Abstract
Background/Aims
Compact lipiodol uptake without enhancement on multiphasic helical computed tomography (CT) has been suggested as a radiologic response criterion in hepatocellular carcinoma (HCC) patients treated with transarterial chemoembolization (TACE) and subsequent partial hepatectomy. However, its usefulness has not been fully investigated in the explanted liver.
Methods
Between 1998 and 2007, 81 patients with HCC underwent 1-9 sessions of TACE followed by liver transplantation (LT). Thirty-nine tumors in 29 patients showed a radiologic response on CT performed prior to LT. The radiologic response criteria and the duration of the response were evaluated to predict total necrosis in the explanted liver.
Results
Among the 39 tumors, 34 nodules (87.2%) exhibited total pathological necrosis. While 13 out of 16 tumors (81.3%) with a radiologic response for 6 months or less were completely necrotic, 21 out of 23 tumors (91.3%) with a radiologic response of longer than 6 months showed total necrosis.
Conclusions
Our results suggested that the radiologic response criteria based on serial CT images might be useful for predicting total necrosis of TACE-pretreated HCC in LT.
Keywords: Explanted liver, Pathology, Computed tomography, Radiologic response
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major global health problem responsible for approximately one million deaths annually.1-3 The recommended curative treatments for HCC include liver transplantation (LT), surgical resection, radiofrequency ablation, and percutaneous ethanol injection.4 LT is an ideal treatment option, as it simultaneously cures HCC and replaces the premalignant cirrhotic liver.5-8 Furthermore, LT is the only curative therapy for HCC when neither surgical resection nor percutaneous ablation is feasible. Considering the limited organ supply, high cost, and considerable risk associated with LT, only those patients with a high probability of survival benefit should be selected to receive the procedure. Milan criteria (solitary HCC<5 cm or no more than 3 nodules smaller than 3 cm) have been accepted in conventional clinical practice as selection criteria for LT. The 5-year survival rate after LT exceeds 70% in patents within this criteria.4,9 Several groups have reported a 5-year survival rate of approximately 50% after LT in patients with HCC when expanded criteria based on the pathology of the explanted liver were used.10,11
Transarterial chemoembolization (TACE) is the most frequently applied palliative treatment modality for HCC and is an important bridging therapy before LT,12,13 particularly in areas where the transplant waiting list time exceeds 6 months including our country.4 TACE has been used prior to LT in an attempt to delay tumor growth or to achieve down-staging of HCC. An evaluation of the patient's response to TACE is essential in order to predict the outcome after LT. Conventional World Health Organization (WHO) criteria used for the estimation of systemic chemotherapy effects on cancer is not applicable to assess the therapeutic efficacy of TACE.14 Therefore, other imaging criteria have been suggested to estimate the possibility of residual or recurrent tumor after TACE.15-17
Compact lipiodol uptake without enhancement on multiphasic helical computed tomography (CT) has been suggested as radiologic response criteria for HCC treated with TACE.15,18-20 A recent study performed at our institution showed that, when depicting viable tumors in HCC patients who were treated with TACE followed by partial hepatectomy, a review of the multiphasic helical CT together with the previous serial CT images could help to reach a correct diagnosis for those lesions that were incorrectly diagnosed using the reviews of the last CT alone.21 However, most of the previous studies evaluated HCC patients who underwent partial hepatectomy after TACE and it has not been known whether radiologic response criteria using multhphasic helical CT could be used to predict complete necrosis in the explanted liver.16,17,22 Furthermore, the reasonable or sufficient duration of radiologic response to predict complete necrosis has not been fully investigated.
In this study, we reviewed the serial multiphasic helical CT images and pathologic specimens of explanted liver from patients with HCC treated with TACE followed by LT to evaluate the effectiveness of radiologic response criteria using multiphasic helical in predicting complete necrosis in the explanted liver.
MATERIALS AND METHODS
1. Patient selection
From January 1998 to December 2007, 81 patients with hepatocellular carcinoma underwent TACE followed by LT at our hospital. HCC was diagnosed based on pathology or clinical diagnostic criteria for HCC suggested by the Korean Liver Cancer Study Group (KLCSG) and the National Cancer Center (NCC) in Korea. According to this diagnostic criteria, in a patient positive for one or more risk factors (Hepatitis B or C virus positive, or cirrhosis), HCC can be diagnosed if one or more imaging techniques indicate HCC and serum alpha-fetoprotein (AFP) level≥400 ng/mL, or two or more imaging techniques indicate HCC when AFP level does not reach the suggested value. Spiral CT scan, gadolinium/SPIO-enhanced dynamic MRI, or hepatic artery angiography can be used for this purpose.23
Multiphasic helical liver CT images were serially taken every 3-4 months between the TACE and LT procedures. The liver CT images were retrospectively reviewed by a single expert radiologist who was blind to the pathologic findings of the explanted liver. Tumors with compact lipiodol uptake and without any contrast-enhanced areas on a multiphasic helical CT scan were defined as having a radiologic response to TACE. The following lesions were regarded as viable areas on the CT images: (i) a hyperattenuating or isoattenuating lesion seen during the hepatic arterial phase seen as a hypoattenuating lesion during the portal venous phase of the delayed phase, (ii) an area of mixed attenuation seen on the hepatic arterial phase images, which shows a hypoattenuating portion on either the portal venous phase or the delayed phase, and (iii) a nodule that was seen as isoattenuating on the hepatic arterial phase, the portal venous phase, or the delayed phase images, but seen as hypoattenuating on the unenhanced images.15
Twenty-nine patients with a single or multiple tumors fulfilling these radiologic response criteria on multiphasic helical CT taken just prior to liver transplantation were recruited. The response duration was defined as the time interval between the first CT showing a radiologic response and the last CT keeping the initial radiologic response.
2. Multphasic helical CT
CT was performed with a helical scanner (HiSpeed Advantage; GE Medical Systems, Milwaukee, WI, USA) with 5 mm section collimation, 2.5 mm interval, and 39.37 mm/sec table speed during a 25-30 second single breath-hold helical acquisition. After an IV injection of 120 mL nonionic iodinated contrast material (Iopamiro 300, Bracco, Milano, Italy; Ultravist 300, Schering, Berlin, Germany) at a speed of 3 mL/sec, the hepatic arterial phase, the portal venous phase, and the delayed phase images were obtained with delays of 30, 60, and 180 seconds, respectively.21
3. TACE
Initially, selective angiography was performed in all patients. After identifying the tumor-feeding artery, transarterial chemoembolization was performed with a mixture of iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin; Kyowa Hakko Kogyo, Tokyo, Japan) in all patients. For each 1-cm diameter of the tumor, 3 mg of doxorubicin hydrochloride and 1 cc of iodized oil were used. The injection was continued until stasis was identified in the feeding artery.21
4. Pathologic analysis and evaluation of predictive factors for complete necrosis
The explanted specimens of the tumors were reviewed by a single experienced liver pathologist. The pathologic specimens were sectioned at 5-mm thickness and the extent of tumor necrosis was estimated as a proportion of the area showing complete necrosis over the total tumor area. The percentage of tumor necrosis was determined after evaluating all of the sections.21
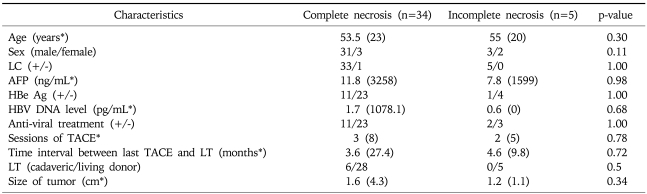
We evaluated the characteristics of patients and tumors and investigated any possible predictive factors for pathologic complete necrosis on explanted liver.
5. Statistical analysis
The Chi-square test and Student's t-test were used for data analysis. All p values were two-tailed and p values less than 0.05 were considered statistically significant. Data analyses were performed using statistics package SAS Enterprise Guide 3.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Patient and tumor characteristics
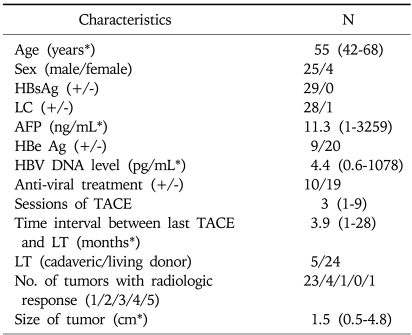
A total of 39 tumors from 29 patients showed compact lipiodol uptake on multiphasic helical CT taken before LT. These patients included 25 males and 4 females, with a median age of 55 (range, 42 to 68). All of the patients had hepatitis B virus (HBV) infection and 28 patients (96.6%) showed cirrhosis on pathologic examination of explanted liver. One to 9 sessions (median 3) of TACE were performed before LT. The last TACE session was performed 1 to 28 months (median 3.9) prior to LT. Five patients received cadaveric liver and 24 patients received a liver from a living donor. The number of tumors showing radiologic response was as follows: one in 23 patients (79.3%), two in 4 patients (13.9%), three in 1 patient (3.4%), and five in 1 patient (3.4%). The tumor size based on the pathology of explanted liver was 0.5 cm to 4.8 cm (median 1.5) (Table 1).
Table 1.
Baseline Characteristics of 29 Patients and 39 Tumors
LC, liver cirrhosis; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; LT, liver transplantation.
*Data are shown as median (range).
2. Pathologic complete necrosis and predictive factors
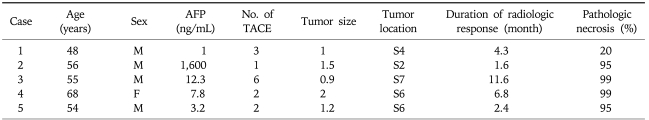
We examined the pathology of 39 tumors with a radiologic response in the explanted liver. While 34 nodules (87.2%) showed total necrosis, 5 nodules (12.8%) still showed a viable portion on the explanted specimen. The detailed characteristics of 5 cases of HCC showing incomplete necrosis independent of radiologic response are summarized in Table 2. A representative case of HCC showing complete necrosis in the explanted liver and one showing incomplete necrosis are shown in Figs. 1 and 2, respectively.
Table 2.
Summary of Five Cases of Hepatocellular Carcinoma Showing Incomplete Necrosis on Pathology Despite Showing a Radiologic Response
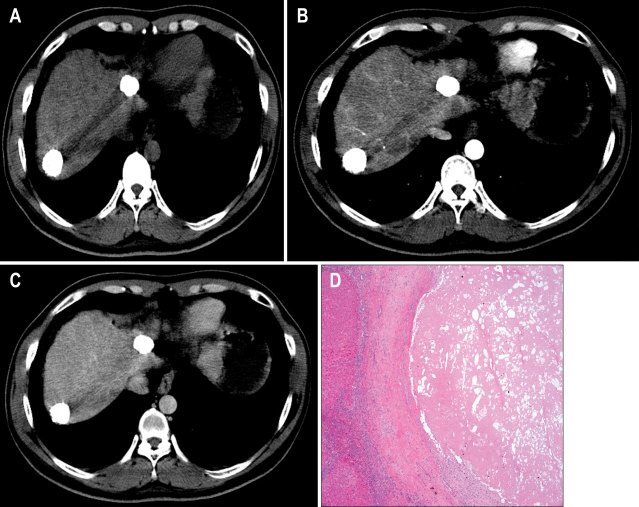
Fig. 1.
Two hepatocellular carcinoma (HCC) nodules with a radiologic response of 39.1 months showing complete necrosis in the explanted liver. (A-C) Computed tomography (CT) showing HCC nodules of 2.5 cm and 2.6 cm with complete lipiodol uptake without enhancement (A, precontrast imaging; B, arterial phase; C, delayed phase). (D) Total tumor necrosis was observed in the explanted liver (H&E stain, ×50).
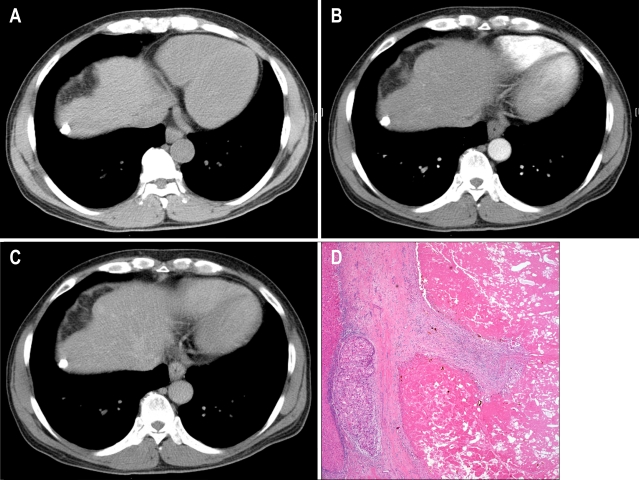
Fig. 2.
A hepatocellular carcinoma nodule with a radiologic response of 2.4 months showing incomplete necrosis in the explanted liver. (A-C) CT showing a 1.2-cm hepatocellular carcinoma (HCC) nodule with complete lipiodol uptake without enhancement (A, precontrast imaging; B, arterial phase; C, delayed phase). (D) The tumor shows a 95% necrotic portion and a 5% viable portion in the explanted liver (H&E stain, ×50).
We investigated any possible factors that could predict complete necrosis in the tumor nodules showing a radiologic response. Tumors with complete necrosis and those with incomplete necrosis showed no significant differences in the source patients' age, sex, serum AFP level, HBeAg positivity, HBV DNA level, or presence of antiviral treatment; the tumors also did not differ significantly in size (Table 3).
Table 3.
Comparison of Tumors with Complete Necrosis and Those with Incomplete Necrosis
LC, liver cirrhosis; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; LT, liver transplantation.
*Data are shown as median (range).
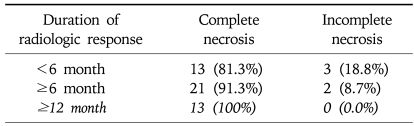
The duration of radiologic response in nodules with complete necrosis tended to be higher than those with incomplete necrosis (13.6±13.8 vs 5.2±4.0 months), although the difference was not statistically significant (p=0.20). While 13 (81.3%) of 16 tumors with a radiologic response for 6 months or less were completely necrotic, 21 (91.3%) of 23 tumors with a radiologic response longer than 6 months showed total necrosis. All 13 tumors (100%) with a radiologic response over 12 months were completely necrotic on the pathologic specimen (Table 4).
Table 4.
Complete Necrosis According to the Duration of Radiologic Response to Transarterial Chemoembolization
DISCUSSION
TACE is one of the common bridging treatments prior to LT in general clinical practice for HCC. In HCC patients who underwent TACE and were expecting LT, accurate staging based on reliable response criteria is invaluable to predict the outcome after LT and to determine whether he or she will remain on the waiting list or not. Since the extent of necrosis after TACE in HCC patients is poorly correlated with the reduction in tumor diameter, the WHO criteria or Response Evaluation Criteria in Solid Tumors (RECIST) conventionally used for the evaluation of the response to systemic chemotherapy cannot be applied for these patients.17,21,24,25
Hence, several studies have suggested other radiologic response criteria based on CT imaging to assess the effect of TACE on HCC. A study suggested that CT could be a suitable tool for the evaluation of the efficacy of TACE in patients receiving the treatment followed by partial hepatectomy based on the assumption that the portion of tumor that retains iodized oil was necrotic. The radiologic tumor necrosis rate calculated on this assumption showed good correlation with the pathologic necrosis rate.17 In addition, volumetric and morphologic CT criteria were reported to be useful for the assessment of prognosis and therapeutic success.22 New radiologic response criteria that included the extent of tumor necrosis was recently suggested by the European Association for the Study of the Liver (EASL).26 The recent report from our center demonstrated that a review of serial multiphasic helical CT images of viable tumor in HCC patients treated with TACE could help to render a correct diagnosis for those lesions that were incorrectly diagnosed depending solely on the review of the latest CT.21 Compact lipiodol uptake without enhancement on multiphasic helical CT has been suggested in previous studies as response criteria to predict complete necrosis for HCC patients receiving partial hepatectomy after TACE. However, its usefulness has not been fully investigated for the explanted liver. Furthermore, the adequate duration of radiologic response to ensure complete pathologic necrosis was not known.16,17,22
To our best knowledge, our study is the first to show that complete lipiodol uptake without enhancement on serial multiphasic helical CT could be useful in predicting complete pathologic necrosis in the explanted liver for HCC patients treated with TACE followed by LT. In our study, a total of 39 tumors in 29 patients who received 1 to 9 sessions of TACE before LT showed compact lipiodol uptake without enhancement on multiphasic helical CT. Thirty-five tumors (87.2%) showed complete necrosis in the explanted specimen. Although the difference was not statistically significant, complete necrosis tended to be higher in those with longer radiologic response (81.3% in ≤6 months, 91.3% in >6 months, 100% in >12 months).
Our study has a few weak points. Inclusion in this study was done on tumor basis, not on patient basis. Any nodules showing radiologic response were included and evaluated, even though the corresponding patient has another nodule without radiologic response. Newly developed HCC nodule in patients with existing tumor showing radiologic response could lead to some bias on data; i.e., high serum AFP or additional sessions of TACE. However, if the patients with nodules without radiologic response were excluded in this study, it would be very difficult to get sufficient number of patients for any useful data. In practice, patients with no viable HCC may require LT unless they show severe decompensation. Hence, we included the subjects on tumor basis. In addition, this study shares a limitation with others performed in single institution. Comparing the characteristics of tumors with complete necrosis to those with incomplete necrosis in explanted liver specimens, the duration of radiologic response in the complete necrosis group tended to be longer than that in the incomplete necrosis group (13.6±13.8 vs 5.2±3 months); however, the difference was not statistically significant since the incomplete necrosis group was too small.
Nevertheless, our study has some strong points. In this single center study including patients within the last 10 years, TACE was performed according to a recent standardized method and multiphasic helical CT was also done with up-to-date equipment and a standard protocol, to avoid the confounding effects of diverse treatments and imaging. In addition, CT findings were evaluated by a single expert radiologist not informed of the pathologic findings, while the pathologic specimens were reviewed by a single experienced liver pathologist. Above all, our study is the first to show that radiologic response, possibly a durable one, is useful to predict complete necrosis in the explanted liver from HCC patients after TACE.
Our results suggested that compact lipiodol uptake without enhancement on multiphasic helical CT could be useful criteria to predict total necrosis of HCC nodules in explanted liver treated with TACE prior to LT. Further studies are warranted to clarify the role of durable radiologic response on serial CT as a predictor for complete necrosis.
References
- 1.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five continents. vol. VII. Lyon: IARC Scientific Publication; 1997. [Google Scholar]
- 2.Verhoef C, Visser O, de Man RA, de Wilt JH, Ijzermans JN, Janssen-Heijnen ML. Hepatocellular carcinoma in the Netherlands incidence, treatment and survival patterns. Eur J Cancer. 2004;40:1530–1538. doi: 10.1016/j.ejca.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–285. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–228. doi: 10.1097/00000658-199109000-00005. discussion 228-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno P, Jaurrieta E, Figueras J, et al. Orthotopic liver transplantation: treatment of choice in cirrhotic patients with hepatocellular carcinoma? Transplant Proc. 1995;27:2296–2298. [PubMed] [Google Scholar]
- 8.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508–518. doi: 10.1097/01.sla.0000090449.87109.44. discussion 518-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO handbook of reporting results of cancer treatment. Geneva: World Health Organization; 1979. [Google Scholar]
- 15.Kim HC, Kim AY, Han JK, et al. Hepatic arterial and portal venous phase helical CT in patients treated with transcatheter arterial chemoembolization for hepatocellular carcinoma: added value of unenhanced images. Radiology. 2002;225:773–780. doi: 10.1148/radiol.2253011346. [DOI] [PubMed] [Google Scholar]
- 16.Choi BI, Kim HC, Han JK, et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709–713. doi: 10.1148/radiology.182.3.1311116. [DOI] [PubMed] [Google Scholar]
- 17.Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 18.Lim JH, Choi D, Kim SH, et al. Detection of hepatocellular carcinoma: value of adding delayed phase imaging to dual-phase helical CT. AJR Am J Roentgenol. 2002;179:67–73. doi: 10.2214/ajr.179.1.1790067. [DOI] [PubMed] [Google Scholar]
- 19.Hwang GJ, Kim MJ, Yoo HS, Lee JT. Nodular hepatocellular carcinomas: detection with arterial-, portal-, and delayed-phase images at spiral CT. Radiology. 1997;202:383–388. doi: 10.1148/radiology.202.2.9015062. [DOI] [PubMed] [Google Scholar]
- 20.Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1–15. doi: 10.3348/kjr.2002.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang KM, Choi D, Lim HK, et al. Depiction of viable tumor in hepatocellular carcinoma treated with transarterial chemoembolization: multiphasic helical CT with review of the previous serial CT images. Korean J Radiol. 2005;6:153–160. doi: 10.3348/kjr.2005.6.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogl TJ, Trapp M, Schroeder H, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 23.Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 24.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]