Abstract
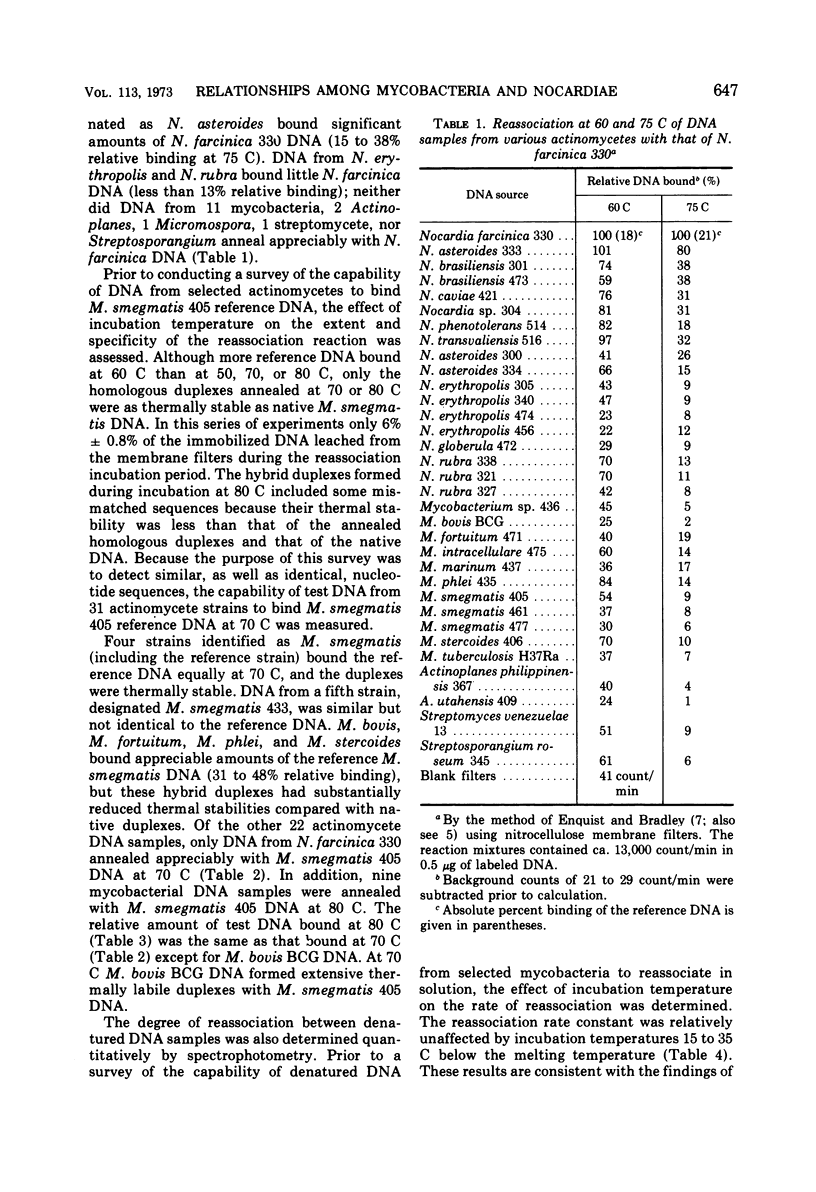
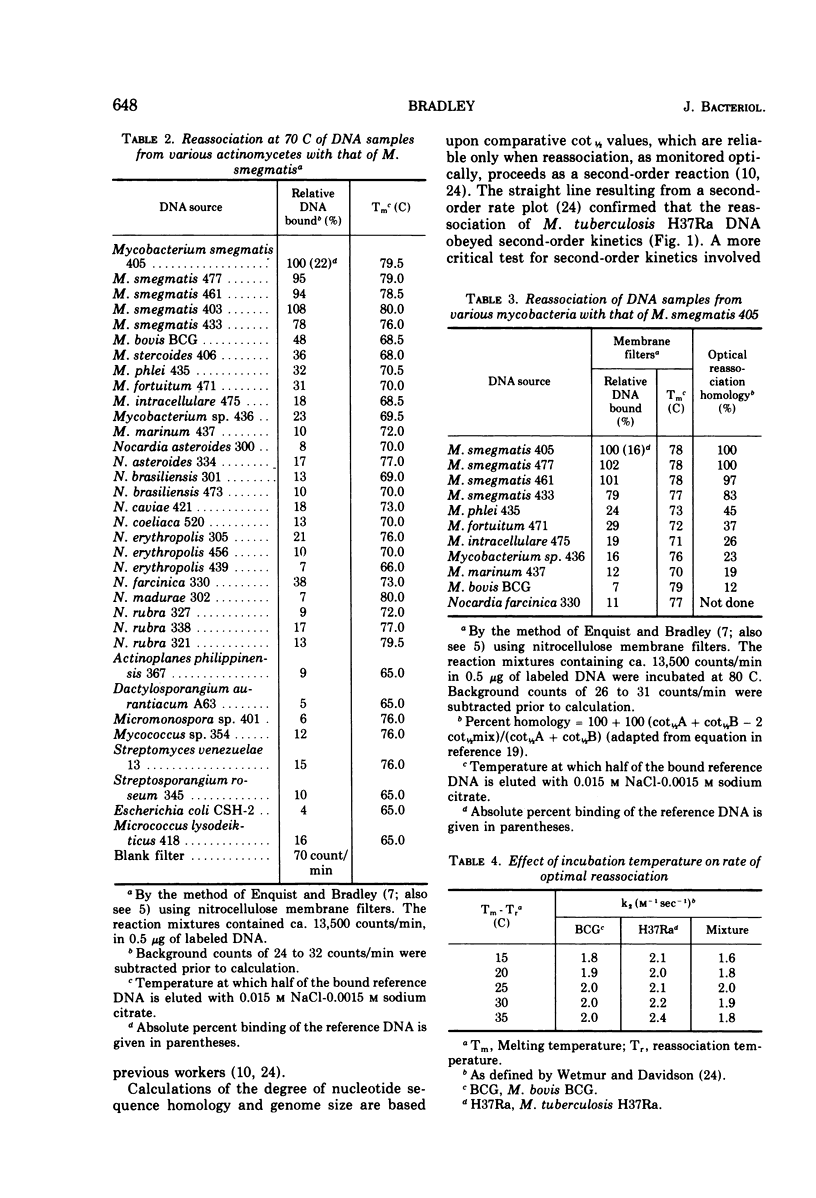
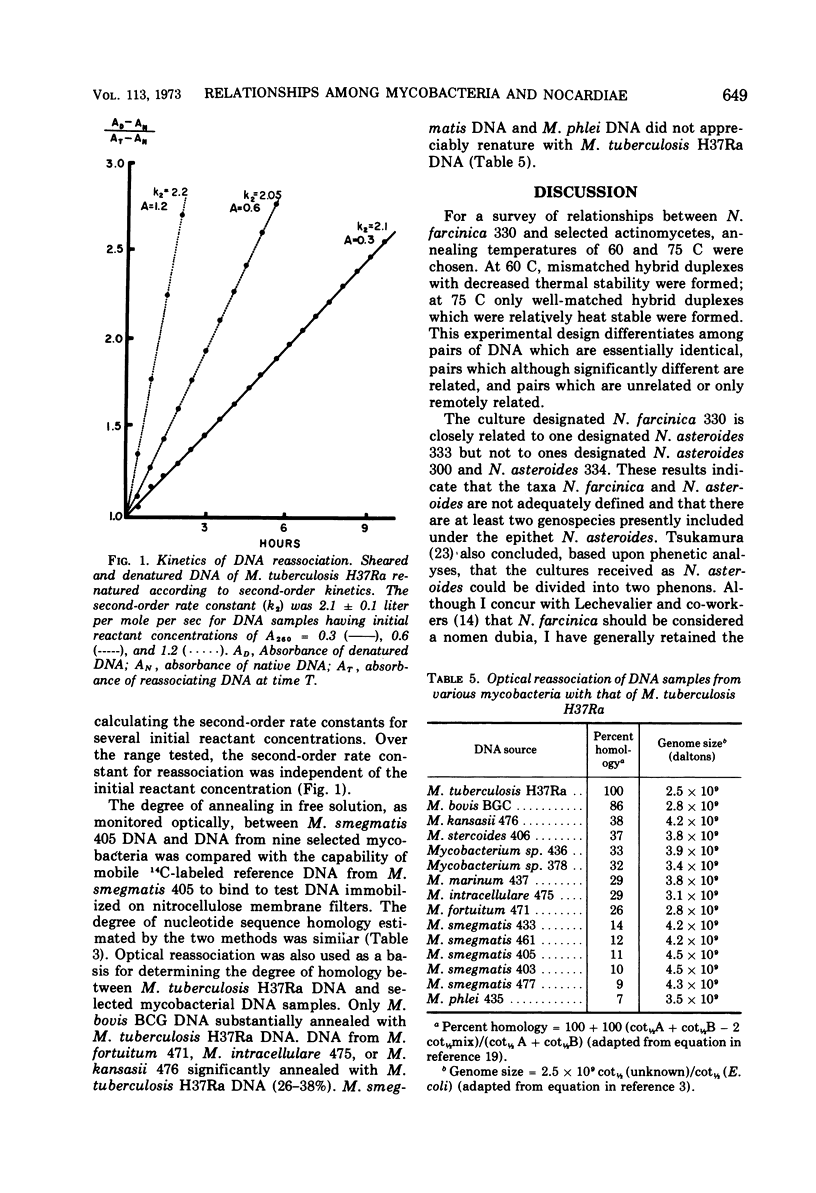
The degree of renaturation between Nocardia farcinica 330 deoxyribonucleic acid (DNA) and DNA from 17 nocardial strains and 11 mycobacterial strains, and between Mycobacterium smegmatis 405 DNA and DNA from 11 mycobacterial strains and 14 nocardial strains was determined by using the nitrocellulose membrane filter technique. These results indicated that some cultures designated N. farcinica were identical to some cultures designated N. asteroides but that other strains called N. asteroides were distinctly different. The species M. smegmatis was homogeneous and distinct from the other species examined. Data comparing the extent of nucleotide sequences shared by M. smegmatis 405 DNA and DNA from nine other mycobacterial strains, as determined by the membrane filter technique and by monitoring DNA reassociation optically, were similar. The extent of reassociation between M. tuberculosis H37Ra DNA and DNA from 14 mycobacterial strains was determined optically. DNA from M. bovis BCG was 86% homologous with M. tuberculosis H37Ra DNA. The other mycobacterial DNA preparations examined were less than 40% homologous with M. tuberculosis H37Ra DNA. The genome sizes of the mycobacteria examined ranged between 2.5 × 109 daltons and 4.5 × 109 daltons.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Brownell G. H. Genophore homologies among compatible nocardiae. J Bacteriol. 1972 Feb;109(2):720–729. doi: 10.1128/jb.109.2.720-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Cattoir H., Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970 Jan;12(1):133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Farina G., Bradley S. G. Reassociation of deoxyribonucleic acids from Actinoplanes and other actinomycetes. J Bacteriol. 1970 Apr;102(1):30–35. doi: 10.1128/jb.102.1.30-35.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON R. E., MIHM J. M. The type species of the genus Nocardia. J Gen Microbiol. 1962 Jan;27:1–10. doi: 10.1099/00221287-27-1-1. [DOI] [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gross W. M., Wayne L. G. Nucleic acid homology in the genus Mycobacterium. J Bacteriol. 1970 Nov;104(2):630–634. doi: 10.1128/jb.104.2.630-634.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lechevalier M. P., Horan A. C., Lechevalier H. Lipid composition in the classification of nocardiae and mycobacteria. J Bacteriol. 1971 Jan;105(1):313–318. doi: 10.1128/jb.105.1.313-318.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson A. M., Bradley S. G., Enquist L. W., Cruces G. Genetic homologies among Streptomyces violaceoruber strains. J Bacteriol. 1969 Sep;99(3):702–706. doi: 10.1128/jb.99.3.702-706.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Gregory K. F. Methods for the determination of deoxyribonucleic Acid homologies in streptomyces. J Bacteriol. 1970 Dec;104(3):1086–1094. doi: 10.1128/jb.104.3.1086-1094.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewfik E. M., Bradley S. G. Characterization of deoxyribonucleic acids from streptomycetes and nocardiae. J Bacteriol. 1967 Dec;94(6):1994–2000. doi: 10.1128/jb.94.6.1994-2000.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. Numerical taxonomy of the genus Nocardia. J Gen Microbiol. 1969 Jun;56(3):265–287. doi: 10.1099/00221287-56-3-265. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



