 In 1983, these improved outcomes led to the decision at a National Institutes of Health Consensus Development Conference that liver transplantation was no longer experimental procedure and deserved broader application in clinical practice.4 This meeting initiated the modern era of liver transplantation and resulted in the propagation of liver transplantation across the United States and around the world.
Since the early 1980s, there have been significant advances in all aspects of liver transplantation, including recipient selection, donor management, operation technique, immunosuppression, and postoperative management of liver recipients. These changes, which have marked the evolution from an experimental technique to established and routine therapy, have resulted in enormous improvements in outcome. The overall 1-year survival for adult and pediatric deceased donor liver transplantation (DDLT) is now expected to be in excess of 85%, with 5- and 10-year survival in excess of 70% and 60%, respectively.5-8
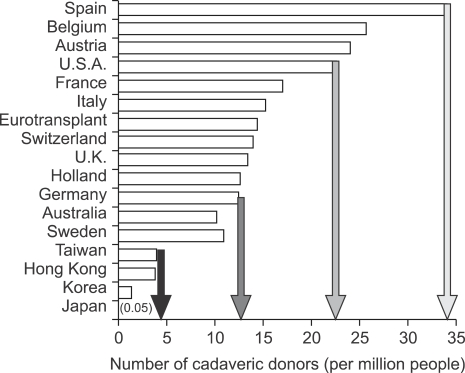
The success of liver transplantation as treatment for most types of acute and chronic liver failure has led to increased referrals for transplantation in the setting of a relatively fixed supply of cadaveric donor organs.1 At the end of 2006, more than 17,000 patients were listed for liver transplantation in the United States.9 Despite performance of more than 6,000 liver transplantations annually in the United States during the past several years,9,10 only one thirds of candidates received liver transplantation and almost 2,000 deaths have occurred annually in patients listed for liver transplantation during past 6 years.1 The persistent shortage of donor organs is obviously insufficient to meet the growing demand for liver transplantation. This kind of imbalance between the supply of donor organ and the patients' numbers demanding liver transplantation is extremely severe in the region of East Asia including Korea, Japan, Hong Kong and Taiwan (Fig. 1). The disparity between supply and demand might be related to inadequate understanding for organ donation after brain death mainly influenced by Confucianism and also insufficient legal supporting system. Although the first successful living donor liver transplantation (LDLT) was performed for pediatric recipient using lateral sector (Couinaud's segment 2 and 3) by Strong RW in 1989, the above situation in the East Asia led surgeons having humanistic enthusiasm to concentrate on developing and performing LDLT as an alternative.
Fig. 1.
Number of deceased donor livers available in various countries in 2000. Reprinted from de Villa VH, Lo CM, Chen CL. Ethics and rationale of living-donor liver transplantation in Asia. Transplantation 2003;75(3 Suppl):S2-S5.
The first successful liver transplantation in Korea was performed using deceased donor whole-size liver by Kim ST in 1988.11 In the early 1990s, liver transplantation was slowly developed as a feasible option in the treatment of end-stage liver disease in Korea mainly because of the severe shortage of deceased donor liver grafts. In 1994, Lee SG performed the first successful pediatric LDLT in 6-month old female biliary atresia child with her father's left lateral sector.12
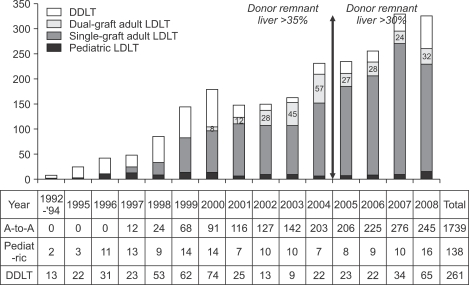
The success in pediatric liver transplantation and the shortage of organs provided the necessary incentive to attempt living donations for adult recipients.13 The accumulated knowledge of the sophisticated hepatobiliary surgery as well as the experiences of DDLT and pediatric LDLT finally made us to perform the first successful adult LDLT using left lobe in 1997 February.14 The emerging awareness of the importance of graft volume and low graft-to-recipient weight ratio led us to initiate right lobe LDLT in adults from 1997 July.13 On the contrary to Hong Kong Group,15 we designed modified right lobe graft to reduce the risk to the donor after right lobe donation by leaving middle hepatic vein to the donor's remaining liver, and reconstruction of divided but sizable-middle hepatic vein branches to prevent congestion of anterior sector in the recipient's side.16,17 This innovative design of right lobe graft has contributed to rapid expansion of our adult living donor liver transplantation program (Fig. 2). In addition, in order to alleviate the small for size graft problem to the recipients and donor risk from harvesting right lobe from suboptimal donor simultaneously, dual-grafts LDLT was initiated in 2000 March.18 Thereafter, the donor rejection rate due to inadequate volume or excessive steatosis was reduced from 40% to 20%.19
Fig. 2.
Annual number of liver transplantations performed at the Asan Medical Center, Ulsan University College of Medicine. The reduction in the accepted remnant liver volume from >35% to >30% of the donor's total liver volume in 2005 resulted in a decrease in the proportion of dual-graft LDLTs. In 2008 there was a marked increase in organ donations, which was fueled by organ donation from a famous boxer in early 2008, hence reducing the number of LDLTs.
LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation.
The aim of this review is to give an update on the present status of liver transplantation mainly in adults, and to highlight some recent developments on indications for transplantation, patient selection, perioperative care, immunosuppression, and long-term management of liver transplant recipients.
INDICATIONS AND CONTRAINDICATIONS
Cirrhosis accounts for more than 80% of transplants performed in adults, and the most important indications for liver transplantation in the United States are hepatitis C (21%), alcoholic liver disease (16%), cholestatic liver disease including primary biliary cirrhosis and sclerosing cholangitis (17%). Other indications are chronic hepatitis (hepatitis B, autoimmune hepatitis), metabolic disease (Wilson's disease, nonalcoholic steatohepatitis), fulminant hepatic failure, and non-metastatic hepatocellular carcinoma (HCC).20 However, the most common indications of liver transplantation in Korea are hepatitis B (81%) including HCC (21.5%) and then fulminant hepatic failure (7%), alcoholic liver disease (4%) and hepatitis C (3%). Recently the proportion of hepatitis C and alcoholic liver disease is slowly increasing and the indications of liver transplantation become resembling to Western countries year by year (Fig. 3).21 Major pediatric indications for liver transplantation include biliary atresia and metabolic diseases.
Fig. 3.
Comparison of indications for liver transplantation between Korea and the United States.
HBV, hepatitis B virus; LC, liver cirrhosis; HCC, hepatocellular carcinoma; FHF, fulminant hepatic failure; HCV, hepatitis C virus; SBC, secondary biliary cirrhosis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; CCC, cholangiocarcinoma; Re-LT, retransplantation; PSC, primary sclerosing cholangitis; BD, bile duct.
Patients with hepatitis B cirrhosis and high viremia were not eligible for transplantation in the past because of the high risk of recurrence after transplantation with consequent rapid graft loss. Since the availability of antiviral medication, high viremia is treatable and transplantation has become a more realistic option with excellent graft and patient survival that is even superior to that of many other indications.22 After transplantation, antiviral treatment, often including hepatitis B immune globulin, is continued to prevent recurrent infection.23
HCC is a well-recognized indication for transplantation when the patients have Child B and C liver cirrhosis at the same time. Generally, only patients who meet the Milan criteria of a single tumor up to 5 cm or up to three tumors with less than 3 cm and no major vascular invasion such as portal and hepatic vein, as determined by imaging studies,24 are approved by the transplant organizations in most countries. Survival rates for these patients are comparable to those for cirrhosis leading to transplant without a complicating HCC recurrence. Preoperative base-line metastatic work-up includes bone scan and chest computed tomography (CT). Recently, positron emission tomography (PET) scan also tends to be included because of the usefulness to find undetected malignancy and to avoid legal issue. Discussion at present focuses on expansion of these strict criteria, as on the one hand the removed liver often shows more tumor lesions than expected, and on the other hand patients with somewhat larger lesions often do well.25 Yao et al. have shown that patients who have single tumor 6.5 cm in diameter or smaller or three or fewer tumors with largest being 4.5 cm or less in diameter and a total tumor burden of 8.0 cm or less (the so-called "UCSF criteria") achieved results that were not different from those of patients belonging to Milan criteria.26 Lee et al. proposed Asan Medical Center (AMC) criteria from the results of LDLT for HCC, based on explant pathology, that were largest tumor diameter ≤5 cm, number ≤6, and no gross vascular invasion. It had similar prognostic power but highest discriminatory power than Milan and UCSF criteria.27 Finally, radiofrequency ablation and chemoembolization have become bridging therapies to transplantation while patient is waiting on the liver transplant list.28,29
Chronic hepatitis C virus (HCV) infection is the leading indication for liver transplantation in United States, and the number of transplantation for this indication is expected to increase dramatically over the next 10 to 20 years.30,31 Retransplantation for patients who develop allograft dysfunction as a result of recurrent hepatitis C remains controversial, because outcomes are often poor,32 and antiviral therapy of recurrent HCV infection has relatively low efficacy.33
For alcoholic liver disease, the prerequisites for transplantation in most centers are alcohol abstinence for at least six months and active treatment for alcohol dependency before transplantation.34,35
ALF, also called "fulminant hepatic failure," and the more indolent variant, subfulminant hepatic failure, are characterized by the development of liver failure manifested by coagulopathy, jaundice, and encephalopathy leading to coma in the absence of chronic liver disease.36 ALF accounts for 5% to 6% of all liver transplantations.9 Acetaminophen hepatotoxicity is the leading cause of ALF, whereas idiosyncratic drug-induced liver injury is the major cause of subfulminant hepatic failure.36 Patients who have ALF can recover spontaneously, but those with subfulminant hepatic failure are expected to have 100% mortality without transplantation.10,36 Transplantation indications ALF are usually based on King's College criteria,37 and/or Clichy criteria.38 However, it is often recommended that patients with ALF who fail to meet King's College criteria still be considered for liver transplantation, because spontaneous recovery is not guaranteed.1 Timely referral and liver transplantation is of paramount importance, because death from sepsis and cerebral edema may occur within days of onset of stage 3 or 4 hepatic encephalopathy.2,10,36 Lee et al.39 described that the timely LDLT using appropriate graft type sufficing at least more than 40% of patient's standard liver volume with minimal steatosis resulted in remarkably improved survival rate for adult ALF patients in this region where deceased organ donation is very scarce.
Graft failure with the need for retransplantation accounts for an increasing number of transplantations. Shortly after transplantation, this mainly concerns primary nonfunction of liver grafts and hepatic artery thrombosis, later biliary complications and recurrent hepatitis C can become indications for retransplantation.25
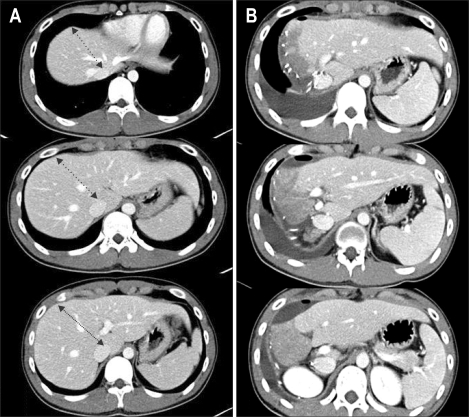
In general, the past few years are characterized by an increasingly shorter list of absolute contraindications and a growing list of indications for liver transplantation. Specific type of Budd-Chiari syndrome and severe portal vein thrombosis and/or stenosis, previously considered as a anatomic abnormality precluding liver transplantation, were no longer contraindication for liver transplantation. For Budd-Chiari syndrome, thrombosis and/or stenosis extending to intrapericardial inferior vena cava (IVC) and almost orifice of right atrium, Lee et al. replaced the diseased stenotic retrohepatic vena cava of the recipient with a large caliber Dacron interposition graft between right atrium and infraheaptic IVC (Fig. 4).13 Portal venous thrombosis, sclerosis and size discrepancy between the graft and recipient's portal vein (PV) are other issues that make it difficult or impossible to perform standard end-to-end anastomosis. These problems are usually overcome by the use of PV thrombectomy and venoplasty, and additionally portal vein stenting by using intraoperative portogram if necessary (Fig. 5).40 Sometimes interposition vascular graft from superior mesenteric vein or left renal vein when large splenorenal shunt is accompanied, should be used to supply mesenteric blood to the graft, which is essential for graft regeneration (Fig. 6).41,42
Fig. 4.
LDLT with replacement of the inferior vena cava using a Dacron interposition graft for Budd-Chiari syndrome. (A) Preoperative CT scan showing typical findings of Budd-Chiari syndrome: retrohepatic obliteration of the inferior vena cava (multiple arrows), and portal vein stenosis and a large coronary collateral vein (black and white arrowheads, respectively). (B) Intraoperative photograph showing replacement of the inferior vena cava with a Dacron interposition graft between the right atrium and the suprarenal inferior vena cava (white arrowhead), and middle hepatic vein tributaries reconstructed using cryopreserved cadaveric iliac vein grafts. (C) Two-year postoperative CT scan revealing a patent replaced inferior vena cava, right hepatic vein, and portal vein without stenosis (white arrowhead, white arrow, and black arrowhead, respectively).
LDLT, living donor liver transplantation.
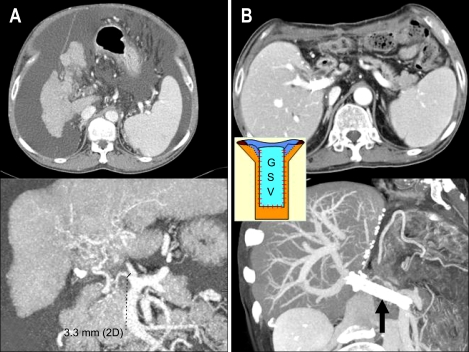
Fig. 5.
Portal vein plasty with the great saphenous vein and intraoperative stent placement for severe portal vein stenosis. (A) Preoperative CT scan showing severe portal vein stenosis and abundant ascites. (B) The liver graft regenerated well and ascites had disappeared by the 2-month postoperative follow-up, as revealed by this CT scan, after intraoperative portal vein plasty with an autogenous great saphenous vein (GSV, indicated by the separate figure in the small box) and additional stent placement (black arrow).
Fig. 6.
Renoportal anastomosis using a cadaveric fresh iliac vein graft for an obliterated portal vein, and large spontaneous splenorenal shunts. (A) Preoperative CT scan showing obliterated portal vein stenosis and large spontaneous splenorenal shunts draining most of the splanchnic blood flow into the left renal vein (LRV, black arrow). (B) Intraoperative photography showing an interposition cadaveric fresh iliac vein graft (IPG) anastomosed to the left renal vein. The black arrow indicates the interruption suture running between the inferior vena cava (IVC) and the left renal vein in order to prevent portal flow stealing. (C) Patent interposition iliac vein graft between the left renal vein and the grafted portal vein. A well-regenerated liver is visible on the 2-year postoperative follow-up CT scan.
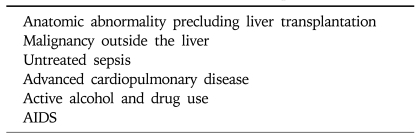
Most generally accepted absolute contraindications to transplantation are noted in Table 1. Transplant centers are now increasingly offering liver transplantation to carefully selected older patients and human immune-deficiency virus (HIV)-infected individuals in the absence of acquired immune deficiency syndrome (AIDS).43
Table 1.
Contraindications to Liver Transplantation
LISTING AND TIMING OF LIVER TRANSPLANTATION
The presence of cirrhosis alone is not sufficient to warrant transplantation. Although the high level of morbidity and mortality in chronic liver disease is related to complications of cirrhosis, the well-compensated cirrhotic patient can remain stable for many years. Fattovich et al.44 reported a 91% 5-year survival rate in a cohort of 384 cirrhotic HCV patients. However, once an index manifestation of decompensation had occurred such as variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and occurrence of HCC, etc., survival dropped to 50% at 5 years, suggesting that referral for liver transplantation should be considered once an index complication has occurred.
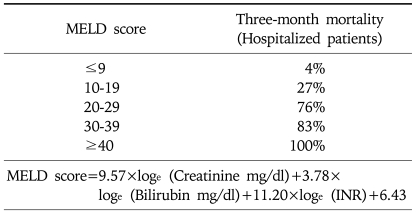
Since the application of the Model of End-Stage Liver Disease (MELD) score for organ allocation in 2002 by the Unite Network for Organ Sharing (UNOS), cirrhotic patient has to meet minimal listing criteria for placement on the deceased donor waiting list (Child-Turcotte-Pugh score of at least 7 for most causes of cirrhosis). Once approved for listing, the patients is prioritized according to the MELD score.45 This score, based on objective laboratory values, predicts the 3-month mortality of patients awaiting liver transplantation. The MELD score incorporates serum creatinine, bilirubin, and prothrombin time (INR). The formula for the MELD score and 3-month mortality is available on the Internet at www.mayoclinic.org/meld/mayomodel6.html (Table 2).46 The driving force of this system is disease severity, and there is no inherent benefit to early listing. In fact, listing too early may waste time and resources and cause the patient anxiety. A MELD score 10 or any complication of portal hypertension is an appropriate indication for transplant evaluation.43
Table 2.
Model for End-Stage Liver Disease Score
Reprinted from Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-470.
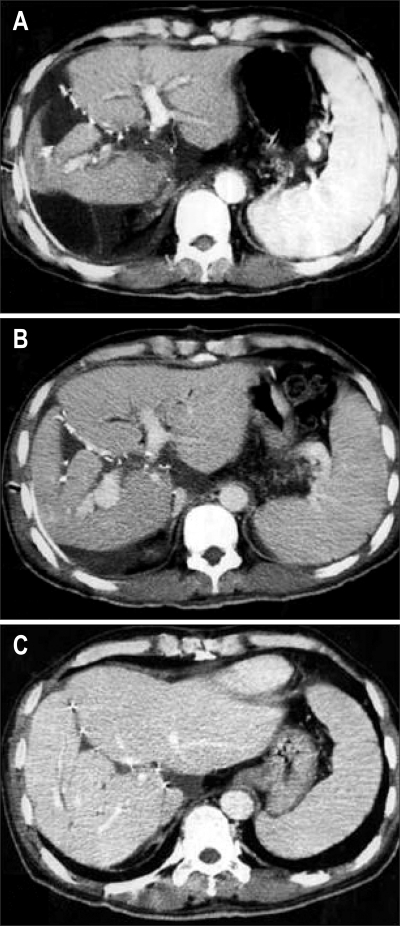
If cirrhotic patients have more than MELD score of 30, 3-month survival will be less than 20%, so-called "acute-on-chronic liver failure (ACLF), UNOS 2A" state. The patients show similar clinical findings to ALF, but manifest underlying liver cirrhosis and related portal hypertension. Treatment strategies are timely liver transplantation using good quality graft including deceased donor whole liver or living donor partial liver graft having more than 50% of recipient's standard liver volume and minimal fatty change. At our institution, 16% of the adult LDLT was urgently performed for ACLF patients and the 1-year and 5-year survival rates were 84% and 79% respectively. These successful results indicate that ACLF patients are one of the most appropriate candidates for adult LDLT (Fig. 7).47
Fig. 7.
CT scan showing changes between before and after liver transplantation. (A) HBV-related acute-on-chronic liver failure patient with huge ascites, altered mentality, and high MELD score (40 points). (B) Good regeneration of dual liver grafts is visible and the ascites have disappeared on this 1-month postoperative follow-up CT scan.
RECIPIENT OPERATIONS
1. Deceased donor whole liver transplantation45
The recipient operation consists of total hepatectomy of the native liver followed by implantation of the donor liver. The native hepatectomy usually begins with division of the ligamentous attachment of the liver, followed by skeletonization of the hilar structures (bile duct, hepatic artery, and portal vein) to prepare for implantation of the new liver. The IVC is encircled above and below the liver to achieve full vascular control. Committing the patient to transplant, diseased liver is removed with retrohepatic IVC. The donor liver is surgically prepared for implantation on the back table, then brought onto the operative field. Anastomoses are constructed between the donor liver and recipient in the following sequence: suprahepatic IVC, infrahepatic IVC, and PV anastomosis. Once the PV is anastomosed, the clamp is removed in sequence and the liver is perfused in PV inflow.
The hepatic artery is typically connected to the recipient hepatic artery with large luminal diameter at the junction of the gastroduodenal artery. Thereafter, bile duct is reconstructed using an end-to-end choledochocholedochostomy. If the recipient bile duct is not appropriate for end-to-end reconstruction, a Roux-en-Y choledochojejunostomy is performed.
2. Living donor liver transplantation
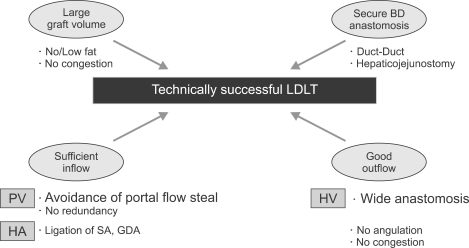
The surgical technique for recipients is based on whole liver resection, with preservation of the IVC removed for whole DDLT.48 However, it is more sophisticated operation and needs much more careful and delicate dissection than DDLT, because living donor partial liver graft having much smaller-sized hepatic artery, vein and PV should be implanted. For technically successful operation, it is important to make a large and long opening along the sides of the hepatic veins and important to maintain satisfactory portal, biliary and hepatic arterial sources for the reconstruction (Fig. 8).49 Anastomosis is performed in the following order: hepatic vein, PV and then hepatic artery. The provision of adequate outflow is indispensable for graft function; thus, it is necessary to obtain a wide orifice and an enough length of the hepatic vein for anastomosis.50 Hepatic arterial reconstruction is technically difficult due to the existence of short, thin and small hepatic arteries on the liver graft, particularly in the East Asian people. The Kyoto group introduced microvascular surgery and report excellent outcomes.51 It becomes standard technique for hepatic anastomosis currently.
Fig. 8.
Interrelationship between graft volume, hepatic inflow and outflow, and bile-duct anastomosis for determining the technically successful living donor liver transplantation (LDLT).
Bile duct reconstruction is usually performed last. The preferred technique in adult LDLT has been shifted from hepaticojejustomy to duct-to-duct anastomosis.49 Duct-to-duct anastomosis can preserve physiologic biloenteric and bowel continuity, thus preventing delayed bowel movement, and also allows for endoscopic access to the biliary tree for diagnostic and therapeutic instrumentation and management and prevent ascending cholangitis.52
LIVER DONORS
The principal condition for liver donor is ABO blood type compatibility. Histocompatibility is not important for liver transplantation as much as kidney transplantation, and do not determine whether we do transplant or not. Absolute contraindications to organ donation are infectious disease and active malignancy that can cause death of the recipient through transmission. Infectious diseases include Creutzfeldt-Jakob disease, kuru, Gerstmann-Strussler-Scheinker syndrome, and fatal insomnia, HIV virus infection, disseminated and invasive infection by other viruses, mycobacterium, or fungi, and systemic infection by methicillin-resistant staphylococci. However, low grade skin cancer, as basal cell carcinoma, and many squamous cell carcinomas, carcinoma in situ (uterine and cervical), and primary brain tumors without extracranial metastases do not exclude donation.53,54
1. Donor selection
1) Deceased liver donor
Selection of an appropriate donor is crucial to the successful outcome of DDLT. Among the most prominent donor characteristics that may influence the development of initial poor function or primary nonfunction in the recipient include old donor age, prolonged ischemia, hypotension and excessive inotropic support, non-heart-beating donors and steatosis.55 Following characteristics described an ideal liver donor: 50 years or younger; no hepatobiliary disease; hemodynamic and respiratory stability (systolic blood pressure >100 mmHg, and central venous pressure >5 cm/H2O); an acceptable PaO2 and hemoglobin level; no severe abdominal trauma, systemic infection, or cancer; diuresis greater than 50 mL/h and normal creatinine; and finally, a dopamine requirement less than 10 µg/kg/min.56 The critical shortage of deceased donor grafts and the increasing number of recipients awaiting liver transplantation make it extremely difficult to limit organ selection to the use of ideal donors only. The use of donor with extended criteria for organ acceptance has therefore become a necessity in the current era.57
Until now, no consensus exists for what an extended criteria donor (ECD) graft is, but typically it refers to grafts more commonly associated with poor function and decreased survival on average. This includes older donors, donation after cardiac death, grafts from individuals infected with HCV, hepatitis B, steatosis and prolonged cold ischemia time.58,59 Use of ECD increases the pool of available donors and reduces the number of deaths on the waiting list.60 In an audit of Organ Procurement and Transplantation Network (OPTN) data from over 12,000 recipients between June 2002 and June 2005, 2,873 grafts (24%) were from ECDs. ECD donors are used most commonly for the recipients with low MELD scores (<15)61 because the combination of high MELD recipients and ECD donors is associated with poor graft function and increased mortality.58,62 Cameron et al.62 insisted ECD grafts should not be used for critically-ill recipients such as advanced age and urgent recipients (ALF or ACLF) due to increment of the risk ratio of death by 50%.
2) Living liver donor
According to Florman et al.63 report, faced with following statistics, death on the waiting list in 2004 was greater than 10%, physicians caring for these patients have endeavored to perform transplants with partial liver grafts from healthy, volunteer liver donors. From this point of view, LDLT was an avoidable and inevitable option for surgeons to treat end stage liver disease patients in the East Asia under extremely rare deceased organ donation regardless of ethical issues about donors.
Organs from living donors offer many potential advantages over organs from deceased donors. The most important advantages of living donation are that it optimizes the timing of transplantation and frees patients from the waiting list. Secondly preservation time is minimal, so there is significantly less ischemic damage to the liver. Therefore, the quality of the donated liver is much better. Most importantly, LDLT increases the global pool of transplantable organs, allowing more people to get transplantation as a life-saving therapy.
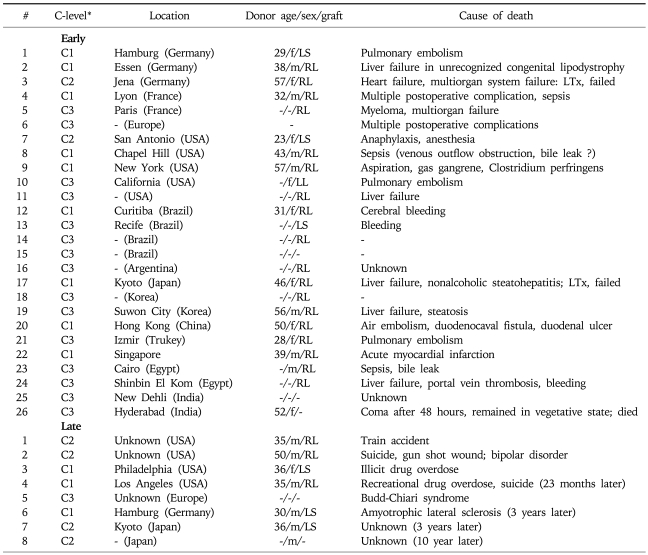
There are, however, a number of disadvantages to LDLT which must be considered carefully. The donor, a perfectly healthy volunteer, faces unequivocal risks of morbidity and even mortality. To date, more than 12,000 living donor hepatic resection have been performed worldwide (ELTR, UNOS, CM Lo, pers. comm., 2006: mortality approaches 0.5% for the right lobe donor in contrast to approximately 0.1% for left lobe donation).64 Table 3 summarizes the worldwide reported donor's mortalities collected from the international literature and from recent reports at professional society meetings.65 Additionally, two donors have required liver transplantation for themselves as the result of operative complications.63
Table 3.
Worldwide Review on Living Liver Donor Mortality Divided in Early (Postoperative) and Late Mortality
f, female; m, male; LS, lateral segment; LL, left lobe; RL, right lobe; LTx, liver transplantation.
*Certainty (C) level based on the source of information
C1, Donor death documented, confirmed, and published in the medical literature by an author representing the transplant center where the fatality occurred; evidence based on direct information; details of the case often provided, e.g., donor age, sex, graft type, date of surgery, cause of death, date of death, survival; C2, Donor death not clearly identified, confirmed or published by transplant center where the fatality occurred; transplant center usually not identified; indirect evidence obtained from secondary publications by other authors or reviews; eventually some information about the case provided; C3, Donor death not identified, confirmed or published; transplant center not identified; evidence based on verbal presentation or personal communication; no further details of the case provided.
Reprinted from Ringe B, Strong RW. The dilemma of living liver donor death: to report or not to report? Transplantation 2008;85:790-793.
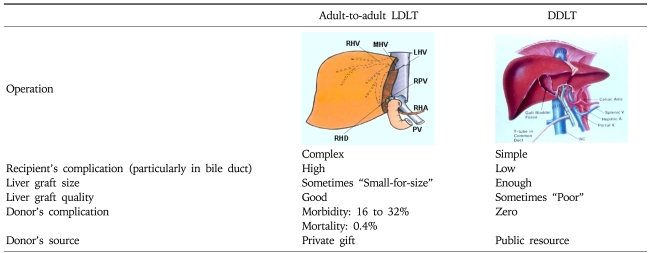
LDLT also carries certain increased risks for the recipients. It is technically more complex than deceased whole liver transplantation. The incidence of biliary complications increased with partial grafts. In addition, the small-for-size syndrome is essentially seen only with partial grafts, when the recipient does not receive enough functional liver mass. Finally, LDLT procedures are considerably more labor-intensive efforts and prone to result in more postoperative complications related to short and small hepatic artery, vein, PV and bile ducts (Table 4).
Table 4.
Differences between Adult-to-adult LDLT and DDLT
LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation.
To be a successful LDLT, appropriate donor evaluation is one of the most crucial parts.66 The goal of evaluation process is to exclude donors with an increased risk for morbidity and mortality, while at the same time assuring that a suitable graft for the recipient can be obtained. The person who is willing to be a living liver donor, should satisfy the following guiding ethical principles including altruism, the absence of coercion or monetary reward, patient autonomy, beneficence, and nonmaleficence.67 Additionally, he is fully informed of the risks and benefits as a donor, and fully informed of the risks, benefits, and alternative treatment available to the recipient,68 because donor safety is utmost important issue. There can be no exceptions to this rule, regardless of the consequences for the recipient, even death.
Most living donors are in excellent health. Although there is no definitive age cutoff, typically ages between 18 and 55 years are preferred.65 At our institution, upper age limit is 60 years, but biological age is more important than donor's chronologic age. However, careful caution is necessary for older age donor (>50 years) who is associated with a fourfold risk of complications.69 Lower age limit can be lowered to 16 years only when the recipient is the donor's parent.19
Fatty liver is dangerous for both donor and recipient. The Vancouver Forum participants64 suggested that donor liver biopsy should be performed if blood specimen liver tests are abnormal and if steatosis or other abnormalities are noted on imaging studies. Additionally, the histological findings that should preclude living liver donation were also clearly defined during Forum. They include (i) portal or sinusoidal fibrosis, (ii) nonalcoholic steatohepatitis, (iii) steatosis >20% (only for right liver), and (iv) portal inflammation or necroinflammatory changes.64 Liver biopsy could unveil unexpected liver pathology (as high as 21%),65 and body mass index is an inaccurate indicator of steatosis, so many centers advocate routine liver biopsy in the donor evaluation protocol.70 In our experience, routine application of preoperative donor liver biopsy make us avoid invalid donor operation,19 and biopsy related donor complication has been minimized with help of the hands of experienced practitioner coupled with careful post-biopsy observation. If the transplant is not urgent, we recommend the potential donor with liver steatosis to reduce body weight and to perform vigorous exercise. Successful reduction of liver steatosis is possible and donation is acceptable.71
Regarding to ABO blood type in LDLT, identical or compatible ABO blood type between recipient and donor is recommended; however, ABO incompatible blood type LDLT has been undertaken successfully in special instance such as infant <1 year of age without presence of isoagglutinins, and in emergency situations where no deceased donor allograft is available.72 Recently, the group of Kyoto,73 Chicago,74 and Gent75 demonstrated the feasibility of adult LDLT across ABO barriers by using different approaches and immunosuppressive protocols. At our institution, the liver donor-exchange program for adult, as in kidney transplantation, has performed to overcome blood group mismatching in 5 donor-recipient pairs since 2003, and also started ABO incompatible adult-to-adult LDLT successfully in 2008.19
By definition, a living donor is a completely healthy person. It is the consensus that potential donors with concomitant medical illness should not be allowed to donate.70 However, the presence of mild systemic disease, such as well controlled mild hypertension or diet-controlled diabetes, is not necessarily a contraindication to donation.76
Extensive blood tests are considered necessary to detect HIV, HCV, or hepatitis B virus (HBV) infection, and laboratory testing for a preexisting hypercoagulable condition especially if the potential donor has a history of venous thrombosis.64,77 The HBcAb positive donor can donate for a recipient affected by HBV-cirrhosis and HBV naïve recipient also with prophylaxis of hepatitis B immunoglobulin.19
Imaging study of the liver is needed to exclude parenchymal liver disease, to identify the vascular and biliary anatomy and to estimate the graft and remnant liver volume. As a 'all in one' procedures to simplify and shorten such time-consuming and costly procedure, use of multidetector CT is helpful to evaluate them in a single diagnostic study,78,79 and is better than magnetic resonance imaging in the ability to accurately assess the biliary anatomy.78 It is generally believed that a remnant liver of at least 30% of the original liver volume with complete venous drainage is safe for donor survival and a graft that provides 40% of the estimated standard liver volume80 or 0.8% of the body weight of the recipient is necessary for the recipient recovery.19,64,81 Volumetric imaging analysis may overestimate the actual liver volume by as much as 10%. The main cause of the overestimation is related to the difference between the vital liver filled with blood in vivo and the graft that is in a state of collapse ex vivo.
2. Donor procurement
1) Deceased liver donor
Whole organ procurement is now a well described procedure. The principles of minimum mobilization to define vascular structures, in situ perfusion with 4℃ preservation solution, and sequential en bloc harvest of organs yield good allograft preservation.
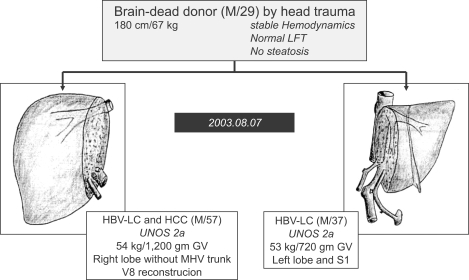
"Split liver" grafting involves the preparation of two allografts from a single donor.82 In most cases, the extended right lobe allograft (segment 4-8) is used in an adult or large child, while the left lateral segment allograft (segment 2,3) is transplanted into a small recipient. At our institution, split liver transplantation for two adult recipients has been performed successfully to more than 16 adult recipients.83 The whole liver is divided into right lobe without middle hepatic vein (MHV), same to modified right lobe graft in LDLT, and left liver with IVC and common hepatic artery by using in situ or ex vivo technique (Fig. 9).
Fig. 9.
Split liver transplantation to two adult recipients in order to expand the donor pool at Asan Medical Center. This is the first time that this procedure was performed in Korea, in August 2003.
2) Living liver donor
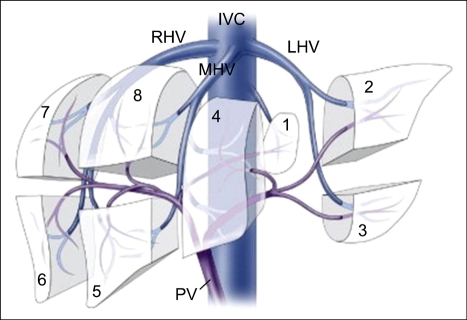
The performance of LDLT relies on an understanding of the vascular and biliary anatomy of the liver. The left hepatic lobe consists of left lateral sector (Counaud segment 2 and 3) and medial sector (Counaud segment 4); and Counaud segment 5,6,7 and 8 compose the right hepatic lobe (Fig. 10). Counaud segment 1 is the caudate lobe. For most people the left lateral sector comprise 20% of their total liver volume, the left lobe 40%, and the right lobe 60%. In general, the common grafts that can be used from a living donor for transplantation include the left lateral sector, the left lobe (with or without caudate lobe), and the right lobe (Fig. 11).
Fig. 10.
Segmental anatomy of the liver using the Couinaud's segments. Reprinted from Brown RS Jr. Live donors in liver transplantation. Gastroenterology 2008;134:1802-1813.
Fig. 11.
Most commonly used graft for adult-to-adult LDLT. (A) Right lobe graft, (B) Left lobe graft.
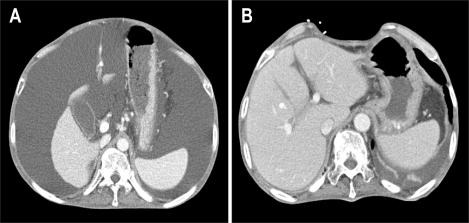
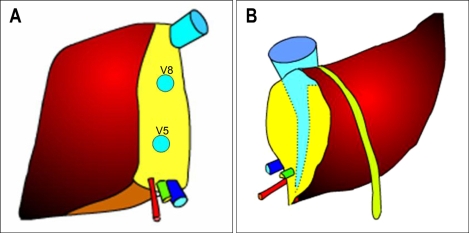
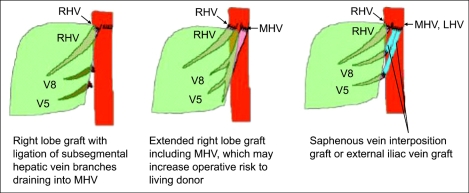
The argument for the need of including the MHV in right lobe graft continues ever since its inception. Hong Kong group advocates for right lobe with MHV. They believe that MHV is the important drainage vein of segments 5 and 8, and sometimes 6.84,85 Right lobe graft without MHV will lead to graft congestion, drainage of hepatic artery blood via the right anterior portal vein, persistence of portal hypertension, and excessive blood flow into the right posterior sector leading to serious graft damage. However, the most important shortcoming of this liver graft is hampering the donor safety (Fig. 12). Actually graft type of the donor deaths after liver donation from Hong Kong, Japan, and Korea were all right lobe with MHV grafts.65 In addition, the majority of programs in the United States shows that this is too large a liver resection for a healthy donor, who does not essentially require surgery, and increase the risk of small-for-size syndrome and the subsequent postoperative liver failure in the donor.76 Marcos et al. advocates for right lobe without MHV, because donor safety could be ensured, intrahepatic venous collaterals are present in between the middle and right hepatic vein branches,86 and the incidence of graft congestion is very low. However, collaterals between the right hepatic vein and MHV are small and late in development and are not present in every case.87 Lee et al. noted world first about the importance of congestion and its drainage in the anterior sector of right lobe graft without MHV,87 and initiated modified right lobe graft, which is right lobe graft without MHV but restoring the continuity of sizable segment 5 and 8 (≥5 mm) venous drainage in the graft by using venous conduit (Fig. 13).17,88 After clinical application of this graft type, 6-months recipient survival rate improved from 71% to more than 90% at our institution.89 In some rare instance, right posterior sector (Counaud segment 6,7),90 and monosegment graft have been used.91
Fig. 12.
Congestion of the medial sector after procurement of the right lobe with a middle hepatic vein graft from a living liver donor. (A) Preoperative CT scan showing the donor liver and imaginary parenchymal transection line when the right lobe with the middle hepatic vein graft is harvested. (B) CT scan made on the 7th postoperative day, revealing a large area of congestion at the medial sector.
Fig. 13.
A scheme for modified right lobe (MRL) liver grafting. RHV, right hepatic vein; MHV, middle hepatic vein; LHV, left hepatic vein; V5, hepatic venous tributaries from segment 5; V8, hepatic venous tributaries from segment 8.
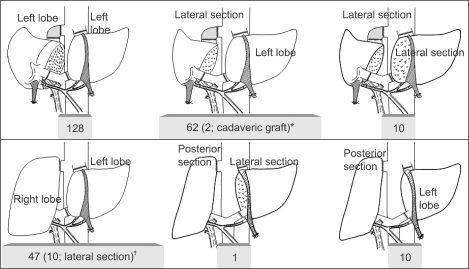
During recipient and donor workup for adult LDLT, heartbreaking cases who can not be transplanted from a single donor due to graft-recipient size mismatching, unacceptable right-to-left lobe volume discrepancy and excessive hepatic steatosis, are not uncommon. Under those circumstance, Lee et al. has performed dual-graft adult LDLT in which two left liver (left lateral sector or left lobe) grafts are procured from two donors and implanted in one recipient, in order to achieve maximal donor safety through minimal resection of liver mass while increasing recipient actual graft volume (Fig. 14).19,92,93 Sometimes right lobe or right posterior sector graft also has been used for right-sided liver graft of dual-graft LDLT because of the recipient body size or anatomic variation of donor liver (Fig. 15). At our institution, various types of single liver graft together with dual-grafts has been used to satisfy both donor safety and recipient metabolic demands (Fig. 16).
Fig. 14.
Postoperative follow-up CT scan of the recipient, demonstrating the balanced regeneration of both liver grafts. (A) CT scan taken 5 days after transplantation showing that the second left lobe graft in the right upper abdomen was still small and supported by a tissue expander bag. (B) CT scan made 2 weeks after transplantation, showing the rapid regeneration of both grafts. (C) CT scan made 2 months after transplantation, showing that two regenerated left lobe grafts were in the shape of a normal liver.
Fig. 15.
Two hundred and fifty-eight dual-graft adult-to-adult LDLTs were performed between March 2000 and December 2008 at the Asan Medical Center. *Two recipients received a left lobe from a live donor and a split lateral segment from a deceased donor, respectively, on June 2000 and December 2008, respectively; †Right lobe (from wife) and left lobe (from cousin) dual-graft adult-to-adult LDLT for a large-size recipient was performed successfully in April 2001. Lateral section replaced the left lobe in 10 of 47 recipients.
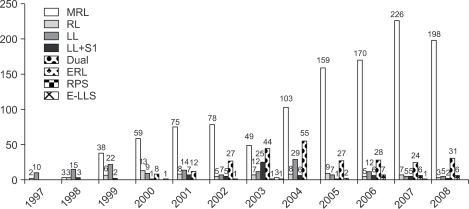
Fig. 16.
Types of graft in 1,739 adult-to-adult LDLTs.
MRL, modified right lobe; RL, right lobe; LL, left lobe; S1, caudate lobe; ERL, extended right lobe; RPS, right posterior sector; E-LLS, extended left lateral segment.
As an incision line for all types of donor hepatectomy, a right subcostal incision with midline extension is adequate and spares the donor morbidity of dividing the rectus muscle bilaterally.63 In addition to conventional open techniques, Cheriqui et al.94 reported for the first time laparoscopic left lateral sector retrievals for pediatric LDLT. Recently at our institution also, laparoscopic left lateral sector donor hepatectomy has been performed routinely for pediatric LDLT. Intraoperative cholangiography by using a radio-opaque marker tagging method and direct bile duct probing through the cystic duct was routinely used to identify an adequate location for bile duct division, minimizing the number of graft duct openings obtained from various donor bile duct anatomies.95 For the parenchymal transection, ultrasound or water-jet dissector are generally used in combination with electrocautery under without hilar occlusion or using only intermittent clamping.96 Graft retrieval is performed without portal cannulation in donor's abdominal cavity. Pinching of the portal vein wall with the cannula in situ or pushing the tube into the right anterior or posterior PV is efficient for uniform flushing of the liver graft. The need for hepatic artery flushing by preservation flushing is controversial. Finally, in case of modified right lobe graft, vascular conduits using recipient's great saphenous vein, PV, umbilical vein, or cryopreserved cadaveric vessels are employed to establish the good outflow of the graft.88,97,98 In case of right lobe with MHV, Hong Kong group preferred to perform venoplasty of the right hepatic vein/MHV to form a large triangular orifice.99 At our center, however, quilt venoplasty using recipient's graft saphenous vein and cryopreserved vessels is used to make a large single orifice.100 Before wound closure, the left liver remnant should be anchored to the anterior abdominal wall to prevent its rotation into the right subphrenic cavity and subsequent outflow obstruction.101
IMMUNOSUPPERSSION AFTER LIVER TRANSPLANTATION
After introduction of cyclosporine, a number of new immunosuppressants (tacrolimus, mycophenolate mofetil, sirolimus, interleukin-2 receptor blocker, humanized monoclonal antibody, etc) are available currently. Large series describing results from the previous years mention acute rejection in 40 to 60% of liver transplant patients.102,103 More recent data from USA for 2003 show acute rejection in as few as 18% of patients (www.unos.org). Vast majority of patients can be treated satisfactorily with boluses of steroids. Chronic rejection is a rare event in liver transplantation, occurring in less than 5% of patients.104
In the majority of liver transplant recipients, combination of two or three different maintenance immunosuppressive drugs is used for prevention of rejection. The calcineurin inhibitors tacrolimus and cyclosporine are the mainstays of immunosuppression in liver transplantation. Over 95% of patients are discharged with a calcineurin inhibitor as a primary immunosuppressant, with tacrolimus being most frequently used (www.unos.org).
Steroids are still almost universally used after liver transplantation. Most patients are discharged with steroids, which are subsequently tapered and weaned in the following months.
Mycophenolate mofetil (MMF), which is antimetabolites such as azathioprine, is also frequently used to reduce calcineurin inhibitor dose and to prevent or limit side effects such as renal dysfunction, hypertension and hyperlipidemia. UNOS data show that 60% of patients are discharged from the hospital on the use of MMF, same in our institute.
Sirolimus (Rapamycin) is a new immunosuppressive agent, which is structurally similar to tacrolimus, and has antifibrotic and antineoplastic characteristics, but lack of nephrotoxicity. However, severe side effects, including delayed wound healing and vascular complications limit its clinical usage.105 In general one can say that currently the most important challenge with regards to immunosuppression in liver transplantation is not to find drugs that are more powerful, but drugs that are less harmful. In the mean time, the present availability of a wide spectrum of effective and specific immunosuppressive drugs allows individualized selection of drugs, thereby limiting serious side effects.25
COMPLICATIONS
1. Early post-transplant complications
Postoperative technical and organic medical complications, primary dysfunction, graft rejection and infections are the major short-term complications (Table 5).106
Table 5.
Allograft Dysfunction and Surgical Complications Occurring in the Immediate Postoperative Period
During the initial 48-72 postoperative hours, abnormal liver biochemistries are typical and reflect a number of insults to the graft, including following harvesting, preservation, and subsequent reperfusion. However, daily routine Doppler ultrasound exam should be performed to exclude vascular complications such as hepatic artery thrombosis, portal and hepatic vein stenosis or obstruction.
Biliary complications are considered the Achilles' heel of LT, particularly in the setting of LDLT. The rate of bile leak or stricture at the anastomotic site or cut edge of the transected liver, were reported in 15-60% of recipients in early, single center reports.76 At our institution, cumulative biliary complication rate after LDLT rose to 20.2% in 5 years, and most stenosis was successfully treated using radiologic intervention.107
Within the first week after LT, liver biochemistry steadily improve as ischemia and reperfusion damage resolves, and also the volume of transplanted liver graft is regenerating. Acute rejection becomes an important and frequent cause of graft dysfunction at 1 week and beyond. Liver biopsy is necessary for confirmatory diagnosis.
Various infectious complications following liver transplantation can occur, and opportunistic infections related to intensive immunosuppresion are relatively common such as cytomegalovirus, Pneumocystis carinii, and fungal infection.
Neurologic dysfunction, hyperglycemia and renal impairment can occur mainly related to the toxic effects of cyclosporine and tacrolimus.
2. Long-term complications
Today, with improved survival in most transplant centers, increasing attention is being given to complications that develop in the long-term, and that are highly related to the immunosuppressive treatment. The most frequent complications are chronic renal failure, hypertension, diabetes mellitus, dyslipidemia, obesity, bone or neurological complications, development of de novo tumors.108
Impaired renal dysfunction before transplantation, chronic use of calcineurin inhibitors and hypertension probably all contribute to the increased risk for chronic renal failure after liver transplantation. The cumulative risk of renal failure has been described to be as high as 20% five years after transplantation.109 Strenuous management of hypertension and withdrawal or reduction of calcineurin inhibitors in an early stage should be considered.
Hypertension, diabetes, hyperlipidemia, and obesity, which are well-known risk factors for cardiovascular disease, occur in up to 75%, 15%, 40-60%, and 30-70% of patients, respectively, long-term after transplantation.110,111 This increase in risk factors is at least partly due to continuous immunosuppressive drugs. This combined with high risk behavior leads to a markedly increased risk for arthrosclerosis and subsequent cardiovascular events.112 Vigorous screening for cardiovascular risk factors and aggressive management is essential for all liver transplant recipients.
Increased risk for osteoporosis in liver transplant recipient results from the combination of low bone mineral density before transplantation due to hepatic osteodystrophy, malnutrition and inactivity and steroid use after transplantation.112 In the present era, with lower dosages of corticosteroids and the availability of bisphosphonates, bone disease can be prevented and treated easier than the earlier era.
Varying degree of neurological complications develop in large proportion of liver transplant recipient mostly secondary to calcineurin inhibitor. Tremor is the most common symptom, and headache, paresthesia, or insomnia is other complaints that can be actually very disabling. Reduction of immunosuppressive drug dose might be helpful.
De novo malignancy has an incidence of 5-16% in different series.113 It is a major cause of late death in liver transplant recipients.114 There is irrefutable evidence of an increased incidence of skin, cervical, and lymphoid tumors after liver transplantation.115 The incidence of oropharyngeal, esophageal, lung, throat, and tongue cancer may also be increased.116 The contributors to this increased risk are high-risk behavior before transplantation (smoking, alcoholism) and the life-long use of immunosuppressive drugs. Consequently, post-transplant management should focus on the elimination of risk factors, as well as minimizing the amount of immunosuppression.
RECURRENT DISEASE AFTER TRANSPLANTATION
In the early 1990s hepatitis B recurrence was observed more than 80% of liver transplant recipients. Currently, effective antiviral prophylaxis with high dose hepatitis B immunoglobulin has virtually eliminated HBV recurrence.117 Overall survival of patients transplanted for HBV-related cirrhosis currently exceeds 90% at one year and 85% at 5 years.23
Recurrence of hepatitis C is almost universally seen. It usually presents gradually in the postoperative courses, but accelerated compared to the pretransplant setting. Protocol biopsies done 5 years after transplant showed that 20% of patients transplanted for HCV already had evidence of allograft cirrhosis.118 During recent years, however, improved survival of patients transplanted for HCV has been seen, with a 10-year patient survival of approximately 60%.119 Recent study using combination of ribavirin and pegylated interferon for recurrent HCV patients after LT showed some promising preliminary results.120
Recurrence of HCC is especially common in patients with a poorly differentiated tumor or macroscopic vascular invasion.120 Various treatment including surgical resection, transarterial chmoembolization, radiofrequency ablation, chemotherapy, and radiotherapy, should be performed for recurrent HCC. The outcome is universally dismal, but long-term survival is possible in a few selected cases received radical treatment.121
Recurrence of autoimmune disease in an organ from a donor is immunologically intriguing. Diagnosis can be difficult due to other potential causes for graft dysfunction. Recurrence of an early stage of primary biliary cirrhosis may occur in a majority of patients transplanted for this indication in the long term, but seldom leads to cirrhosis.122
Recurrence of autoimmune hepatitis is seen in 22% of patients, and is treated as in nontransplant patients.123 Recurrence of primary sclerosing cholangitis occurs in about 11% of patients,123 diagnosis may be difficult because of overlap with nonanastomotic strictures from other causes.
Retransplantation for recurrent disease is a difficult ethical issue faced by transplant teams in an era of intractable organ shortage. Perioperative risk, survival, quality of life, as well as the presence of comorbidities such as renal failure related to immunosuppression toxicity all need to be weighed in the decision to retransplant.43
RECIPIENT OUTCOMES
The survival rates after DDLT are expected more than 85% and 75% at 1-year and 5-years post-transplantation, respectively.124 The survival rate after LDLT was much lower than DDLT at the time of beginning. However, LDLT has evolved to be a valuable strategy in reducing waiting list mortality for pediatric patients and adult patients with chronic liver disease, ALF and HCC. In the East Asia including Korea, Japan, Hong Kong, where deceased organ donation is very rare, marked improvement of graft survival around 85% had achieved with help of accumulation of knowledge about portal inflow modulation, adequate graft size and venous outflow drainage, and technical innovations in LDLT during early period of 2000s.17,125,126 A recent retrospective analysis of the Adult-to-Adult LDLT cohort study (A2ALL) group clearly showed, that graft failure rate correlated with experience; i.e., centers with more than 20 LDLT had a significantly lower graft failure risk.126 Accordingly, more recent studies reported similar patient survival rates among living donor and deceased donor liver transplant recipient.61,127,128 A2ALL consortium and from UNOS data base, however, showed that LDLT was not the most appropriate solution for the patients with a MELD score >30, so-called, ACLF or chronic liver disease with severe decompensation,129,130 because the post-transplantation survival rates are poor (57%), compared to 82% survival rate in deceased donor liver transplant recipients who were on same severity.131,132 Recently patient survival rate after adult LDLT at our institution is 90.7% and 85.2% at 2-year and 5-years post-transplantation,19 and A2ALL's recommendation for ACLF patients is invalid because our large LDLT series showed 84% and 79% survival rate for high MELD (>30) recipients at 1-year and 5-years post-transplantation,47 comparable results to DDLT.131 It could be only possible with the technical refinement by transplant surgeons and the 24 hours intensive postoperative care by dedicating young surgeons, because postoperative courses of LDLT using partial liver graft is a dynamic process necessitating rapid graft regeneration and it can cause unpredictable disastrous events more frequently than DDLT.
CONCLUSIONS
Liver transplantation has revolutionized the management of acute and chronic liver diseases. The scarcity of donor organ is still the factors limiting its use. Expansion of the donor pool with increasing use of extended criteria organs has increased greatly over the last few decades. In the East Asia, LDLT has been a good alternative to DDLT regardless of disease severity and currently the outcome is not different between them. With improved survival in most transplant centers, increased attention should be given to complications that develop in the long-term.
References
- 1.Ahmed A, Keeffe EB. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007;11:227–247. doi: 10.1016/j.cld.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Murray KR, Carithers RL., Jr AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 3.Consensus Conference: Indications for Liver Transplantation, January 19 and 20, 2005, Lyon-Palais Des Congrès: text of recommendations (long version) Liver Transpl. 2006;12:998–1011. doi: 10.1002/lt.20765. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Conference Statement: liver transplantation--June 20-23, 1983. Hepatology. 1984;4:107S–110S. [PubMed] [Google Scholar]
- 5.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon RD, Fung J, Tzakis AG, et al. Liver transplantation at the University of Pittsburgh, 1984 to 1990. Clin Transpl. 1991:105–117. [PMC free article] [PubMed] [Google Scholar]
- 7.Abbasoglu O, Levy MF, Brkic BB, et al. Ten years of liver transplantation: an evolving understanding of late graft loss. Transplantation. 1997;64:1801–1807. doi: 10.1097/00007890-199712270-00030. [DOI] [PubMed] [Google Scholar]
- 8.Asfar S, Metrakos P, Fryer J, et al. An analysis of late deaths after liver transplantation. Transplantation. 1996;61:1377–1381. doi: 10.1097/00007890-199605150-00016. [DOI] [PubMed] [Google Scholar]
- 9.United Network for Organ Sharing (UNOS) Richmond (VA): UNOS; 1995. [cited 2006 Feb 12]. United Network for Organ Sharing: Organ donation and transplantation [Internet] Available from: http://www.unos.org. [Google Scholar]
- 10.Yu AS, Keeffe EB. Liver transplantation. In: Zakim D, Boyer TB, editors. Hepatology: a textbook of liver disease. 4th ed. Philadelphia: Saunders; 2003. pp. 1617–1656. [Google Scholar]
- 11.Kim ST. Liver transplantation. Korean J Gastroenterol. 1993;25:9–17. [Google Scholar]
- 12.Lee SG, Lee YJ, Park KM, et al. Living-related donor liver transplantation: the Seoul experience. Transplant Proc. 1996;28:2383–2384. [PubMed] [Google Scholar]
- 13.Lee SG, Hwang S, Kim KH, et al. Toward 300 liver transplants a year. Surg Today. 2009;39:367–373. doi: 10.1007/s00595-008-3917-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee SG, Lee YJ, Park KM, et al. Adult-to-adult living donor liver transplantation. J Korean Surg Soc. 1998;55:719–725. [Google Scholar]
- 15.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–269. doi: 10.1097/00000658-199709000-00005. discussion 269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SG, Lee YJ, Park KM, et al. Anterior segment congestion of right liver lobe graft in living donor liver transplantation and its strategy to prevent congestion. J Korean Soc Transplant. 1999;13:213–220. [Google Scholar]
- 17.Lee SG, Park KM, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation. 2002;74:54–59. doi: 10.1097/00007890-200207150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lee SG, Hwang S, Park KM, et al. An adult-to-adult living donor liver transplant using dual left lobe grafts. Surgery. 2001;129:647–650. doi: 10.1067/msy.2001.114218. [DOI] [PubMed] [Google Scholar]
- 19.Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920–927. doi: 10.1002/lt.20734. [DOI] [PubMed] [Google Scholar]
- 20.Seaberg EC, Belle SH, Beringer KC, et al. Liver Transplantation in the United States from 1987-1998: updated results from the Pitt-UNOS liver transplant registry. In: Cecka JM, Terasaki PI, editors. Clinical transplants 1998. Los Angeles: UCLA Tissue Typing laboratory; 1999. pp. 17–37. [PubMed] [Google Scholar]
- 21.Lee SG. Current situation of liver transplantation. In: Park YH, Kim SH, Lee KW, editors. Hepato-biliary-pancreatic surgery. 2nd ed. Seoul: Eui-Hak Publishing; 2006. pp. 553–562. [Google Scholar]
- 22.Kim WR, Poterucha JJ, Kremers WK, Ishitani MB, Dickson ER. Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl. 2004;10:968–974. doi: 10.1002/lt.20217. [DOI] [PubMed] [Google Scholar]
- 23.Hwang S, Lee SG, Ahn CS, et al. Prevention of hepatitis B recurrence after living donor liver transplantation: primary high-dose hepatitis B immunoglobulin monotherapy and rescue antiviral therapy. Liver Transpl. 2008;14:770–778. doi: 10.1002/lt.21440. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 25.Verdonk RC, van den Berg AP, Slooff MJ, Porte RJ, Haagsma EB. Liver transplantation: an update. Neth J Med. 2007;65:372–380. [PubMed] [Google Scholar]
- 26.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 27.Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellualr carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 28.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752–1764. doi: 10.1053/j.gastro.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Freeman RB., Jr Transplantation for hepatocellular carcinoma: the Milan criteria and beyond. Liver Transpl. 2006;12:S8–S13. doi: 10.1002/lt.20936. [DOI] [PubMed] [Google Scholar]
- 30.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curry MP. Hepatitis B and hepatitis C viruses in liver transplantation. Transplantation. 2004;78:955–963. doi: 10.1097/01.tp.0000140927.63952.58. [DOI] [PubMed] [Google Scholar]
- 32.Kotlyar DS, Campbell MS, Reddy KR. Recurrence of diseases following orthotopic liver transplantation. Am J Gastroenterol. 2006;101:1370–1378. doi: 10.1111/j.1572-0241.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 33.Triantos C, Samonakis D, Stigliano R, Thalheimer U, Patch D, Burroughs A. Liver transplantation and hepatitis C virus: systematic review of antiviral therapy. Transplantation. 2005;79:261–268. doi: 10.1097/01.tp.0000149696.76204.38. [DOI] [PubMed] [Google Scholar]
- 34.Lim JK, Keeffe EB. Liver transplantation for alcoholic liver disease: current concepts and length of sobriety. Liver Transpl. 2004;10:S31–S38. doi: 10.1002/lt.20267. [DOI] [PubMed] [Google Scholar]
- 35.DiMartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- 36.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 37.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 38.Bernuau J, Samuel D, Durand F, et al. Criteria for emergency liver transplantation in patients with acute viral hepatitis and factor V (FV) below 50% of normal: a prospective study. Hepatology. 1991;14:49A. [Google Scholar]
- 39.Lee SG, Ahn CS, Kim KH. Which types of graft to use in patients with acute liver failure? (A) Auxiliary liver transplant (B) Living donor liver transplantation (C) The whole liver. (B) I prefer living donor liver transplantation. J Hepatol. 2007;46:574–578. doi: 10.1016/j.jhep.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Moon DB, Lee SG, Ahn CS, et al. Application of intraoperative cine-portogram to detect spontaneous portosystemic collaterals missed by intraoperative Doppler exam in adult living donor liver transplantation. Liver Transpl. 2007;13:741–746. doi: 10.1002/lt.21252. [DOI] [PubMed] [Google Scholar]
- 41.Marubashi S, Dono K, Nagano H, et al. Living-donor liver transplantation with renoportal anastomosis for patients with large spontaneous splenorenal shunts. Transplantation. 2005;80:1671–1675. doi: 10.1097/01.tp.0000185087.93572.1d. [DOI] [PubMed] [Google Scholar]
- 42.Moon DB, Lee SG, Ahn CS, et al. Technical modification of reno-portal anastomosis in living donor liver transplantation for patients with obliterated portal vein and large spontaneous splenorenal shunts. Hepatogastroenterology. 2008;55:2193–2199. [PubMed] [Google Scholar]
- 43.Lopez PM, Martin P. Update on liver transplantation: indications, organ allocation, and long-term care. Mt Sinai J Med. 2006;73:1056–1066. [PubMed] [Google Scholar]
- 44.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 45.Koffron A, Stein JA. Liver transplantation: indications, pretransplant evaluation, surgery, and posttransplant complications. Med Clin North Am. 2008;92:861–888. doi: 10.1016/j.mcna.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 47.Moon DB, Lee SG, Hwang S, et al. Living donor liver transplantation for acute-on-chronic liver failure: is it justified? Liver Transpl. 2008;14:S241. [Google Scholar]
- 48.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugawara Y, Makuuchi M. Living donor liver transplantation: present status and recent advances. Br Med Bull. 2006;75-76:15–28. doi: 10.1093/bmb/ldh058. [DOI] [PubMed] [Google Scholar]
- 50.Lee SG. Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J Hepatobiliary Pancreat Surg. 2006;13:131–138. doi: 10.1007/s00534-005-1019-7. [DOI] [PubMed] [Google Scholar]
- 51.Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation: its surgical advantages compared with conventional procedures. Transplantation. 1992;54:263–268. doi: 10.1097/00007890-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Dulundu E, Sugawara Y, Sano K, et al. Duct-to-duct biliary reconstruction in adult living donor liver transplantation. Transplantation. 2004;78:574–579. doi: 10.1097/01.tp.0000128912.09581.46. [DOI] [PubMed] [Google Scholar]
- 53.Detry O, Honore P, Hans MF, Delbouille MH, Jacquet N, Meurisse M. Organ donors with primary central venous system tumor. Transplantation. 2000;70:244–248. [PubMed] [Google Scholar]
- 54.Kauffman HM, McBride MA, Cherikh WS, Spain PC, Delmonico FL. Transplant tumor registry: donors with central nervous system tumors. Transplantation. 2002;73:579–582. doi: 10.1097/00007890-200202270-00017. [DOI] [PubMed] [Google Scholar]
- 55.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 56.Lionaz C, Gonzalez EM. Marginal donors in liver transplantation. Hepatogastroenterology. 2000;47:256–263. [PubMed] [Google Scholar]
- 57.Fondevila C, Ghobrial RM. Donor selection and management. In: Busuttil RW, Klintmalm GB, editors. Transplantation of the liver. 2nd ed. Philadelphia: Elsevier Saunders; 2005. pp. 515–528. [Google Scholar]
- 58.Silberhumer GR, Pokorny H, Hetz H, et al. Combination of extended donor criteria and changes in the Model for End-stage Liver Disease score predict patient survival and primary dysfunction in liver transplantation: a retrospective analysis. Transplantation. 2007;83:588–592. doi: 10.1097/01.tp.0000255319.07499.b7. [DOI] [PubMed] [Google Scholar]
- 59.Alkofer B, Samstein B, Guarrera JV, et al. Extended-donor criteria liver allografts. Semin Liver Dis. 2006;26:221–233. doi: 10.1055/s-2006-947292. [DOI] [PubMed] [Google Scholar]
- 60.Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7:1265–1270. doi: 10.1111/j.1600-6143.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 61.Said A, Lucey MR. Liver transplantation: an update 2008. Curr Opin Gastroenterol. 2008;24:339–345. doi: 10.1097/MOG.0b013e3282f8e27e. [DOI] [PubMed] [Google Scholar]
- 62.Cameron AM, Ghobrial RM, Yersiz H, et al. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243:748–753. doi: 10.1097/01.sla.0000219669.84192.b3. discussion 753-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Florman S, Miller CM. Live donor liver transplantation. Liver Transpl. 2006;12:499–510. doi: 10.1002/lt.20754. [DOI] [PubMed] [Google Scholar]
- 64.Bar ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373–1385. doi: 10.1097/01.tp.0000216825.56841.cd. [DOI] [PubMed] [Google Scholar]
- 65.Nadalin S, Malagò M, Radtke A, et al. Current trends in live liver donation. Transpl Int. 2007;20:312–330. doi: 10.1111/j.1432-2277.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 66.Mullhaupt B, Dimitroulis D, Gerlach JT, Clavien PA. Hot topics in liver transplantation: organ allocation-extended criteria donor-living donor liver transplantation. J Hepatol. 2008;48:S58–S67. doi: 10.1016/j.jhep.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Wright L, Faith K, Richardson R, et al. Ethical guidelines for the evaluation of living organ donors. Can J Surg. 2004;47:408–413. [PMC free article] [PubMed] [Google Scholar]
- 68.Abecassis M, Adams M, Adams P, et al. Consensus statement on the live organ donor. JAMA. 2000;284:2919–2926. doi: 10.1001/jama.284.22.2919. [DOI] [PubMed] [Google Scholar]
- 69.Patel S, Orloff M, Tsoulfas G, et al. Living-donor liver transplantation in the United States: identifying donors at risk for perioperative complications. Am J Transplant. 2007;7:2344–2349. doi: 10.1111/j.1600-6143.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 70.Fan ST. Live donor liver transplantation in adults. Transplantation. 2006;82:723–732. doi: 10.1097/01.tp.0000235171.17287.f2. [DOI] [PubMed] [Google Scholar]
- 71.Hwang S, Lee SG, Jang SJ, et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. 2004;10:721–725. doi: 10.1002/lt.20172. [DOI] [PubMed] [Google Scholar]
- 72.Heffron T, Welch D, Pillen T, et al. Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis. Liver Transpl. 2006;12:972–978. doi: 10.1002/lt.20760. [DOI] [PubMed] [Google Scholar]
- 73.Yoshizawa A, Sakamoto S, Ogawa K, et al. New protocol of immunosuppression for liver transplantation across ABO barrier: the use of Rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc. 2005;37:1718–1719. doi: 10.1016/j.transproceed.2005.03.148. [DOI] [PubMed] [Google Scholar]
- 74.Testa G, Benedetti E. Adult living-donor liver transplantation with ABO-incompatible grafts. Transplantation. 2008;85:681–686. doi: 10.1097/TP.0b013e3181665172. [DOI] [PubMed] [Google Scholar]
- 75.Troisi R, Noens L, Montalti R, et al. ABO-mismatch adult living donor liver transplantation using antigen-specific immunoadsorption and quadruple immunosuppression without splenectomy. Liver Transpl. 2006;12:1412–1417. doi: 10.1002/lt.20727. [DOI] [PubMed] [Google Scholar]
- 76.Brown RS., Jr Live donors in liver transplantation. Gastroenterology. 2008;134:1802–1813. doi: 10.1053/j.gastro.2008.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valentin-Gamazo C, Malago M, Karliova M, et al. Experience after the evaluation of 700 potential donors for living donor liver transplantation in a single center. Liver Transpl. 2004;10:1087–1096. doi: 10.1002/lt.20223. [DOI] [PubMed] [Google Scholar]
- 78.Schroeder T, Malago M, Debatin JF, Goyen M, Nadalin S, Ruehm SG. "All-in-one" imaging protocols for the evaluation of potential living liver donors: comparison of magnetic resonance imaging and multidetector computed tomography. Liver Transpl. 2005;11:776–787. doi: 10.1002/lt.20429. [DOI] [PubMed] [Google Scholar]
- 79.Schroeder T, Radtke A, Kuehl H, Debatin JF, Malago M, Ruehm SG. Evaluation of living liver donors with an all-inclusive 3D multi-detector row CT protocol. Radiology. 2006;238:900–910. doi: 10.1148/radiol.2382050133. [DOI] [PubMed] [Google Scholar]
- 80.Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336–340. doi: 10.1001/archsurg.135.3.336. [DOI] [PubMed] [Google Scholar]
- 81.Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 82.Renz JF, Yersiz H, Reichert PR, et al. Split-liver transplantation: a review. Am J Transplant. 2003;3:1323–1335. doi: 10.1046/j.1600-6135.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 83.Hwang S, Lee SG, Park KM, et al. A case report of split liver transplantation for two adult recipient in Korea. Transplant Proc. 2004;36:2736–2740. doi: 10.1016/j.transproceed.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 84.Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137–148. doi: 10.1097/01.sla.0000077921.38307.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimoto T, Sugawara Y, Kishi Y, et al. Reconstruction of the middle hepatic vein tributary in right lateral sector graft. Liver Transpl. 2005;11:309–313. doi: 10.1002/lt.20349. [DOI] [PubMed] [Google Scholar]
- 86.Marcos A, Orloff M, Mieles L, et al. Functional venous anatomy for right-lobe grafting and techniques to optimize outflow. Liver Transpl. 2001;7:845–852. doi: 10.1053/jlts.2001.27966. [DOI] [PubMed] [Google Scholar]
- 87.Lee SG, Park KM, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. doi: 10.1097/00007890-200103270-00021. [DOI] [PubMed] [Google Scholar]
- 88.Lee S, Park K, Heang S, et al. Anterior segment congestion of a right liver lobe graft in living-donor liver transplantation and strategy to prevent congestion. J Hepatobiliary Pancreat Surg. 2003;10:16–25. doi: 10.1007/s10534-002-0789-5. [DOI] [PubMed] [Google Scholar]
- 89.Moon DB, Lee SG. Adult-to-adult living donor liver transplantation at the Asan Medical Center. Yonsei Med J. 2004;45:1162–1168. doi: 10.3349/ymj.2004.45.6.1162. [DOI] [PubMed] [Google Scholar]
- 90.Sugawara Y, Makuuchi M, Takayama T, et al. Liver Transplantation using a right lateral sector graft from a living donor to her granddaughter. Hepatogastroenterology. 2001;48:261–263. [PubMed] [Google Scholar]
- 91.Karahara M, Kaihara S, Oike F, et al. Living donor liver transplantation with monosegment. Transplantation. 2003;76:694–696. doi: 10.1097/01.TP.0000079446.94204.F9. [DOI] [PubMed] [Google Scholar]
- 92.Lee SG, Hwang S, Park KM, et al. An adult-to-adult living donor liver transplant using dual left lobe grafts. Surgery. 2001;129:647–650. doi: 10.1067/msy.2001.114218. [DOI] [PubMed] [Google Scholar]
- 93.Lee SG, Hwang S, Park KM, et al. Seventeen adult-to-adult living donor liver transplantations using dual grafts. Transplant Proc. 2001;33:3461–3463. doi: 10.1016/s0041-1345(01)02491-5. [DOI] [PubMed] [Google Scholar]
- 94.Cheriqui D, Soubrane D, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392–396. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 95.Lee SG, Park KM, Hwang S, et al. Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 2002;25:277–284. doi: 10.1016/S1015-9584(09)60192-5. [DOI] [PubMed] [Google Scholar]
- 96.Miller CM, Masetti M, Cautero N, et al. Intermittent inflow occlusion in living liver donors: impact on safety and remnant function. Liver Transpl. 2004;10:244–247. doi: 10.1002/lt.20071. [DOI] [PubMed] [Google Scholar]
- 97.Sugawara Y, Makuuchi M, Sano K, et al. Vein reconstruction modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180–185. doi: 10.1097/01.SLA.0000048444.40498.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cattral MS, Greig PD, Muradali D, Grant D. Reconstruction of middle hepatic vein of a living-donor right lobe liver graft with recipient left portal vein. Transplantation. 2001;71:1864–1866. doi: 10.1097/00007890-200106270-00028. [DOI] [PubMed] [Google Scholar]
- 99.Liu CL, Zhao Y, Lo CM, Fan ST. Heaptic venoplasty in right lobe live donor liver transplantation. Liver Transpl. 2003;9:1265–1272. doi: 10.1016/j.lts.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 100.Hwang S, Lee SG, Ahn CS, et al. Outflow vein reconstruction of extended right lobe graft using quilt venoplasty technique. Liver Transpl. 2006;12:156–158. doi: 10.1002/lt.20574. [DOI] [PubMed] [Google Scholar]
- 101.Ogata S, Kianmanesh R, Belghiti J. Doppler assessment after right hepatectomy confirms the need to fix the remnant left liver in the anatomical position. Br J Surg. 2005;92:592–595. doi: 10.1002/bjs.4861. [DOI] [PubMed] [Google Scholar]
- 102.Demitris AJ, Ruppert K, Dvorchik I, et al. Real-time monitoring of acute liver-allograft rejection using the Banff schema. Transplantation. 2002;74:1290–1296. doi: 10.1097/00007890-200211150-00016. [DOI] [PubMed] [Google Scholar]
- 103.Haddad EM, McAlister VC, Renouf E, et al. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;4:CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jain A, Demetris AJ, Kashyap R, et al. Does tacrolimus offer virtual freedom from chronic rejection after primary liver transplantation? Risk and prognostic factors in 1,048 liver transplantations with a mean follow-up of 6 years. Liver Transpl. 2001;7:623–630. doi: 10.1053/jlts.2001.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trotter JF. Sirolimus in liver transplantation. Transplant Proc. 2003;35:193S–200S. doi: 10.1016/s0041-1345(03)00234-3. [DOI] [PubMed] [Google Scholar]
- 106.Mazariegos GV, Molmenti EP, Kramer DJ. Early complications after orthotopic liver transplantation. Surg Clin North Am. 1999;79:109–129. doi: 10.1016/s0039-6109(05)70009-8. [DOI] [PubMed] [Google Scholar]
- 107.Hwang S, Lee SG, Sung KB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl. 2006;12:831–838. doi: 10.1002/lt.20693. [DOI] [PubMed] [Google Scholar]
- 108.Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001;7:S13–S21. doi: 10.1053/jlts.2001.29167. [DOI] [PubMed] [Google Scholar]
- 109.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 110.Sethi A, Stravitz RT. Review article: medical management of the liver transplant recipient - a primer for non-transplant doctors. Aliment Pharmacol Ther. 2007;25:229–245. doi: 10.1111/j.1365-2036.2006.03166.x. [DOI] [PubMed] [Google Scholar]
- 111.Muñoz SJ, Elgenaidi H. Cardiovascular risk factors after liver transplantation. Liver Transpl. 2005;11:S52–S56. doi: 10.1002/lt.20602. [DOI] [PubMed] [Google Scholar]
- 112.Neal DA, Tom BD, Luan J, et al. Is there disparity between risk and incidence of cardiovascular disease after liver transplant? Transplantation. 2004;77:93–99. doi: 10.1097/01.TP.0000100685.70064.90. [DOI] [PubMed] [Google Scholar]
- 113.Vallejo GH, Romero CJ, de Vicente JC. Incidence and risk factors for cancer after liver transplantation. Crit Rev Oncol Hematol. 2005;56:87–99. doi: 10.1016/j.critrevonc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 114.Saigal S, Norris S, Muiesan P, Rela M, Heaton N, O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482–487. doi: 10.1053/jlts.2002.32977. [DOI] [PubMed] [Google Scholar]
- 115.Penn I, Halgrimson CG, Starzl TE. De novo malignant tumors in organ transplant recipients. Transplant Proc. 1971;3:773–778. [PMC free article] [PubMed] [Google Scholar]
- 116.Jain A, DiMartini A, Kashyap R, Youk A, Rohal S, Fung J. Long-term follow-up after liver transplantation for alcoholic liver disease under tacrolimus. Transplantation. 2000;70:1335–1342. doi: 10.1097/00007890-200011150-00012. [DOI] [PubMed] [Google Scholar]
- 117.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 118.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitits C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 119.Belli LS, Burroughs AK, Burra P, et al. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733–740. doi: 10.1002/lt.21093. [DOI] [PubMed] [Google Scholar]
- 120.Oton E, Barcena R, Moreno-Planas JM, et al. HCV recurrence after liver transplantation: viral and histologic response to full-dose PEG-interferon and ribavirin. Am J Transplant. 2006;6:2348–2355. doi: 10.1111/j.1600-6143.2006.01470.x. [DOI] [PubMed] [Google Scholar]
- 121.Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 122.Jacob DA, Neumann UP, Bahra M, et al. Long-term follow-up after recurrence of primary biliary cirrhosis after liver transplantationin 100 patients. Clin Transplant. 2006;20:211–220. doi: 10.1111/j.1399-0012.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 123.Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systemic review. Liver Transpl. 2006;12:1813–1824. doi: 10.1002/lt.20910. [DOI] [PubMed] [Google Scholar]
- 124.Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. The survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886–897. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 125.Soejima Y, Shimada M, Suehiro T, et al. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581–586. doi: 10.1053/jlts.2003.50114. [DOI] [PubMed] [Google Scholar]
- 126.Lo CM, Fan ST, Liu CL, et al. Lessons learned from one hundred right lobe living donor liver transplantations. Ann Surg. 2004;240:151–158. doi: 10.1097/01.sla.0000129340.05238.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shiffman ML, Saab S, Feng S, et al. Liver and Intestine transplantation in the United States, 1995-2004. Am J Transplant. 2006;6:1170–1187. doi: 10.1111/j.1600-6143.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 128.Dumortier J, Adham M, Ber C, et al. Impact of adult-to-adult living donor liver transplantation on access to transplantation and patients' survival: an 8-year single-center experience. Liver Transpl. 2006;12:1770–1775. doi: 10.1002/lt.20895. [DOI] [PubMed] [Google Scholar]
- 129.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berg CL, Gillespie BW, Merion RM, et al. A2ALL Study Group. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Testa G, Malago M, Nadalin S, et al. Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340–346. doi: 10.1053/jlts.2002.32941. [DOI] [PubMed] [Google Scholar]
- 132.Kam I. Adult-adult right hepatic lobe living donor liver transplantation for status 2a patients: too little, too late. Liver Transpl. 2002;8:347–349. doi: 10.1053/jlts.2002.33194. [DOI] [PubMed] [Google Scholar]