Abstract
Adult celiac disease is a chronic intestinal disorder that has been estimated to affect up to 1-2% of the population in some nations. Awareness of the disease has increased, but still it remains markedly underdiagnosed. Celiac disease is a pathologically defined condition with several characteristic clinical scenarios that should lead the clinician to suspect its presence. Critical to diagnosis is a documented responsiveness to a gluten-free diet. After diagnosis and treatment, symptoms and biopsy-proven changes may recur and appear refractory to a gluten-free diet. Recurrent symptoms are most often due to poor diet compliance, a ubiquitous and unrecognized gluten source, an initially incorrect diagnosis, or an associated disease or complication of celiac disease. Some patients with persistent symptoms and biopsy-proven changes may not have celiac disease at all, instead suffering from a sprue-like intestinal disease, so-called unclassified sprue, which is a specific entity that does not appear to respond to a gluten-free diet. Some of these patients eventually prove to have an underlying malignant cause, particularly lymphoma. The risk of developing lymphoma and other malignancies is increased in celiac disease, especially if initially diagnosed in the elderly, or late in the clinical course of the disease. However, recent studies suggest that the risk of gastric and colon cancer is low. This has led to the hypothesis that untreated celiac disease may be protective, possibly due to impaired absorption and more rapid excretion of fat or fat-soluble agents, including hydrocarbons and other putative cocarcinogens, which are implicated in the pathogenesis of colorectal cancer.
Keywords: Adult celiac disease, Malignancy in celiac disease, Lymphoma, Small intestinal cancer, Colonic neoplasms
INTRODUCTION
Celiac disease has become increasingly recognized. In Finland, a prevalence of up to 2% has been noted in recent decades.1 In adults, the disorder often presents with chronic diarrhea, weight loss and malabsorption. However, some have no diarrhea and weight loss is not evident. Instead, iron deficiency or alterations in blood chemistry values (e.g., low serum albumin) occur. Or, a closely-linked clinical disorder may be present (e.g., autoimmune thyroid disease, insulin-dependent diabetes, dermatitis herpetiformis). More recently, there has been an increased recognition that some neurological disorders, such as dementia in the elderly, should even be considered.2 Finally, positive screening blood tests (e.g., endomysial or tissue transglutaminase antibodies), though not diagnostic, may lead to suspicion of celiac disease.
DIAGNOSIS OF CLASSICAL CELIAC DISEASE
Diagnosis of celiac disease rests on 2 specific, and sequential, criteria: first, typical biopsy changes need to be shown in proximal small intestine before treatment; and second, an unequivocal clinical and/or pathological response must be documented in response to a gluten-free diet.3 With a gluten-free diet, the diarrhea should resolve and weight gain should occur, at least sufficient to provide a convincing clinical diagnosis. However, with limited symptoms, repeated biopsies may be needed to demonstrate histological improvement. Recently, it has become appreciated that over 50% of celiacs may now be classified as overweight or obese.4 In these, weight gain has also been shown with a gluten-free diet.
Serologic tests may be used for screening, but cannot be solely relied upon for diagnosis.5,6 Interestingly, the fate of different celiac antibodies in genetically at-risk children on a normal diet have been examined and shown to spontaneously disappear.7 False negative serological tests also occur (e.g., concomitant IgA deficiency). So, if there is clinical suspicion of celiac disease in an adult, then a biopsy should be done. Clearly, a biopsy will be needed to determine if the serologically-based suspicion of celiac disease was correct since false-positive blood tests also occur. In a recent study, for instance, no biopsy evidence of celiac disease was present despite very high tissue transglutaminase values.8
Histopathological changes in classic celiac disease are typically present in the proximal rather than distal small bowel and have been classified elsewhere based on the degree of altered architecture.3 Other different classification methods have been reported. For example, the Marsh classification9 as modified by Oberhuber et al.10 has found its way into the clinical realm, but a recent histopathological evaluation concluded that this particular schema was cumbersome and intraobserver agreement was reduced.11
Most often, a "severe (flat) mucosal lesion" is found (Figs. 1 and 2). This has also been labeled the "flat destructive" or "Marsh 3 lesion". The villi are rudimentary. Lamina propria lymphoid cell elements are increased, particularly plasma cells and lymphocytes. Intraepithelial lymphocytes are also increased. The surface epithelium may appear more cuboidal (rather than columnar). Crypt epithelium is hyperplastic with increased numbers of cells and an increased mitotic index. Subcellular changes (e.g., increased epithelial vacuolization) occur and the glycocalyx is altered (i.e., shown with fluorescein-linked lectins).12 With a strict gluten-free diet, these histopathological changes revert to normal.3 Comparable biopsy sites will eventually improve towards normal (Fig. 3), but these changes may require prolonged periods,13 especially in older adults.14
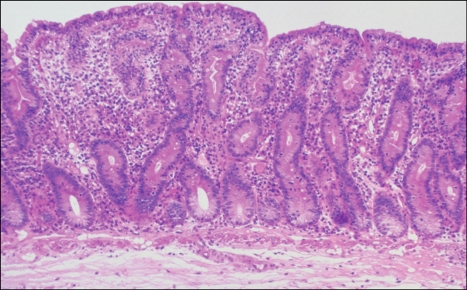
Fig. 1.
Biopsy-proven changes of untreated celiac disease. Villi are "flattened" and rudimentary, while crypts are expanded and hyperplastic with increased numbers of epithelial cells and an increased mitotic index. The cellularity of the lamina propria is enhanced, and there is an increased number of plasma cells and lymphocytes (Adapted from Freeman HJ. Pearls and pitfalls in the diagnosis of adult celiac disease. Can J Gastroenterol 2008;22:273-280).
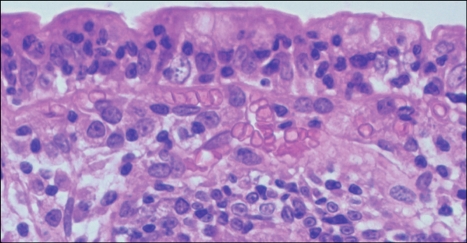
Fig. 2.
High-magnification photograph of the field shown in Fig. 1 showing increased numbers of intraepithelial lymphocytes (Adapted from Freeman HJ. Pearls and pitfalls in the diagnosis of adult celiac disease. Can J Gastroenterol 2008; 22:273-280).
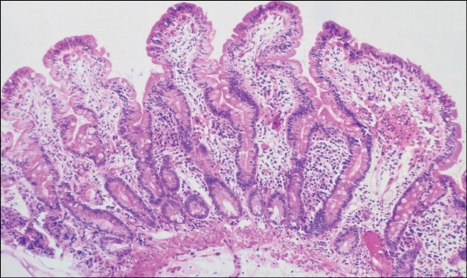
Fig. 3.
Biopsy specimen showing normalization of biopsyproven changes following implementation of a gluten-free diet. The villi are elongated, the crypt shortened, and the cellularity of the lamina propria is much reduced (Adapted from Freeman HJ. Pearls and pitfalls in the diagnosis of adult celiac disease. Can J Gastroenterol 2008;22:273-280).
In some, less severe architectural changes have been noted. In part, this may reflect increased use of serological detection methods. The villous architecture may be minimally altered and labeled a "mild lesion" (i.e., "infiltrative" or "Marsh 1 lesion"). However, epithelial cells show some loss of polarity and intraepithelial lymphocytes are increased.3,9 In a "moderate lesion" (i.e., "infiltrative hyperplastic" or "Marsh 2 lesion"), definite changes in the architecture of the villus are visualized. Less severe changes may also occur in dermatitis herpetiformis and asymptomatic first-degree relatives.3,9 If less severe changes are detected, however, studies to exclude other non-celiac causes, particularly infections (e.g., giardiasis, cryptosporidiosis) are critical. Epithelial lymphocytosis alone with normal small bowel architecture may respond to a gluten-free diet in about 10%, suggesting that, occasionally, an increased intraepithelial lymphocyte count alone may suggest celiac disease.15 Some have claimed that the celiac disease can be defined without quantitative changes in lymphocyte numbers using immunohistochemical markers alone. However, these reported qualitative changes need to be confirmed.16
Pathological changes in celiac disease also occur along the length of the small bowel but may be poorly appreciated.17 These may have particular importance in assessing the response to a gluten-free diet. The extent of these histological changes generally seems to correlate with the severity of the clinical presentation. In classical celiac disease with diarrhea and weight loss, severe pathological changes may extend well into the jejunum. In the ileum, variably severe, often patchy, changes may also occur, but usually, ileal biopsies are normal. Some believe that this "proximal-to-distal" gradient in severity of pathological changes reflects higher concentrations of dietary gluten (or its derivative peptides) in the proximal small bowel. Alternatively, changes may be "indirect" due to immune-mediated effects driven by re-circulating memory T-cells.18 Even though the ileum is often normal, prior studies have shown that the ileum in celiac disease is very sensitive to gluten peptides infused through long intestinal tubes.17 With extensive small intestinal involvement, diarrhea and malabsorption of many nutrients may result. In contrast, deficits may be very limited if the extent of pathological change is limited (e.g., duodenum alone). In this setting, diarrhea and weight loss may not be evident. In some, only isolated iron deficiency may be present, in part, due to the major localization of celiac disease in the duodenum, the principal site for iron absorption.
Treatment with a strict gluten-free diet normally should result in resolution of the diarrhea and significant weight gain. As the clinical state improves, biopsies normalize, initially in more distal sites of small bowel involvement, and later, in the proximal small bowel.3 To verify that a gluten-free diet biopsy response has occurred, however, prolonged periods of gluten restriction may be necessary, even months or years, before normal proximal duodenal mucosa can be defined.13 Repeated endoscopic biopsies may sometimes be done, usually from duodenum. If normalization of duodenal biopsies fails to occur within a few months, a potential clinical pitfall may result. The disease may be erroneously labeled "refractory", even though a diet-induced response may have been initiated, but only in more distally involved small bowel.19
OCCULT AND LATENT CELIAC DISEASE
Celiac disease may be clinically occult and may not be detected even until late adult years.20 In some with subclinical disease, only isolated deficiencies of specific nutrients (i.e., iron, calcium) may develop without diarrhea or weight loss. Typical intestinal symptoms are absent or minimal and some have termed this presentation as "silent". Some initially present with the skin disorder, dermatitis herpetiformis,21,22 autoimmune thyroid disease,23 insulin-dependent diabetes,24 a small intestinal carcinoma25 or lymphoma.26 Occult celiac disease has also been associated with other intestinal disorders including: collagenous colitis,27 lymphocytic colitis,28 lymphocytic sclerosing cholangitis,29 collagenous and lymphocytic gastritis.30
Latent celiac disease is a separate form of subclinical disease initially reported in dermatitis herpetiformis,31 and later in small intestinal lymphoma.32 In these reports, the small intestine was initially biopsy-normal. Then, pathological changes of variable severity were induced with a high-gluten diet showing that the mucosa was gluten-sensitive (as volunteers fed high gluten diets do not develop histological changes). These gluten-induced changes also improved in both reports with a gluten-free diet.
ROLE OF ENDOSCOPY IN DIAGNOSIS
The diagnosis of celiac disease is pathologically-based. Detection is optimized by submission of quality biopsies and close communication with an expert histopathologist. Endoscopic changes represent macroscopic changes only, are not specific and reflect the disappearance of normal mucosal patterns.33-35 These cannot be relied upon for a precise diagnosis. A smooth tubular surface with loss of normal folds, "scalloped folds", a mucosal "mosaic" pattern with bulbar micronodularity are descriptive changes that are often used. "Scalloped folds", however, have also been reported in Crohn's disease with duodenal involvement.36 Similar macroscopic changes have been reported with emerging methodologies including capsule and double-balloon enteroscopy. These mucosal changes that occur with direct visualization, however, may be limited and their correlation with microscopic alterations is limited. Conversely, and most important, a "normal" endoscopic appearance is not sufficient to exclude celiac disease. Experienced endoscopists, for example, estimated that about 10% of their celiacs were only recognized because random biopsies from the duodenum were taken, not because endoscopic abnormalities were visualized.37
OTHER CAUSES OF SEVERE ("FLAT") OR VARIABLY SEVERE LESIONS
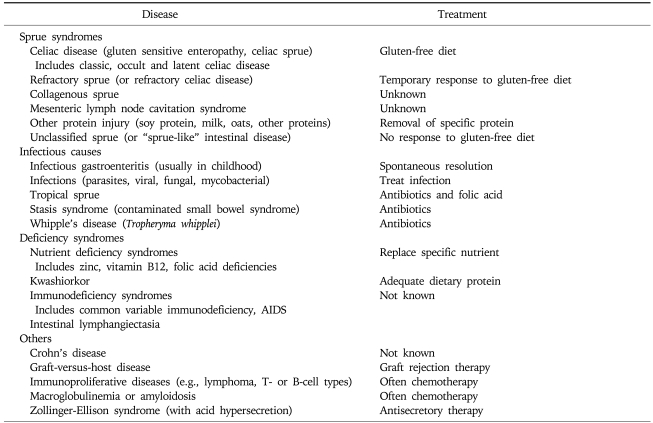
Other causes of severe or variably severe small bowel biopsy changes may be associated with diarrhea or malabsorption (Table 1). But, only celiac disease responds to a strict gluten-free diet. Oats may be safely consumed, but also can be contaminated by other grains.38 In addition, oats alone has been reported to induce abnormalities in villous architecture.39
Table 1.
Causes of a Severe "Flat" or Variably Severe Biopsy Lesion*
*Modified from Freeman HJ. Small intestinal mucosal biopsy for investigation of diarrhea and malabsorption in adults. Gastrointest Endosc Clin N Am 2000;10:739-53, vii.
Infections can produce a spectrum of histopathological changes, appearing like classical celiac disease. However, these changes are usually temporary and generally respond to antibiotic treatment. These may occur in adults, but less often than in children. These may be due to an undetected viral agent or a bacterial infection that spontaneously resolved without treatment.40 In adults, architectural disturbance may be quite severe, but is usually variable. In giardiasis, for example, a common protozoan pathogen, up to 15% may have changes that are so architecturally abnormal as to mimic celiac disease (at least, until the organisms are detected in fecal material, luminal aspirates or the biopsy specimen). Other protozoans should also be excluded and include: Isospora belli, Cryptospordium parvum, Cyclospora cayetanensis and other microsporidians such as Enterocytozoon bieneusi. Acquired immune deficiency states that follow transplantation or HIV (retroviral) infection may also be associated with severe biopsy changes.41 In the latter setting, the precise cause of pathologic changes may be difficult to determine because multiple infectious agents may be present. The retroviral agent per se may be directly responsible, or alternatively, indirectly due to the immunologic dysfunction, superimposed malnutrition or another infectious organism. Mycobacterium avium-intracellulare infection, often seen with AIDS, is quite distinctive with lamina propria foamy macrophages that stain positive for acid-fast organisms.42 These are also periodic acid-schiff positive reminiscent of Whipple's disease, but with Trophyrema whipplei (the Whipple's bacillus), acid-fast stains are negative. Parasites can also cause severe biopsy changes, but many can be morphologically identified in the biopsy material (e.g., Strongyloides stercoralis, hookworm, Schistosoma or Capillaria species). Some viral agents (e.g., cytomegalovirus) may be seen in the small bowel, often in the setting of an immune compromised state.43
Other disorders may cause diarrhea and/or malabsorption with severe biopsy changes like celiac disease. In some, distinct features are evident: lymphangiectasia, macroglobulinemia, amyloidosis, abetalipo-proteinemia, lipid storage disorders, radiation injury, and drugs (e.g., neomycin, busulfan, methotrexate, sulindac, azathioprine).44 Although Crohn's disease may cause mucosal granulomas in the duodenum,45 severe architectural changes without granulomas similar to untreated celiac disease may occur in the proximal small intestine.46 In Crohn's disease, however, these pathological findings do not improve with a gluten-free diet.
REFRACTORY DISEASE AND SPRUE-LIKE INTESTINAL DISEASE
In celiac disease, diarrhea or malabsorption may recur. Even though the mucosa may normalize with gluten restriction, severe pathologic changes may then later recur.19 A list of specific entities should be considered by the clinician (Table 2). Most often, symptoms and recurrent biopsy changes are due to poor compliance to the gluten-free diet; sometimes, this is completely inadvertent since gluten is ubiquitous and may be found in various ingested items, such as pill capsules or communion wafers. There may also be a difference in tolerance to gluten with a minimum threshold in some groups. A superimposed cause (e.g., infection) or superimposed deficiency of one or more nutrients (e.g., folic acid, zinc) may be critical in causing independent histological changes that are difficult to differentiate from changes of celiac disease. In long-standing celiac disease, pancreatic exocrine insufficiency may also occur, especially if concomitant malnutrition is evident.13 In some, it is also possible that the original diagnosis was not correct and some other cause for the severe biopsy changes was present. Finally, another related disorder (e.g., collagenous colitis) or a particularly sinister complication (e.g., lymphoma) may cause recurrent symptoms.
Table 2.
Causes of Refractory or Recurrent Disease*
*Failure to initially respond to a gluten-free diet suggests "sprue-like" intestinal disease or unclassified sprue, not refractory celiac disease.
Rarely, an uncommon disorder, collagenous sprue, may complicate adult celiac disease.47 Sometimes, it may be appear during the investigation of malabsorption or a diarrhea disorder, without any evidence of pre-existent celiac disease. Panmalabsorption with diarrhea, weight loss, electrolyte abnormalities and marked nutritional disturbance may be seen. Small bowel biopsies reveal a pathologically distinctive sub-epithelial band of collagen. Long term nutritional support with parenteral nutrition may be needed for survival. Antibodies to endomysium in collagenous sprue may reflect an immunological link to celiac disease.48 Rarely, lymphoma may supervene in collagenous sprue.49,50 Occasionally, another distinctive syndrome may complicate celiac disease with recurrent small bowel changes of variable severity, splenic hypofunction, and mesenteric lymph node cavitation.51 In this disorder, lymphoma has also been recorded.52
Sprue-like intestinal disease (or unclassified sprue) refers to a severe ("flat") or variably severe (moderate to mild) mucosal lesion that has never responded to a gluten-free diet.19 Although some have labeled this entity as "refractory celiac disease", response to a gluten-free diet has not been shown and so cannot be truly labeled with "celiac disease". Usually, additional biopsies are abnormal despite a gluten-free diet. Most remain symptomatic and fail to clinically respond to a gluten-free diet. In some dietary compliance may be difficult to prove and histological improvement may be difficult to document, especially with duodenal biopsies alone. This group likely represents a heterogeneous collection of small bowel disorders, a "wastebasket group" with no defined cause. Some may have a "resistant form" of celiac disease, while others prove to have a "difficult-to-diagnose" lymphoma. In some, an abnormal subset of intraepithelial lymphocytes with morphologically normal, but phenotypically abnormal lymphocytes was reported. These intriguing findings could provide a prognostic marker for later lymphoma development but added confirmatory studies are needed.
INTESTINAL MALIGNANCIES AND RISK IN CELIAC DISEASE
Intestinal malignancies occur during the clinical course of adult celiac disease.53-56 Often, these are lymphomas that complicate already well established celiac disease. But, in some, lymphoma as well as other malignancies, such as small bowel adenocarcinoma, actually precede recognition of celiac disease,25,26 providing further evidence for an intimate clinical linkage.
The precise risk of malignancy, particularly lymphoma, in adult celiac disease has been difficult to determine because there are many confounding variables. In adults with severe biopsy changes in proximal small intestine, the overall lymphoma risk was about 8 to 10% in a tertiary care setting.55,57 Age at the time of the initial diagnosis of celiac disease seems to be a critical risk factor. If celiac disease was first diagnosed later in life, lymphoma was detected more often than if the diagnosis of celiac disease was defined earlier. Duration on the gluten-free diet may be important. Interestingly, a long-term cohort study of 285 children with celiac disease treated with a gluten-free diet described only a single small bowel lymphoma.58 Recent reports have also suggested that this excess lymphoma risk in celiac disease has actually decreased, related possibly to use of serological screening tests for celiac disease in those with limited symptoms.59,60
LYMPHOMA DIAGNOSIS IN CELIAC DISEASE
The diagnosis of lymphoma in celiac disease may be challenging and sometimes presentations may be dramatic because most lymphomas in celiac disease occur in small intestine. Usually, these occur in jejunum, but localization in the ileum, also occurs.54 Duodenal lymphoma may develop while associated gastric and colonic lymphomas have been reported.54 Ulcerating or stenosing and obstructing tumors are often seen.61 Occasionally, however, the lymphoma may be multifocal or diffuse with only mucosal localization. Concomitant nodal involvement may be present.54 In some, a definite pathological diagnosis of lymphoma may be particularly difficult in the presence of either small intestinal (including duodenal) erosions or ulcers.26 Crohn's ulceration or a label of "ulcerative jejunoileitis" may be recorded.26 Some of these ulcerating lesions, however, later prove to contain frankly neoplastic lymphoma cells that may be difficult to appreciate in severely inflamed small intestine. Free perforation of the small intestine may be due to lymphoma, especially if celiac disease is known to be present or suspected.61 Even if there is a high degree of suspicion, lymphoma may still be notoriously difficult to diagnose, despite multiple endoscopic "pinch" or suction small intestinal biopsies.52 In some that eventually prove to have a lymphoma, full thickness small intestinal biopsies may not have provided a definitive pathological diagnosis, especially if only focal mucosal lymphomatous involvement was present. In recent years, tissue evaluated by flow cytometry and immunohistochemical studies or PCR have been added to the armamentarium of tools to facilitate diagnosis.62
LYMPHOMA TYPES IN CELIAC DISEASE
Lymphomas may be classified based on pathological and immunophenotypical features. Both B-cell and T-cell lymphomas occur in celiac disease. However, detection of a T-cell type more often leads to clinical suspicion of possible adult celiac disease. Primary intestinal T-cell lymphoma is recognized under the WHO classification system as enteropathy-associated T-cell lymphoma (ETL or EATL). They are very uncommon and represent an estimated 5% of all gastrointestinal lymphomas.62,63 Indeed, in a recent population-based study from the Netherlands, the disease was most common in the proximal small intestine, usually defined by surgical resection, more prevalent in males than females and rare with a reported incidence of 0.1 per 100,000 inhabitants per year.64 Previously, these lymphomas were thought to be histiocytic in origin (and labeled malignant histiocytosis), but their origin now appears to be primarily from T-cells, particularly intraepithelial lymphocytes.62,63 In celiac disease (without lymphoma), intraepithelial lymphocytes express the following antigens (among others): CD3 surface + and CD 8+. In a subset of patients that seem clinically refractory to a gluten-free diet, intraepithelial lymphocytes have a different form of T-cell phenotypic expression: CD 3 shows intracytoplasmic (i.e., not surface) expression while CD 8 expression may be absent. Some studies have suggested that this may reflect a specific form of refractory disease (type 2) with a poor prognosis and a possible precursor lesion for development of lymphoma.65-68 Defective synthesis of the T-cell receptor chains appears to be responsible for the loss of surface T-cell expression.69
Intestinal T-cell lymphomas are also a heterogeneous group. For example, a natural killer-like T-cell lymphoma of the intestine may occur that appears to have a distinct immunophenotype. This entity is not known to be associated with celiac disease, progresses rapidly and has a poor prognosis.70,71 Also, cases with both B-cell and T-cell type lymphomas have been described in the single individuals with celiac disease.72,73
Even lymphomas with T-cell immunophenotypic features have been defined in extra-intestinal sites complicating celiac disease but without lymphomatous intestinal involvement. These are rare and include hepatosplenic type T-cell lymphoma74 or lymphoma occurring in other embryologically-related or gut-derived sites, including the thyroid gland75 or bronchopulmonary and pleural sites.
Recent studies also provide evidence for an increased risk for other lymphoma types. In a pathological review of tumor materials from celiacs, there was an apparent aggregation of autoimmune and inflammatory disorders, female sex and B-cell lymphoma.76 More than double the risk for B-cell lymphoma was recorded with the most common type classified as a diffuse large B-cell lymphoma. In the same study, T-cell lymphomas had an approximate 50-fold risk along with a poorer prognosis (reflected in mean survival time after diagnosis and 5-year survival rate.76
LYMPHOMA TREATMENT
Lymphoma treatment in celiac disease does not substantially differ from lymphoma treatment in the absence of celiac disease. Usually, surgery, radiation and chemotherapy have been used. Most believe that treatment results are best in those with an early diagnosis.77 Novel treatments, such as cladribine, a purine analogue that induces T-cell depletion,78 and alemtuzumab, a biological agent,79 along with autologous stem cell transplantation80 are being considered and evaluated.
Newly diagnosed lymphoma patients should be screened for celiac disease, especially if chronic diarrhea or weight loss are evident. A diagnosis of celiac disease is more readily established prior to lymphoma treatment (since chemotherapy or radiation may induce small intestinal changes). Concomitant recognition of underlying celiac disease may also have important nutritional implications.
OTHER CANCERS
Malignant disorders may occur elsewhere in the gastrointestinal tract in celiac disease. For some of these, the rate may be increased. Small bowel adenocarcinoma is normally rare, but may be more frequently recognized in celiac disease. Some have suggested that these may be due to an adenoma-carcinoma sequence,54 but others have reported that the risk of duodenal adenoma is not increased in celiac disease.81 Most occur in the proximal small intestine and may cause bleeding or obstruction. If complete resection can be accomplished, the prognosis is better than if a lymphoma were present.82
Some European studies have also noted an increased risk of esophageal and pharyngeal carcinoma.53,83 This has not been confirmed in American centers, but in one report,57 a single hypopharyngeal squamous cell carcinoma was detected in a celiac patient with a prior lymphoma. In the same series, other esophageal or gastric cancers were not detected, in spite of repeated endoscopic studies during the course of diagnosis and treatment of celiac disease, but Barrett's esophagus, a known precursor of esophageal adenocarcinoma, was reported.57 Possibly, environmental or other confounding factors in different geographic areas are responsible.
Colorectal cancer risk was marginally increased, mainly in the ascending and transverse colon,84 in a population-based celiac cohort, but not in the closely allied disorder, dermatitis herpetiformis.84 Other studies have not detected an increased colorectal cancer risk in celiac disease,55,57,85 especially in celiac disease with a diagnosis initially established late in life.55,57 Possibly, untreated celiac disease protects from colon cancer. Dietary fat or fat soluble agents, including hydrocarbons or other putative co-carcinogens that have been implicated in colon cancer pathogenesis, may be poorly absorbed and rapidly excreted. Alternatively, immunological changes (e.g., increased intraepithelial lymphocytosis) may prohibit development of epithelial malignancies. Further studies to elucidate this issue are needed.
FUTURE DIRECTIONS
Mechanisms involved in the development of malignancy in celiac disease are poorly understood, including both lymphoma as well as epithelial malignancies. The small intestinal mucosa involved with changes attributed to celiac disease may still show histological improvement with a gluten-free diet, even after intestinal lymphoma is detected.26,32 There are many potential confounding variables that may alter the pathogenesis of lymphoma in a celiac population and influence risk measurements in different countries. These include genetic, infectious (e.g., EB virus) and other dietary and epidemiological variables. Duration of gluten restriction and degree of compliance to a gluten-free diet are be specific factors that are difficult to measure precisely, but seem critical to malignant change in celiac disease. Other malignancies, particularly epithelial malignancies, such as gastric or colon cancer, seem to occur much less often, even if celiac disease is only first recognized later in life. Possibly, untreated celiac disease protects against other cancers, such as colon cancer. Dietary fat or fat-soluble agents, such as hydrocarbons or other putative co-carcinogens, implicated in colon carcinogenesis may be poorly absorbed or rapidly excreted in celiac disease with diarrhea and impaired absorption. Immunological alterations, associated with increased intraepithelial lymphocyte numbers in celiac disease and the epithelial lymphocytosis reported in the stomach or colon of celiac,28,29 may directly or indirectly inhibit development of epithelial malignancies, particularly at these sites. Further studies are needed to elucidate these observations in adults with celiac disease.
References
- 1.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 2.Freeman HJ. Neurological disorders in adult celiac disease. Can J Gastroenterol. 2008;22:909–911. doi: 10.1155/2008/824631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewin KJ, Riddell RH, Weinstein WM. Gastrointestinal pathology and its clinical implications. New York: Igaku-Shoin; 1992. [Google Scholar]
- 4.Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101:2356–2359. doi: 10.1111/j.1572-0241.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Gillett HR, Freeman HJ. Serological testing in screening for adult celiac disease. Can J Gastroenterol. 1999;13:265–269. doi: 10.1155/1999/194367. [DOI] [PubMed] [Google Scholar]
- 6.Dickey W, Hughes DF, McMillan SA. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am J Gastroenterol. 2000;95:712–714. doi: 10.1111/j.1572-0241.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 7.Simell S, Hoppu S, Hekkala A, et al. Fate of five celiac disease-associated antibodies during normal diet in genetically at-risk children observed from birth in a natural history study. Am J Gastroenterol. 2007;102:2026–2035. doi: 10.1111/j.1572-0241.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 8.Freeman HJ. Strongly positive tissue transglutaminase antibody assays without celiac disease. Can J Gastroenterol. 2004;18:25–28. doi: 10.1155/2004/912053. [DOI] [PubMed] [Google Scholar]
- 9.Marsh MN. Mucosal pathology in gluten sensitivity. In: Marsh MN, editor. Coeliac disease. Oxford: Blackwell Scientific Publications; 1992. pp. 136–191. [Google Scholar]
- 10.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Corazza GR, Villanacci V, Zambelli C, et al. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838–843. doi: 10.1016/j.cgh.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Freeman HJ. Topography of lectin binding sites in celiac sprue. Can J Gastroenterol. 1992;6:271–276. [Google Scholar]
- 13.Freeman HJ, Whittaker JS. Non-alcoholic chronic pancreatitis with pancreatic calcification: presenting manifestation of occult celiac disease. Can J Gastroenterol. 1994;8:319–322. [Google Scholar]
- 14.Tursi A, Brandimarte G, Giorgetti GM, et al. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: a 2-year prospective study. Endoscopy. 2006;38:702–707. doi: 10.1055/s-2006-925178. [DOI] [PubMed] [Google Scholar]
- 15.Kakar S, Nehra V, Murray JA, Dayharsh GA, Burgart LJ. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol. 2003;98:2027–2033. doi: 10.1111/j.1572-0241.2003.07631.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaukinen K, Maki M, Partanen J, Sievanen H, Collin P. Celiac disease without villous atrophy: revision of criteria called for. Dig Dis Sci. 2001;46:879–887. doi: 10.1023/a:1010729207320. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald WC, Brandborg LL, Flick AL, Trier JS, Rubin CE. Studies of celiac sprue. IV. The response of the whole length of the small bowel to a gluten-free diet. Gastroenterology. 1964;47:573–589. [PubMed] [Google Scholar]
- 18.MacDonald TT. T cell-mediated intestinal injury. In: Marsh MN, editor. Coeliac disease. Oxford: Blackwell Scientific Publications; 1992. pp. 283–304. [Google Scholar]
- 19.Freeman HJ. Refractory celiac disease and sprue-like intestinal disease. World J Gastroenterol. 2008;14:828–830. doi: 10.3748/wjg.14.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman HJ. Adult celiac disease in the elderly. World J Gastroenterol. 2008;14:6911–6914. doi: 10.3748/wjg.14.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brow JR, Parker F, Weinstein WM, Rubin CE. The small intestinal mucosa in dermatitis herpetiformis. I. Severity and distribution of the small intestinal lesion and associated malabsorption. Gastroenterology. 1971;60:355–361. [PubMed] [Google Scholar]
- 22.Weinstein WM, Brow JR, Parker F, Rubin CE. The small intestinal mucosa in dermatitis herpetiformis. II. Relationship of the small intestinal lesion to gluten. Gastroenterology. 1971;60:362–369. [PubMed] [Google Scholar]
- 23.Freeman HJ. Celiac-associated autoimmune thyroid disease. A study of 16 patients with overt hypothyroidism. Can J Gastroenterol. 1995;9:242–246. [Google Scholar]
- 24.Gillett PM, Gillett HR, Israel DM, et al. High prevalence of celiac disease in patients with type 1 diabetes detected by antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol. 2001;15:297–301. doi: 10.1155/2001/640796. [DOI] [PubMed] [Google Scholar]
- 25.Freeman HJ. Occult celiac disease in an octogenarian presenting with a small intestinal adenocarcinoma. Can J Gastroenterol. 1994;8:354–357. [Google Scholar]
- 26.Freeman HJ, Weinstein WM, Shnitka TK, Piercey JR, Wensel RH. Primary abdominal lymphoma. Presenting manifestation of celiac sprue or complicating dermatitis herpetiformis. Am J Med. 1977;63:585–594. doi: 10.1016/0002-9343(77)90204-2. [DOI] [PubMed] [Google Scholar]
- 27.Freeman HJ. Collagenous colitis as the presenting feature of biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:664–668. doi: 10.1097/01.mcg.0000135363.12794.2b. [DOI] [PubMed] [Google Scholar]
- 28.Wolber R, Owen D, Freeman H. Colonic lymphocytosis in patients with celiac sprue. Hum Pathol. 1990;21:1092–1096. doi: 10.1016/0046-8177(90)90144-t. [DOI] [PubMed] [Google Scholar]
- 29.Wolber R, Owen D, DelBuono L, Appelman H, Freeman H. Lymphocytic gastritis in patients with celiac sprue or spruelike intestinal disease. Gastroenterology. 1990;98:310–315. doi: 10.1016/0016-5085(90)90819-m. [DOI] [PubMed] [Google Scholar]
- 30.Freeman HJ. Collagenous mucosal inflammatory diseases of the gastrointestinal tract. Gastroenterology. 2005;129:338–350. doi: 10.1053/j.gastro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein WM. Latent celiac sprue. Gastroenterology. 1974;66:489–493. [PubMed] [Google Scholar]
- 32.Freeman HJ, Chiu BK. Multifocal small bowel lymphoma and latent celiac sprue. Gastroenterology. 1986;90:1992–1997. doi: 10.1016/0016-5085(86)90272-6. [DOI] [PubMed] [Google Scholar]
- 33.Jabbari M, Wild G, Goresky CA, et al. Scalloped valvulae conniventes: an endoscopic marker of celiac sprue. Gastroenterology. 1988;95:1518–1522. doi: 10.1016/s0016-5085(88)80071-4. [DOI] [PubMed] [Google Scholar]
- 34.Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol. 2002;97:933–938. doi: 10.1111/j.1572-0241.2002.05612.x. [DOI] [PubMed] [Google Scholar]
- 35.Olds G, McLoughlin R, O'Morian C, Sivak MV., Jr Celiac disease for the endoscopist. Gastrointest Endosc. 2002;56:407–415. doi: 10.1016/s0016-5107(02)70047-3. [DOI] [PubMed] [Google Scholar]
- 36.Culliford A, Markowitz D, Rotterdam H, Green PH. Scalloping of duodenal mucosa in Crohn's disease. Inflamm Bowel Dis. 2004;10:270–273. doi: 10.1097/00054725-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Freeman HJ. Survey of gastroenterologists on the diagnosis and treatment of adult patients with celiac disease in British Columbia. Can J Gastroenterol. 1998;12:149–152. doi: 10.1155/1998/534216. [DOI] [PubMed] [Google Scholar]
- 38.Janatuinen EK, Kemppainen TA, Julkunen RJ, et al. No harm from five year ingestion of oats in coeliac disease. Gut. 2002;50:332–335. doi: 10.1136/gut.50.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundin KE, Nilsen EM, Scott HG, et al. Oats induced villous atrophy in coeliac disease. Gut. 2003;52:1649–1652. doi: 10.1136/gut.52.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber DS, Blacklow NR, Trier JS. The mucosal lesion of the proximal small intestine in acute infectious non-bacterial gastroenteritis. N Engl J Med. 1973;288:1318–1323. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- 41.Koch J, Owen RL. Small intestine pathogens in AIDS: conventional and opportunistic. Gastrointest Endosc Clin N Am. 1998;8:869–888. doi: 10.1016/S1052-5157(18)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.AIDS with Mycobacterium avium-intracellulare lesions resembling those of Whipple's disease. N Engl J Med. 1983;309:1323–1325. doi: 10.1056/NEJM198311243092113. [DOI] [PubMed] [Google Scholar]
- 43.Freeman HJ, Shnitka TK, Piercey JR, Weinstein WM. Cytomegalovirus infection of the gastrointestinal tract in a patient with late onset immunodeficiency syndrome. Gastroenterology. 1977;73:1397–1403. [PubMed] [Google Scholar]
- 44.Ziegler TR, Fernandez-Estivariz C, Gu LH, Fried MW, Leader LM. Severe villus atrophy and chronic malabsorption induced by azathioprine. Gastroenterology. 2003;124:1950–1957. doi: 10.1016/s0016-5085(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 45.Sukhabote J, Freeman HJ. Granulomatous (Crohn's) disease of the upper gastrointestinal tract: a study of 22 patients with mucosal granulomas. Can J Gastroenterol. 1993;7:605–609. [Google Scholar]
- 46.Schuffler MD, Chaffee RG. Small intestinal biopsy in a patient with Crohn's disease of the duodenum. The spectrum of abnormal findings in the absence of granulomas. Gastroenterology. 1979;76:1009–1014. [PubMed] [Google Scholar]
- 47.Weinstein WM, Saunders DR, Tytgat GN, Rubin CE. Collagenous sprue: an unrecognized type of malabsorption. N Engl J Med. 1970;283:1297–1301. doi: 10.1056/NEJM197012102832401. [DOI] [PubMed] [Google Scholar]
- 48.Freeman HJ. Hyposplenism, antiendomysial antibodies and lymphocytic colitis in collagenous sprue. Can J Gastroenterol. 1999;13:347–350. doi: 10.1155/1999/596427. [DOI] [PubMed] [Google Scholar]
- 49.Robert ME, Ament ME, Weinstein WM. The histologic spectrum and clinical outcome of refractory and unclassified sprue. Am J Surg Pathol. 2000;24:676–687. doi: 10.1097/00000478-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Freeman HJ. Collagenous sprue associated with an extensive T-cell lymphoma. J Clin Gastroenterol. 2003;36:144–146. doi: 10.1097/00004836-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Matuchansky C, Colin R, Hemet J, et al. Cavitation of mesenteric lymph nodes, splenic atrophy, and a flat small intestinal mucosa. Report of six cases. Gastroenterology. 1984;87:606–614. [PubMed] [Google Scholar]
- 52.Freeman HJ, Chiu BK. Small bowel malignant lymphoma complicating celiac sprue and the mesenteric lymph node cavitation syndrome. Gastroenterology. 1986;90:2008–2012. doi: 10.1016/0016-5085(86)90275-1. [DOI] [PubMed] [Google Scholar]
- 53.Holmes GK, Stokes PL, Sorahan TM, Prior P, Waterhouse JA, Cooke WT. Coeliac disease, gluten-free diet, and malignancy. Gut. 1976;17:612–619. doi: 10.1136/gut.17.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79–S86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 55.Freeman HJ. Lymphoproliferative and intestinal malignancies in 214 patients with biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:429–434. doi: 10.1097/00004836-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Brousse N, Meijer JW. Malignant complications of coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:401–412. doi: 10.1016/j.bpg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Freeman HJ. Neoplastic disorders in 100 patients with adult celiac disease. Can J Gastroenterol. 1996;10:163–166. [Google Scholar]
- 58.Solaymani-Dodaran M, West J, Logan RF. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population-based cohort study. Am J Gastroenterol. 2007;102:864–870. doi: 10.1111/j.1572-0241.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y, Kristinsson SY, Goldin LR, Bjorkholm M, Caporaso NE, Landgren O. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136:91–98. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mearin ML, Catassi C, Brousse N, et al. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol. 2006;18:187–194. doi: 10.1097/00042737-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Freeman HJ. Free perforation due to intestinal lymphoma in biopsy-defined or suspected celiac disease. J Clin Gastroenterol. 2003;37:299–302. doi: 10.1097/00004836-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Meijer JW, Mulder CJ, Goerres MG, Boot H, Schweizer JJ. Coeliac disease and (extra)intestinal T-cell lymphomas: definition, diagnosis and treatment. Scand J Gastroenterol Suppl. 2004;(241):78–84. doi: 10.1080/00855920410014605. [DOI] [PubMed] [Google Scholar]
- 63.Isaacson PG, O'Connor NT, Spencer J, et al. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985;2:688–691. doi: 10.1016/s0140-6736(85)92930-7. [DOI] [PubMed] [Google Scholar]
- 64.Verbeek WH, Van De Water JM, Al-Toma A, Oudejans JJ, Mulder CJ, Coupe VM. Incidence of enteropathy--associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol. 2008;43:1322–1328. doi: 10.1080/00365520802240222. [DOI] [PubMed] [Google Scholar]
- 65.Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413–424. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Verkarre V, Romana SP, Cellier C, et al. Recurrent partial trisomy 1q22-q44 in clonal intraepithelial lymphocytes in refractory celiac sprue. Gastroenterology. 2003;125:40–46. doi: 10.1016/s0016-5085(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 67.Zettl A, deLeeuw R, Haralambieva E, Mueller-Hermelink HK. Enteropathy-type T-cell lymphoma. Am J Clin Pathol. 2007;127:701–706. doi: 10.1309/nw2bk1dxb0eqg55h. [DOI] [PubMed] [Google Scholar]
- 68.Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 69.Tjon JM, Verbeek WH, Kooy-Winkelaar YM, et al. Defective synthesis or association of T-cell receptor chains underlies loss of surface T-cell receptor-CD3 expression in enteropathy-associated T-cell lymphoma. Blood. 2008;112:5103–5110. doi: 10.1182/blood-2008-04-150748. [DOI] [PubMed] [Google Scholar]
- 70.Yuan CM, Stein S, Glick JH, Wasik MA. Natural killer-like T-cell lymphoma of the small intestine with a distinct immunophenotype and lack of association with gluten- sensitive enteropathy. Arch Pathol Lab Med. 2003;127:e142–e146. doi: 10.5858/2003-127-e142-NKTLOT. [DOI] [PubMed] [Google Scholar]
- 71.Muram-Zborovski T, Loeb D, Sun T. Primary intestinal intraepithelial natural killer-like T-cell lymphoma: case report of a distinct clinicopathologic entity. Arch Pathol Lab Med. 2009;133:133–137. doi: 10.5858/133.1.133. [DOI] [PubMed] [Google Scholar]
- 72.Nava VE, Cohen P, Bishop M, Fowler D, Jaffe ES, Ozdemirli M. Enteropathy-type T-cell lymphoma after intestinal diffuse large B-cell lymphoma. Am J Surg Pathol. 2007;31:476–480. doi: 10.1097/01.pas.0000213391.49698.57. [DOI] [PubMed] [Google Scholar]
- 73.Makishima H, Komiyama Y, Asano N, Momose K, Nakamura S, Ishida F. Peripheral T-cell lymphoma following diffuse large B-cell lymphoma associated with celiac disease. Intern Med. 2008;47:295–298. doi: 10.2169/internalmedicine.47.0500. [DOI] [PubMed] [Google Scholar]
- 74.Freeman HJ. Fulminant liver failure with necrotizing foci in the liver, spleen and lymph nodes in celiac disease due to malignant lymphoma. Can J Gastroenterol. 1996;10:225–229. [Google Scholar]
- 75.Freeman HJ. T cell lymphoma of the thyroid gland in celiac disease. Can J Gastroenterol. 2000;14:635–636. doi: 10.1155/2000/582364. [DOI] [PubMed] [Google Scholar]
- 76.Smedby KE, Akerman M, Hildebrand H, Glimelius B, Ekbom A, Askling J. Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut. 2005;54:54–59. doi: 10.1136/gut.2003.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciccocioppo R, Perfetti V, Corazza GR. Treating ETTCL: a matter of early diagnosis and chemotherapy strategies. Dig Liver Dis. 2007;39:642–645. doi: 10.1016/j.dld.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Al-Toma A, Goerres MS, Meijer JW, et al. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol. 2006;4:1322–1327. doi: 10.1016/j.cgh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Vivas S, Ruiz de Morales JM, Ramos F, Suarez-Vilela D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N Engl J Med. 2006;354:2514–2515. doi: 10.1056/NEJMc053129. [DOI] [PubMed] [Google Scholar]
- 80.Al-Toma A, Verbeek WH, Visser OJ, et al. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig Liver Dis. 2007;39:634–641. doi: 10.1016/j.dld.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Rampertab SD, Fleischauer A, Neugut AI, Green PH. Risk of duodenal adenoma in celiac disease. Scand J Gastroenterol. 2003;38:831–833. doi: 10.1080/00365520310004515. [DOI] [PubMed] [Google Scholar]
- 82.Howdle PD, Jalal PK, Holmes GK, Houlston RS. Primary small-bowel malignancy in the UK and its association with coeliac disease. QJM. 2003;96:345–353. doi: 10.1093/qjmed/hcg058. [DOI] [PubMed] [Google Scholar]
- 83.Selby WS, Gallagher ND. Malignancy in a 19-year experience of adult celiac disease. Dig Dis Sci. 1979;24:684–688. doi: 10.1007/BF01314465. [DOI] [PubMed] [Google Scholar]
- 84.Askling J, Linet M, Gridley G, Halstensen TS, Ekstrom K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428–1435. doi: 10.1053/gast.2002.36585. [DOI] [PubMed] [Google Scholar]
- 85.Dickey W. Colon neoplasia co-existing with coeliac disease in older patients: coincidental, probably; important, certainly. Scand J Gastroenterol. 2002;37:1054–1056. doi: 10.1080/003655202320378257. [DOI] [PubMed] [Google Scholar]