Abstract
Background/Aims
The optimal timing for interventional endoscopy in bleeding peptic ulcer disease is controversial. This study compared the outcomes between early endoscopy and delayed endoscopy in patients with bleeding peptic ulcer disease.
Methods
We conducted a prospective analysis of data from 90 patients with bleeding peptic ulcer disease who visited the emergency room between May 2006 and September 2007. Patients were categorized into two groups: the early-endoscopy group (admitted during the daytime or at night with prompt endoscopic management) and the delayed-endoscopy group (admitted at night or during weekends, with endoscopic management delayed until the next day). We compared the clinical outcomes of endoscopy between the two groups.
Results
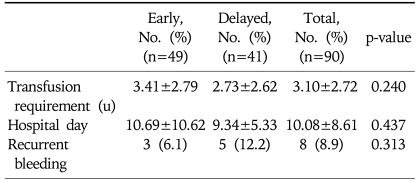
There were 49 patients in the early-endoscopy group and 41 patients in the delayed-endoscopy group. Patient demographics, clinical characteristics, bleeding control modality, and Rockall score did not differ between the two groups. There were also no significant differences between the early- and delayed-endoscopy groups in the re-bleeding rate (3/49 vs 5/41, p=0.313), the duration of hospital stay (10.7 vs 9.3 days, p=0.437), and the total amount of blood transfused (3.4 vs 2.7 units, p=0.240).
Conclusions
The effectiveness of interventional endoscopy for patients with bleeding peptic ulcer disease is not significantly affected by the timing of endoscopy.
Keywords: Bleeding peptic ulcer disease, Interventional endoscopy, Endoscopic timing
INTRODUCTION
Acute upper gastrointestinal hemorrhage (UGIH) is a common emergency in most hospitals.1 Peptic ulcer disease is the most common etiology of UGIH, and more than 40% of acute UGIH cases in North America may be attributed to bleeding peptic ulcers.2 Despite considerable advances in endoscopic hemostatic modalities and pharmacologic treatment, bleeding peptic ulcers still cause significant morbidity and mortality, usually due to advanced patient age and prevalence of concomitant diseases.3 Mortality remains around 12% and may be as high as 20% among elderly patients and those with substantial co-morbidities.1,4 Hemorrhage occurs in 20-30% of patients with peptic ulcer disease. In 70-80% of UGIH cases, bleeding is controlled with conservative management, but endoscopic therapy is beneficial in patients with peptic ulcer disease and active bleeding demonstrating visible vessels.5,6 Currently, endoscopy has been established as an effective treatment for acute UGIH, especially bleeding peptic ulcers, and is the standard of care for patients with this condition.5,6
The optimal timing of interventional endoscopy for bleeding peptic ulcer disease is controversial. In prospective studies, early endoscopy was shown to provide clinical benefit by promoting safe patient predisposition.7,8 In retrospective studies, early endoscopy also resulted in significant reductions in length of stay and the rate of recurrent bleeding or surgery for high- and low-risk groups.9 In a recent study, however, emergency endoscopy performed less than 8 hours after admission showed no definite benefit in comparison with urgent endoscopy performed within eight to 24 hours in high-risk UGIH patients.10
In the daytime, early endoscopic treatment is readily available and safe. However, it is especially difficult to manage incidental problems that might arise at nighttime or during weekends due to lack of sufficient medical personnel, medical facilities, and surgical back-up. In addition, various complications, such as hemorrhage, perforation, and aspiration may result from early endoscopic treatment.11 The aim of this study was to compare the clinical outcomes between early endoscopy and delayed endoscopy in patients with bleeding peptic ulcer disease.
MATERIALS AND METHODS
We conducted a prospective analysis of data from 125 patients with UGIH who visited the emergency room with a suspected bleeding peptic ulcer from May 2006 to September 2007 (age range, 18-80 years). All patients demonstrated UGIH symptoms such as hematemesis, melena, or hematochezia, with coffee-ground or bloody nasogastric aspirate. We enrolled the patients who were diagnosed with peptic ulcer(s) on endoscopy. Exclusion criteria were as follows: (i) cancer bleeding (n=6), (ii) varix bleeding (n=8), (iii) Mallory-Weiss syndrome (n=8), (iv) angiodysplasia (n=2), (v) acute gastric mucosal lesion (AGML) (n=1), and (vi) transference to a different medical facility (n=10). As a consequence, 90 patients were enrolled in our study.
In most studies, the definition of early endoscopy is determined by time to endoscopy after admission. But in our study, the definition of early endoscopy was determined more practically. Patients were categorized into two groups. The early endoscopy group included patients admitted during the daytime or even at night, with prompt endoscopic management. The other group was the delayed endoscopy group patients, who were admitted at night or during the weekends, with delayed endoscopic management until the next day.
We investigated and assessed the following clinical parameters: age, clinical symptoms (hematemesis, melena, hematochezia), comorbidity, medication history (NSAIDs, anti-platelet drugs), history of peptic ulcer or bleeding, and clinical signs and laboratory findings at admission (shock status, initial hemoglobin, etc). We also calculated the Rockall score for all patients.12 Pre-endoscopic scores were made up of three risk factors (age, shock, and comorbidity) and post-endoscopic scores were consisted of endoscopic findings and stigmata of bleeding. Total Rockall scores were calculated as the sum of pre-endoscopic scores and post-endoscopic scores (total score range, 0-10).
Endoscopic procedures were performed by three gastroenterologists consisted of two fellows and one faculty. There was no difference in endoscopist's experiences in performing endoscopic therapy between two groups.
In the delayed endoscopy group, we treated with an 80 mg bolus of intravernous pantoprazole followed by continuous infusion of intravenous pantoprazole 8 mg per hour for 72 hours before and after endoscopy. Early endoscopy group were treated with an 80 mg bolus of intavernous pantoprazole before endoscopy, and continuous infusion of intravenous pantoprazole 8 mg per hour for 72 hours after endoscopy.
Endoscopic findings noted were bleeding site, the size of ulcer, and the stages of ulceration using the Forrest classification. Endoscopic therapy was performed in all patients with Forrest type Ia to IIa ulcers and in some patients with Forrest type IIb or III ulcers. Therapeutic modalities included epinephrine injection, alcohol injection, hemoclip placement, and argon plasma coagulation (APC). According to the amount of bleeding, the extent of ulceration, and the degree of vessel exposure, we used single or combined interventional therapy. Re-bleeding was defined as fresh hematemesis, melena, or hematochezia, and reduction in hemoblobin level in excess of 2 g/dL between 6 hours and 2 weeks after clinical stability.
We compared clinical outcomes, such as the rate of re-bleeding, duration of hospital stay, and the total amount of transfusion between the two groups. This study was carried out in accordance with the Helsinki declaration as revised in 1989 and approved by institutional review board in Hanyang University Guri Hospital.
Statistical analysis
We analyzed demographic characteristics, clinical characteristics, endoscopic findings, therapeutic modalities, and clinical outcomes by categorization of the early and delayed endoscopy groups. Categorical data were compared using Fisher's exact test or a chi-squared test, and comparisons of continuous data were made using the Mann-Whitney test. p values of less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS 12.0 version (SPSS Inc., Chicago, IL, USA).
RESULTS
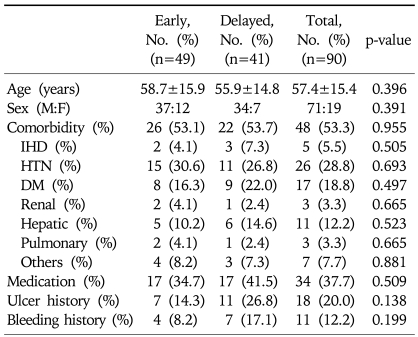
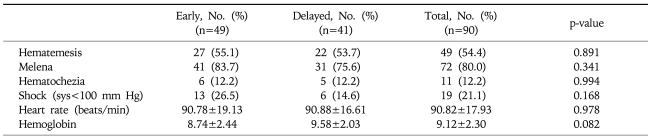
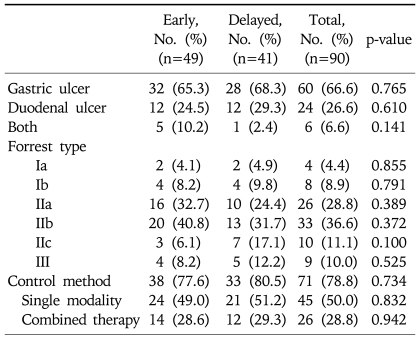
A total of 90 patients with peptic ulcer bleeding were enrolled. Of these, 49 underwent early endoscopy (37 men; mean age, 58.7 years) and 41 underwent delayed endoscopy (34 men; mean age, 55.9 years). The difference between the groups with respect to these demographic characteristics was not statistically significant (Table 1). Clinical characteristics of the patients are shown in Table 2. Major presentations including hematemesis, melena, hematochezia, systolic blood pressure, heart rate, and hemoglobin also showed no statistically significant difference between groups. The mean duration from admission to initial endoscopic examination was 18.63 hours in the delayed endoscopy group, and 4.02 hours in the early endoscopy group. Endoscopic findings are shown in Table 3. Gastric ulcers were more common than duodenal ulcers (66.6%, 26.6%, respectively), but their frequencies did not differ between the two groups. In staging of ulcers by Forrest classification, there was no statistically significant difference between the two groups. Endoscopic treatment was performed in 71 patients (78.8%). All patients with active bleeding (Forrest type Ia and Ib) and with visible vessels (Forrest type IIa) received endoscopic treatment. In Forrest type IIb, adherent clots were removed and endoscopic treatment of the underlying lesion was performed, but if clots were too small and showed no evidence of bleeding, the treatment was not performed. Thirty-eight patients (77.6%) in the early endoscopy group and 33 patients (80.5%) in the delayed endoscopy group underwent endoscopic therapy, but treatment modality showed no significant difference in either group. Clinical outcomes are shown in Table 4. The total Rockall score was not statistically different between the two groups (5.1±1.9 vs 4.9±1.5, p=0.08). The total amount of blood transfused and hospital days were similar in both groups. Furthermore, there was no significant difference in recurrent bleeding rate between the early endoscopy group and delayed endoscopy group (6.1% vs 12.2%, p=0.313).
Table 1.
Demographic Characteristics of the Study Patients
Plus-minus values are means±SD.
IHD, ischemic heart disease; HTN, hypertension; DM, diabetes mellitus.
Table 2.
Clinical Characteristics of the Study Patients
Plus-minus values are means±SD.
Table 3.
Endoscopic Findings and Treatment Modalities
Table 4.
Clinical Outcomes according to the Timing of Endoscopy
Plus-minus values are means±SD.
u, unit.
DISCUSSION
The optimal timing of interventional endoscopy for bleeding peptic ulcer disease has not been clearly established, and no consensus exists regarding the benefits and costs of early endoscopy in UGIH. Previous studies have shown that intensive resuscitation and prompt, aggressive endoscopic therapy reduces mortality in acute nonvariceal UGIH.6 According to a systematic review, the existing data suggests that early endoscopy might be safe and effective for all risk groups.13 On the other hand, Schacher et al. showed that early endoscopy in an emergency room did not improve the clinical outcome in patients with bleeding peptic ulcers.14 Furthermore, in a recent study, emergency endoscopy performed less than 8 hours after admission showed no definite benefit in comparison to urgent endoscopy performed within 8 to 24 hours in high-risk UGIH patients.10 In the current study, the clinical outcomes including transfusion rate, length of hospitalization, and re-bleeding rate did not differ between the early and delayed endoscopy group patients.
There is no general consensus on the definition of early endoscopy in UGIH as yet. In previous studies, the definition of early endoscopy was defined most of the time as the interval from admission to endoscopy, such as under 8 hours,10 under 12 hours,15 and even under 2 hours.16 We applied different definitions to the timing of endoscopy according to when the endoscopy was performed. In our study, "early" endoscopy means that it was performed "promptly", regardless of the time it was performed.
Early endoscopy is difficult to perform in practical situations, because it not only requires endoscopists, physicians, nurses, and an equipment technician, but it also requires a facility investment and surgical back-up. Some studies have shown that early endoscopy might be associated with more complications than delayed endoscopy, such as significant oxygen desaturation.16,17 According to our results, we suggest that early endoscopy need not be unconditionally performed in patients with bleeding peptic ulcers.
Various endoscopic therapies such as epinephrine injection, hemoclip placement, and argon plasma coagulation (APC) were performed in both groups. No single method of endoscopic injection therapy or thermal coaptive therapy is superior to another, and it is reasonable to use combination modalities to treat high-risk stigmata.18 Because the ulcer stage was not significantly different between the two groups, treatment modality also showed no significant difference between the two groups.
In this study, the clinical outcomes, especially recurrent bleeding rate, were not significantly different between the early endoscopy group and delayed endoscopy group. The recurrent bleeding rate in our study was less than that of a previous study (8.9% vs 10%).10 We reasoned that this difference was probably due to the fact that our study included all risk groups, whereas the previous study included only high-risk patients, and the proportion of active bleeding ulcers (Forrest type Ia, Ib) was too small.
There are several limitations to our study. First, we could not carry out out risk stratification according to risk factors such as age and vital signs because of the small sample size. However, prospective design and data of this study are major strengths because previous studies for patients with bleeding peptic ulcer are retrospective in design.19,20 It is nevertheless desirable to perform a future study that includes more patients and analyzes the results by risk stratification. Second, it was difficult to categorize the patients into two groups. Although the patients' demographic and clinical characteristics showed no significant difference between the two groups, a selection bias in the definition of early or delayed endoscopy may exist according to the clinical situation, medical facilities, and the experience of the endoscopist. Third, our results are not always applicable in all patients with UGIH, such as variceal bleeding, because we only considered patients with peptic ulcer bleeding in this study. Though peptic ulcer disease is the most common etiology of UGIH, other etiologies of UGIH cannot be excluded. Hence, we need to pay attention to the individual patient's history, clinical features, and origin of bleeding. Last but not the least, since the incidence of active bleeding ulcers - especially Forrest type Ib - was too small (13.3%), there could be a possibility that clinical outcomes between early and delayed endoscopy groups have actually no difference.
Finally, it was not possible to estimate the influence of proton-pump inhibitor (PPI) therapy. PPI therapy is warranted in all patients with UGIH severe enough to require endoscopic therapy,21 and combination of endoscopic therapy with PPI is indicated for nonbleeding ulcers at endoscopy (Forrest type IIa, IIb) with the intent to reduce re-bleeding and surgery.22 PPI therapy is also recommended in patients with suspected peptic ulcer bleeding associated with hemodynamic instability, and in patients for whom endoscopic evaluation is delayed or unavailable.21 The re-bleeding rate in the delayed endoscopy group was similar to that of early endoscopy group, suggesting the presence of a PPI effect before endoscopy. Several trials reported data for re-bleeding within three days after PPI treatment. There was a significant difference in the 3-day re-bleeding rates in favour of PPI treatment compared to control. Especially, high-dose intravenous PPI treatment reduced the rates of re-bleeding and surgical intervention.23
In conclusion, the effectiveness of interventional endoscopy for patients with bleeding peptic ulcer disease was not significantly affected by the timing of endoscopy. In other words, early endoscopy performed at night showed no definite benefit as compared to delayed endoscopy performed during the day. However, the patient's history and clinical characteristics should be taken into consideration when evaluating whether to perform prompt or delayed endoscopy according to the clinical situation. Further prospective randomized studies will be needed to demonstrate the benefits and costs for UGIH patients according to the timing of endoscopy.
ACKNOWLEDGEMENTS
This work was supported by the research fund of Hanyang University Industrial Digital Park (200700000005845).
References
- 1.Rockall TA, Logan RF, Devlin HB, Northfield TC Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. BMJ. 1995;311:222–226. doi: 10.1136/bmj.311.6999.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boonpongmanee S, Fleischer DE, Pezzullo JC, et al. The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated. Gastrointest Endosc. 2004;59:788–794. doi: 10.1016/s0016-5107(04)00181-6. [DOI] [PubMed] [Google Scholar]
- 3.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717–727. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 4.Chung SC. Peptic ulcer bleeding. Am J Gastroenterol. 2001;96:1–3. doi: 10.1111/j.1572-0241.2001.03444.x. [DOI] [PubMed] [Google Scholar]
- 5.Sacks HS, Chalmers TC, Blum AL, Berrier J, Pagano D. Endoscopic hemostasis. An effective therapy for bleeding peptic ulcers. JAMA. 1990;264:494–499. doi: 10.1001/jama.264.4.494. [DOI] [PubMed] [Google Scholar]
- 6.Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102:139–148. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- 7.Laine L, Cohen H, Brodhead J, Cantor D, Garcia F, Mosquera M. Prospective evaluation of immediate versus delayed refeeding and prognostic value of endoscopy in patients with upper gastrointestinal hemorrhage. Gastroenterology. 1992;102:314–316. doi: 10.1016/0016-5085(92)91816-m. [DOI] [PubMed] [Google Scholar]
- 8.Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy. 1998;30:513–518. doi: 10.1055/s-2007-1001336. [DOI] [PubMed] [Google Scholar]
- 9.Cooper GS, Chak A, Way LE, Hammar PJ, Harper DL, Rosenthal GE. Early endoscopy in upper gastrointestinal hemorrhage: associations with recurrent bleeding, surgery, and length of hospital stay. Gastrointest Endosc. 1999;49:145–152. doi: 10.1016/s0016-5107(99)70478-5. [DOI] [PubMed] [Google Scholar]
- 10.Tai CM, Huang SP, Wang HP, et al. High-risk ED patients with nonvariceal upper gastrointestinal hemorrhage undergoing emergency or urgent endoscopy: a retrospective analysis. Am J Emerg Med. 2007;25:273–278. doi: 10.1016/j.ajem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Blocksom JM, Tokioka S, Sugawa C. Current therapy for nonvariceal upper gastrointestinal bleeding. Surg Endosc. 2004;18:186–192. doi: 10.1007/s00464-003-8155-4. [DOI] [PubMed] [Google Scholar]
- 12.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel BM, Vakil NB, Ofman JJ. Endoscopy for acute non-variceal upper gastrointestinal tract hemorrhage: is sooner better? A systematic review. Arch Intern Med. 2001;161:1393–1404. doi: 10.1001/archinte.161.11.1393. [DOI] [PubMed] [Google Scholar]
- 14.Schacher GM, Lesbros-Pantoflickova D, Ortner MA, Wasserfallen JB, Blum AL, Dorta G. Is early endoscopy in the emergency room beneficial in patients with bleeding peptic ulcer? A "fortuitously controlled" study. Endoscopy. 2005;37:324–328. doi: 10.1055/s-2004-826237. [DOI] [PubMed] [Google Scholar]
- 15.Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. 1996;22:267–271. doi: 10.1097/00004836-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Yen D, Hu SC, Chen LS, et al. Arterial oxygen desaturation during emergent nonsedated upper gastrointestinal endoscopy in the emergency department. Am J Emerg Med. 1997;15:644–647. doi: 10.1016/s0735-6757(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 17.Hill DB, Stokes BD, Gilinsky NH. Arterial oxygen saturation during emergency esophagogastroduodenoscopy. The effects of nasal oxygen. J Clin Gastroenterol. 1994;18:284–286. doi: 10.1097/00004836-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hay JA, Maldonado L, Weingarten SR, Ellrodt AG. Prospective evaluation of a clinical guideline recommending hospital length of stay in upper gastrointestinal tract hemorrhage. JAMA. 1997;278:2151–2156. [PubMed] [Google Scholar]
- 20.Cooper GS, Chak A, Connors AF, Jr, Harper DL, Rosenthal GE. The effectiveness of early endoscopy for upper gastrointestinal hemorrhage: a community-based analysis. Med Care. 1998;36:462–474. doi: 10.1097/00005650-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Erstad BL. Proton-pump inhibitors for acute peptic ulcer bleeding. Ann Pharmacother. 2001;35:730–740. doi: 10.1345/aph.10306. [DOI] [PubMed] [Google Scholar]
- 22.Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol. 2005;100:207–219. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 23.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;25:CD002094. doi: 10.1002/14651858.CD002094.pub3. [DOI] [PubMed] [Google Scholar]