Abstract
Background/Aims
Obesity is reported to be associated with erosive esophagitis (EE). However, the temporal association of obesity and abdominal obesity with EE is unclear. We conducted this study to investigate the temporal association of obesity, especially abdominal obesity with EE.
Methods
Among 1,182 subjects who underwent health screening examinations including upper endoscopy in both 2003 and 2006, a total 1,029 subjects with a normal esophagogastric junction on upper endoscopy in 2003 were enrolled. All subjects completed questionnaires and anthropometric measurements were obtained twice by trained personnels. The patients with newly developed EE were compared to the subjects without newly developed EE.
Results
Among 1,029 subjects, 42 (4.1%) were newly diagnosed with EE and 82 (8.0%) with hiatal hernia. The mean body mass index (BMI) in both examinations was significantly different between the two groups based on the development of erosive esophagitis (p<0.05 in both examinations). The mean waist circumference (WC) in both examinations was also significantly different between the two groups (p<0.01 in both examinations). The multivariate analysis demonstrated that EE was not associated with the BMI in 2003 and the increase of BMI; however, it was associated with the WC in 2003 (Odds ratio, 7.21; 95% CI, 1.78 to 29.19; >90 cm vs <80 cm).
Conclusions
Our study showed that abdominal circumference is an independent risk factor for EE, demonstrating a temporal relationship between abdominal obesity and EE.
Keywords: Abdominal obesity, Erosive esophagitis, Causality
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a major health problem in Western countries; the prevalence of at least weekly episodes of heartburn and/or acid regurgitation has been reported to be 14-24%.1-3 By contrast, GERD is less prevalent in Korea and other Asian countries, ranging from 2.5% to 7.1% for at least weekly symptoms.4-7 In addition, the prevalence of endoscopic erosive esophagitis in Asian populations has been reported to range from 3.4% to 9%, lower than in the West.8-11 GERD is increasing in Korea, which might be attributed to a longer life expectancy, westernized diet, and the increasing prevalence of obesity.10,11
Previous studies have shown a positive association between GERD and obesity in both the Western populations and Asians populations.12-16 Obesity is also a major risk factor for reflux-associated esophageal lesions such as erosive esophagitis, Barrett's esophagus, and esophageal adenocarcinoma.17-22 A large cohort study reported a consistent association between abdominal diameter (independent of BMI) and GERD symptoms in a white male population; however, no such association has been found in Asians.23
A meta-analysis of several studies showed that the findings of overweight and obesity satisfied several criteria for a causal association with GERD and its complications, including esophagitis and esophageal carcinoma.24 However, most of the prior studies used cross-sectional and case-control designs, which make it difficult to assess a temporal association between obesity and events, such as GERD and erosive esophagitis.25
In this study, we investigated the development of endoscopically proven erosive esophagitis in the subjects with a normal esophagogastric junction during the three years of follow-up; we evaluated the risk factors for erosive esophagitis and the temporal association between obesity and the development of erosive esophagitis.
MATERIALS AND METHODS
The study subjects were those who participated in health screening examinations at the health promotion center, Korea University Ansan Hospital from January to December in both 2003 and 2006. These examinations were performed to 4,316 subjects in 2003 and 5,116 in 2006. Among 1,182 subjects who had health check-ups including upper endoscopy in both 2003 and 2006, the exclusion of patients with a previous gastric surgery (n=3), a previous history of GERD (n=5), and medications (n=7) such as proton pump inhibitors, H2-receptor antagonists, and prokinetic drugs was needed. The patients having abnormal endoscopic findings of lower esophagus in 2003 were also excluded; these findings include erosions and ulcers diagnosed as erosive esophagitis (n=24), Barrett's esophagus (n=5), hiatal hernia (n=66), and minimal changes (n=43) such as distal esophageal erythema or hyperemia, congestion, edema, granularity, friability, prominent vascularity, and irregularity in the squamocolumnar junction. A total of 1,029 subjects were finally enrolled in this study.
After obtaining written informed consent the subjects agreed that the information gathered during the study can be used in this study, all subjects were given a self-administered questionnaire prior to endoscopy. The questionnaires inquired about current smoking, alcohol intake, education level, occupation, exercise, and medical history of chronic disease such as hypertension and diabetes mellitus. The education level was classified as low (high school or less) or high (college or more). Exercise was categorized according to total exercise time a week: none, low (less than 2 hours a week), middle (2-3 hours a week), or high (more than 3 hours a week). The serum levels of fasting glucose, cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured. Helicobacter pylori (H. pylori) infection was confirmed by histological examination of the endoscopic biopsy specimens.
Anthropometric parameters including height, weight, waist circumference (WC), and hip circumference were measured at each examination. The body mass index (BMI) was calculated as the ratio of weight (kg) to the square of the height (m2), and according to the modified WHO criteria for the Asia-Pacific guidelines26 categorized as follows: normal (less than 23 kg/m2), overweight (23-24.9 kg/m2), and obese (more than 25 kg/m2). The WC was measured at the midpoint between the lower border of the rib cage and the iliac crest by trained personnel,27 and categorized as follows: less than 80.0 cm, 80.0 to 89.9 cm, and more than 90.0 cm.
Upper endoscopy was performed using a gastroscope (Q240; Olympus Optical Co. Ltd., Tokyo, Japan) on two occasions by the investigators who had finished fellowships of gastroenterology in university hospital and were experts in endoscopy. The endoscopic findings of erosive esophagitis in lower esophagus were based on the longest length of a mucosal break and the confluence of erosions, and were classified using the Los Angeles (LA) classification as grades A-D.28 Minimal changes were not considered to represent erosive esophagitis. The patients with more than LA-A were diagnosed with erosive esophagitis. The presence of hiatal hernia and gastroduodenal lesions including atrophic gastritis, gastric ulcer, and duodenal ulcer were recorded. The study design was reviewed and approved by the Institutional Review Board of Korea University Ansan Hospital (AS09056-001).
The Pearson's chi-square test and independent t-test were used to assess the difference in risk factors between the two groups based on the development of erosive esophagitis. We also examined the difference between the two groups using the chi-square test with respect to the following factors: endoscopic findings, chronic medical disease, blood glucose, lipid, and education level. A paired t-test and analysis of covariance were performed to examine the change in the BMI and WC between the two groups. For the BMI subgroups, the patients with a BMI less than 23.0 kg/m2 were used as a reference group. For the WC subgroups, the patients with a WC of less than 80.0 cm were used as a reference group. Multivariate logistic regression analysis was performed to evaluate the risk factors of erosive esophagitis and several confounding factors. We conducted all analyses using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). Two-sided p values of less than 0.05 were considered to indicate statistical significance.
RESULTS
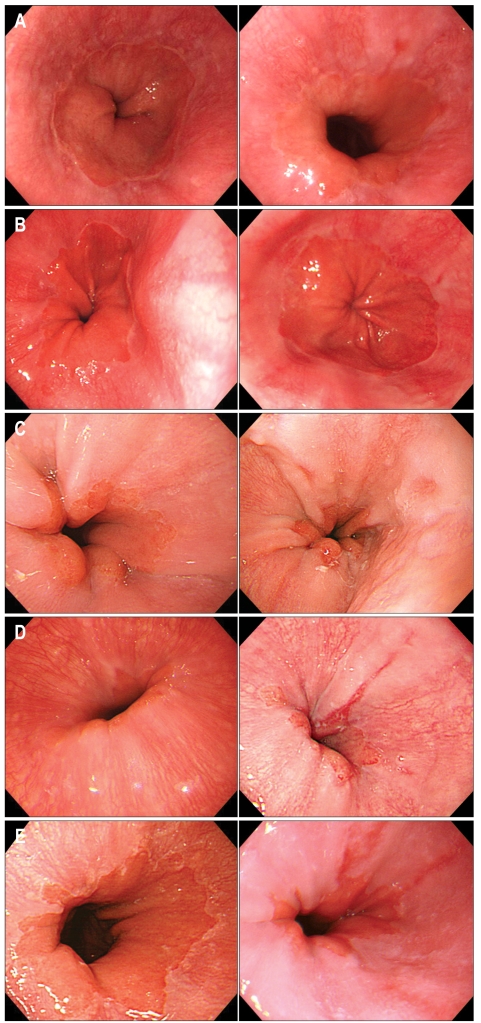
Among 1,029 subjects who met the inclusion criteria, 42 (4.1%) were newly diagnosed with erosive esophagitis in 2006; all of them had mild erosive esophagitis (35 of LA-A and 7 of LA-B) (Fig. 1). Cases of severe erosive esophagitis (LA-C or D) and Barrett's esophagus were not found. Hiatal hernia was newly diagnosed in 82 (8.0%).
Fig. 1.
Paired endoscopic pictures of lower esophagus in 5 cases with newly diagnosed erosive esophagitis (initial picture and follow-up picture 3 years later). Two cases showed newly developed erosive esophagitis LA grade A (A, B) and 3 cases newly developed erosive esophagitis LA grade B (C, D, E).
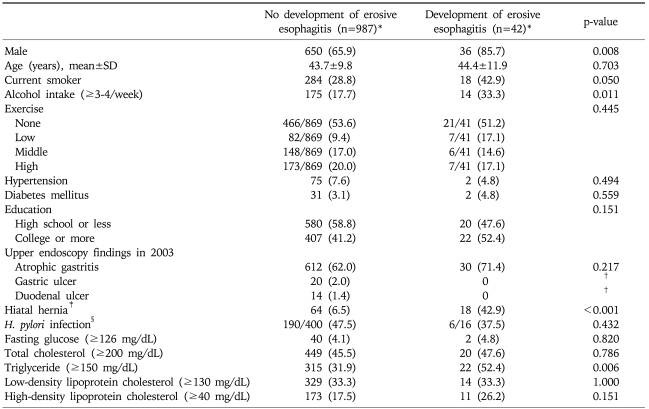
The characteristics of the two groups according to the development of erosive esophagitis were presented in Table 1. The development of erosive esophagitis was significantly increased in males (85.7%). There was a marginal difference in current smoking habits between the two groups (p=0.05). The patients with newly developed erosive esophagitis were more likely to drink alcohol compared to those without newly developed erosive esophagitis (p<0.05). There was no significant difference in education level between the two groups.
Table 1.
Comparisons of Baseline Characteristics between the Two Groups Based on Development of Erosive Esophagitis
SD, standard deviation.
*Data expressed as number (%); †p-value could not be calculated because the patients with newly developed erosive esophagitis didn't have gastric ulcer or duodenal ulcer at the upper endoscopy in 2003; ‡Upper endoscopy finding in 2006; §The histological examination for the diagnosis of H. pylori was performed on some patients who agreed the test.
At upper endoscopy in 2003, peptic ulcers such as gastric and duodenal ulcers were not found in the patients with newly developed erosive esophagitis, and atrophic gastritis was not associated with the development of erosive esophagitis. H. pylori infection rate was not significantly different between the two groups. Newly diagnosed hiatal hernia was significantly different between the two groups (p<0.01).
Chronic diseases such as hypertension and diabetes mellitus were not significantly associated with erosive esophagitis. Neither serum glucose nor lipid levels, with the exception of triglycerides were significantly associated with erosive esophagitis (Table 1).
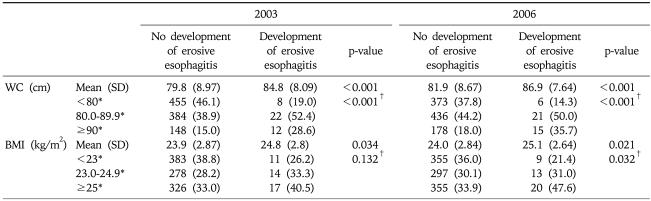
The mean BMIs in both 2003 and 2006 were significantly different between the two groups based on the development of erosive esophagitis (p<0.05 in both examinations). When the subjects were divided into three groups based on BMI (normal, overweight, and obese), erosive esophagitis did not increase with the BMI at the first examination (p for trend=0.13), although erosive esophagitis increased with the BMI at the follow-up examination (p for trend=0.03) (Table 2). The change in BMI (BMI in 2006-BMI in 2003) was not different between the two groups (p for analysis of covariance=0.38). The mean WCs in both examinations were also significantly different between the two groups (p<0.01 in both examinations). When the subjects were divided into three groups based on WC (<80, 80-<90, and ≥90 cm), erosive esophagitis increased with the WC at the first examination (p for trend <0.01) (Table 2). However, the change in WC (WC in 2006-WC in 2003) was not different between the two groups (p for analysis of covariance=0.23).
Table 2.
Distribution of BMI and Abdominal Circumference in 2003 and 2006 between the Two Groups Based on Development of Erosive Esophagitis
SD, standard deviation; WC, waist circumference; BMI, body mass index.
*Data expressed as number (%); †p-value for trend.
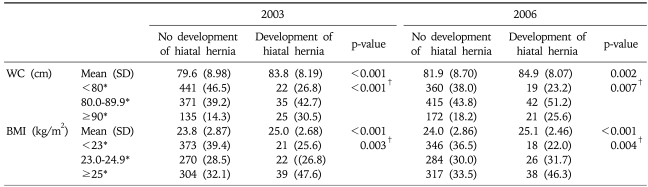
To evaluate the effect of obesity on hiatal hernia, we divided the subjects according to the development of hiatal hernia into the two groups. The mean BMIs in both 2003 and 2006 were significantly different between the two groups (p<0.01 in both examinations). When the subjects were divided into three groups based on BMI, hiatal hernia increased with the BMI at the first examination (p for trend <0.01). The mean WCs in both examinations were significantly different between the two groups. When the subjects were divided into three groups based on WC, hiatal hernia also increased with the WC at the first examination (p for trend <0.01) (Table 3). However, neither the change in WC (WC in 2006 - WC in 2003) nor in BMI (BMI in 2006-BMI in 2003) was different between the two groups based on the development of hiatal hernia.
Table 3.
Distribution of BMI and Abdominal Circumference in 2003 and 2006 between the Two Groups Based on Development of Hiatal Hernia
SD, standard deviation; WC, waist circumference; BMI, body mass index.
*Data expressed as number (%); †p-value for trend.
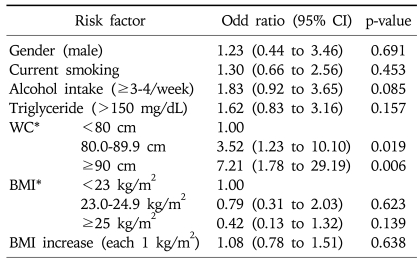
The multiple logistic regression analysis including gender, smoking, alcohol, triglycerides, BMI, WC, and an increase of BMI (each 1 kg/m2) showed that the BMI at the first examination and an increase of BMI were not associated with erosive esophagitis. However, a greater WC at the first examination was strongly associated with erosive esophagitis (OR, 7.21; 95% CI, 1.78 to 29.19; >90 cm vs <80 cm) (Table 4). In addition, erosive esophagitis increased linearly with WC (p for trend <0.05).
Table 4.
Multiple Logistic Analyses of Risk Factors Associated with the Development of Erosive Esophagitis
CI, confidence interval; WC, waist circumference; BMI, body mass index.
*WC and BMI in 2003.
DISCUSSION
As far as we are aware, this is the first study that has investigated the temporal association between obesity and erosive esophagitis through the follow-up of the subjects who had normal esophagogastric junction. Our findings show that abdominal obesity is a significant risk factor of the development of erosive esophagitis. Furthermore, these results demonstrate the temporal association between abdominal obesity and erosive esophagitis.
In our study, 42 (4.1%) subjects were newly diagnosed with erosive esophagitis, although the patients with new onset symptoms related to GERD would not be included. This might be attributed to the high loss to follow up, especially the subjects who were likely to be normal at the follow-up examination. In a recent nationwide study that was performed in the health check-up subjects in Korea, the prevalence rate of erosive esophagitis was 8%, and among the patients, 42% did not present any GERD symptoms.29 In this study, many patients with newly developed erosive esophagitis may also be asymptomatic.
Several studies have showed that GERD symptoms are associated with the BMI,12,14,15,30,31 especially in women.15,30 A large-scale cohort study in women showed that GERD symptoms were positively associated with BMI rather than abdominal obesity as measured by the waist/hip ratio.30 However, the waist/hip ratio is not an accurate measurement of abdominal obesity, since a person with a large waist and large hip has a similar ratio compared with a person with a small waist and small hip. On the contrary, a study from a large integrated health care system suggested that GERD symptoms were positively associated with abdominal obesity as measured by the abdominal diameter independent of the BMI, in a Caucasian male population; however, these findings were not confirmed in African Americans and Asians.23 In Korea, recent studies have demonstrated that obesity, especially abdominal obesity, is a significant risk factor for erosive esophagitis in the patients who underwent upper endoscopy during health check-ups.10,32 Our study showed that the patients with newly developed erosive esophagitis had higher BMI and WC at endoscopic diagnosis than the patients without newly developed erosive esophagitis, which indicates that obesity including abdominal obesity is a significant risk factor for erosive esophagitis. In addition, the abdominal obesity at the first examination was associated with the development of erosive esophagitis. These results demonstrate that abdominal obesity results in the erosive esophagitis.
Several hypotheses have been offered to explain how abdominal obesity can cause GERD. Abdominal fat may cause reflux through an increase in intra-abdominal pressure.33,34 When the intra-abdominal pressure increases, both gastric and esophageal pressures increase and anatomical disruption of the esophagogastric junction occurs. The anatomical disruption of gastroesophageal junction may result in the formation of a hiatal hernia.35 One study showed that obesity was significantly associated with esophagitis, largely through an increase incidence of hiatal hernia.36 This study could not completely evaluate the effect of hiatal hernia on erosive esophagitis because we did not include the subjects who were diagnosed with hiatal hernia at the first examination. However, our findings demonstrate that hiatal hernia is significantly associated with erosive esophagitis and obesity is a risk factor for hiatal hernia, which is consistent with previous studies.36,37 Additionally, this study showed that newly diagnosed hiatal hernia was associated with the obesity at the first examination, which demonstrates that obesity causes hiatal hernia.
The distribution of obesity may be more important than the BMI as a marker of general obesity; this is because abdominal obesity is more strongly related with the metabolic abnormalities of obesity.38 The BMI is not always an accurate estimate of adiposity, particularly in men, mainly because of their greater muscle mass.39 WC, used as an anthropometric surrogate for assessment of abdominal obesity, correlates well with visceral adiposity.40-42 Furthermore, the abdominal diameter has been most strongly associated with visceral adipose tissue among persons with BMI <27 kg/m2.43 This study demonstrated that abdominal obesity, not BMI, increased independently erosive esophagitis, which might be attributed to the fact that most of the subjects had BMI less than 27 kg/m2 (87.1%) and there were a greater number of men (67.0%).
The metabolic activity of visceral fat differs from that of peripheral fat.44 Visceral fat has been strongly associated with increased release of several proinflammatory cytokines such as interleukin 6, tumor necrosis factor-α as well as with lower serum levels of adiponectin, which may play a role in the development of GERD.45,46
A recent meta-analysis demonstrated a dose-response relationship between BMI and the risk of GERD symptoms among both men and women.24 A large-scale cohort study in women showed that weight gain was associated with an increased risk of gastroesophageal reflux symptoms, and weight loss was associated with a decrease in risk.30 However, in our study, both the increase in BMI and WC were not associated with the development of erosive esophagitis, which demonstrated that abdominal obesity was more strongly associated with erosive esophagitis than weight gain.
The strengths of this study include the followings. The study subjects underwent upper endoscopy on two occasions by trained gastroenterologists, and erosive esophagitis was diagnosed on the basis of endoscopic findings. Therefore, the diagnosis of erosive esophagitis was more accurate than if only gastroesophageal reflux symptoms were reported on the questionnaires. Second, the anthropometric measurements by trained personnel at health promotion center were more reliable than self reported measurements. In addition, these measurements were performed twice before and after the development of erosive esophagitis. Therefore, we were able to evaluate the influence of obesity before the development of erosive esophagitis.
This study has several limitations. First, the association of reflux symptoms with erosive esophagitis could not be evaluated, since the reflux symptoms such as heartburn and acid regurgitation were not correctly gathered at both examinations. However, as the subjects were the individuals presenting for health check-ups without a previous history of GERD and medications such as proton pump inhibitors, H2-receptor antagonists, and prokinetic drugs, most of the subjects would be free of symptoms. Second, we did not evaluate the dietary effects on our results, which could confound the association of obesity with erosive esophagitis. It has been reported that dietary fat, rather than obesity itself, is responsible for GERD.47 However, no consistent association has been found between dietary fat and GERD or esophageal adenocarcinoma.21,48,49 Thus, it is unlikely that intake of dietary fat plays a pivotal role in the effect of obesity on GERD. Third, we did not check κ values for the evaluation of inter-observer variations in endoscopic diagnosis. However, all investigators had finished fellowships of gastroenterology in university hospital and were experts in endoscopy.50 Additionally, the five investigators who performed endoscopy in 2006 were fully aware of a method of diagnosing erosive esophagitis and representative figures regarding erosive esophagitis through the educational poster distributed in a Korean nationwide multicenter study.29 Finally, this study was not performed in a population-based manner, since the only subjects who underwent health check-ups in both 2003 and 2006 were enrolled. Some of the subjects examined in 2003 were re-examined in 2006 and the number of aged and female subjects enrolled was relatively small. Further large-scale population-based study is needed to clarify more clearly the temporal association between abdominal obesity and erosive esophagitis.
In summary, abdominal obesity is an independent risk factor for the development of erosive esophagitis. Furthermore, this study establishes the temporal association between abdominal obesity and erosive esophagitis. These results show that abdominal obesity may cause erosive esophagitis, largely through the development of hiatal hernia. In the future, it is necessary to evaluate whether interventions that decrease abdominal circumference prevent the development of erosive esophagitis.
References
- 1.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JY. Systematic review: geographical and ethnic differences in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:705–717. doi: 10.1111/j.1365-2036.2004.02165.x. [DOI] [PubMed] [Google Scholar]
- 3.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 4.Cho YS, Choi MG, Jeong JJ, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Asan-si, Korea. Am J Gastroenterol. 2005;100:747–753. doi: 10.1111/j.1572-0241.2005.41245.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara Y, Higuchi K, Watanabe Y, et al. Prevalence of gastroesophageal reflux disease and gastroesophageal reflux disease symptoms in Japan. J Gastroenterol Hepatol. 2005;20:26–29. doi: 10.1111/j.1440-1746.2004.03521.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong WM, Lai KC, Lam KF, et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther. 2003;18:595–604. doi: 10.1046/j.1365-2036.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong WM, Lai KC, Lam KF, et al. Onset and disappearance of reflux symptoms in a Chinese population: a 1-year follow-up study. Aliment Pharmacol Ther. 2004;20:803–812. doi: 10.1111/j.1365-2036.2004.02198.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang CS, Poon SK, Lien HC, Chen GH. The incidence of reflux esophagitis among the Chinese. Am J Gastroenterol. 1997;92:668–671. [PubMed] [Google Scholar]
- 9.Hung CS, Lee CL, Yang JN, et al. Clinical application of Carlsson\'s questionnaire to predict erosive GERD among healthy Chinese. J Gastroenterol Hepatol. 2005;20:1900–1905. doi: 10.1111/j.1440-1746.2005.03929.x. [DOI] [PubMed] [Google Scholar]
- 10.Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Song CW, Jeen YT, et al. Prevalence of endoscopic reflux esophagitis among Koreans. J Gastroenterol Hepatol. 2001;16:373–376. doi: 10.1046/j.1440-1746.2001.02464.x. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Aros S, Locke GR, 3rd, Camilleri M, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–1806. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang MS, Park DI, Oh SY, et al. Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J Gastroenterol Hepatol. 2007;22:1656–1661. doi: 10.1111/j.1440-1746.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray L, Johnston B, Lane A, et al. Relationship between body mass and gastro-oesophageal reflux symptoms: The Bristol Helicobacter Project. Int J Epidemiol. 2003;32:645–650. doi: 10.1093/ije/dyg108. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi M, Oka H, Hashimoto T, et al. Obesity as a risk factor for GERD in Japan. J Gastroenterol. 2008;43:57–62. doi: 10.1007/s00535-007-2128-7. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 19.Labenz J, Jaspersen D, Kulig M, et al. Risk factors for erosive esophagitis: a multivariate analysis based on the Pro-GERD study initiative. Am J Gastroenterol. 2004;99:1652–1656. doi: 10.1111/j.1572-0241.2004.30390.x. [DOI] [PubMed] [Google Scholar]
- 20.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 21.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Stein DJ, El-Serag HB, Kuczynski J, Kramer JR, Sampliner RE. The association of body mass index with Barrett's oesophagus. Aliment Pharmacol Ther. 2005;22:1005–1010. doi: 10.1111/j.1365-2036.2005.02674.x. [DOI] [PubMed] [Google Scholar]
- 23.Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56:756–762. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 26.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne: Health Communication Australia; 2000. Regional Office for the Western Pacific, International Association for the Study of Obesity, International Obesity Task Force. [Google Scholar]
- 27.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 28.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim N, Lee SW, Cho SI, et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008;27:173–185. doi: 10.1111/j.1365-2036.2007.03561.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA., Jr Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandurkar S, Locke GR, 3rd, Fett S, Zinsmeister AR, Cameron AJ, Talley NJ. Relationship between body mass index, diet, exercise and gastro-oesophageal reflux symptoms in a community. Aliment Pharmacol Ther. 2004;20:497–505. doi: 10.1111/j.1365-2036.2004.02156.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–675. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–755. doi: 10.1136/gut.2006.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Serag HB, Tran T, Richardson P, Ergun G. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol. 2006;41:887–891. doi: 10.1080/00365520500535402. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–2312. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94:2840–2844. doi: 10.1111/j.1572-0241.1999.01426.x. [DOI] [PubMed] [Google Scholar]
- 37.Stene-Larsen G, Weberg R, Froyshov Larsen I, Bjortuft O, Hoel B, Berstad A. Relationship of overweight to hiatus hernia and reflux oesophagitis. Scand J Gastroenterol. 1988;23:427–432. doi: 10.3109/00365528809093890. [DOI] [PubMed] [Google Scholar]
- 38.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 39.von Hafe P, Pina F, Perez A, Tavares M, Barros H. Visceral fat accumulation as a risk factor for prostate cancer. Obes Res. 2004;12:1930–1935. doi: 10.1038/oby.2004.242. [DOI] [PubMed] [Google Scholar]
- 40.Conway JM, Chanetsa FF, Wang P. Intraabdominal adipose tissue and anthropometric surrogates in African American women with upper- and lower-body obesity. Am J Clin Nutr. 1997;66:1345–1351. doi: 10.1093/ajcn/66.6.1345. [DOI] [PubMed] [Google Scholar]
- 41.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness: a critical review. Int J Obes Relat Metab Disord. 1998;22:719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 43.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord. 1998;22:338–342. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]
- 44.International Agency for Research on Cancer. IARC handbook of cancer prevention: weight control and physical activity. Vol. 6. Lyon: IARC Press; 2002. [Google Scholar]
- 45.Barak N, Ehrenpreis ED, Harrison JR, Sitrin MD. Gastrooesophageal reflux disease in obesity: pathophysiological and therapeutic considerations. Obes Rev. 2002;3:9–15. doi: 10.1046/j.1467-789x.2002.00049.x. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe S, Hojo M, Nagahara A. Metabolic syndrome and gastrointestinal diseases. J Gastroenterol. 2007;42:267–274. doi: 10.1007/s00535-007-2033-0. [DOI] [PubMed] [Google Scholar]
- 47.Castell DO. Obesity and gastro-oesophageal reflux: is there a relationship? Eur J Gastroenterol Hepatol. 1996;8:625–626. [PubMed] [Google Scholar]
- 48.El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11–17. doi: 10.1136/gut.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: the NHANES I Epidemiologic Followup Study. First National Health and Nutrition Examination Survey. Ann Epidemiol. 1999;9:424–435. doi: 10.1016/s1047-2797(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]