Abstract
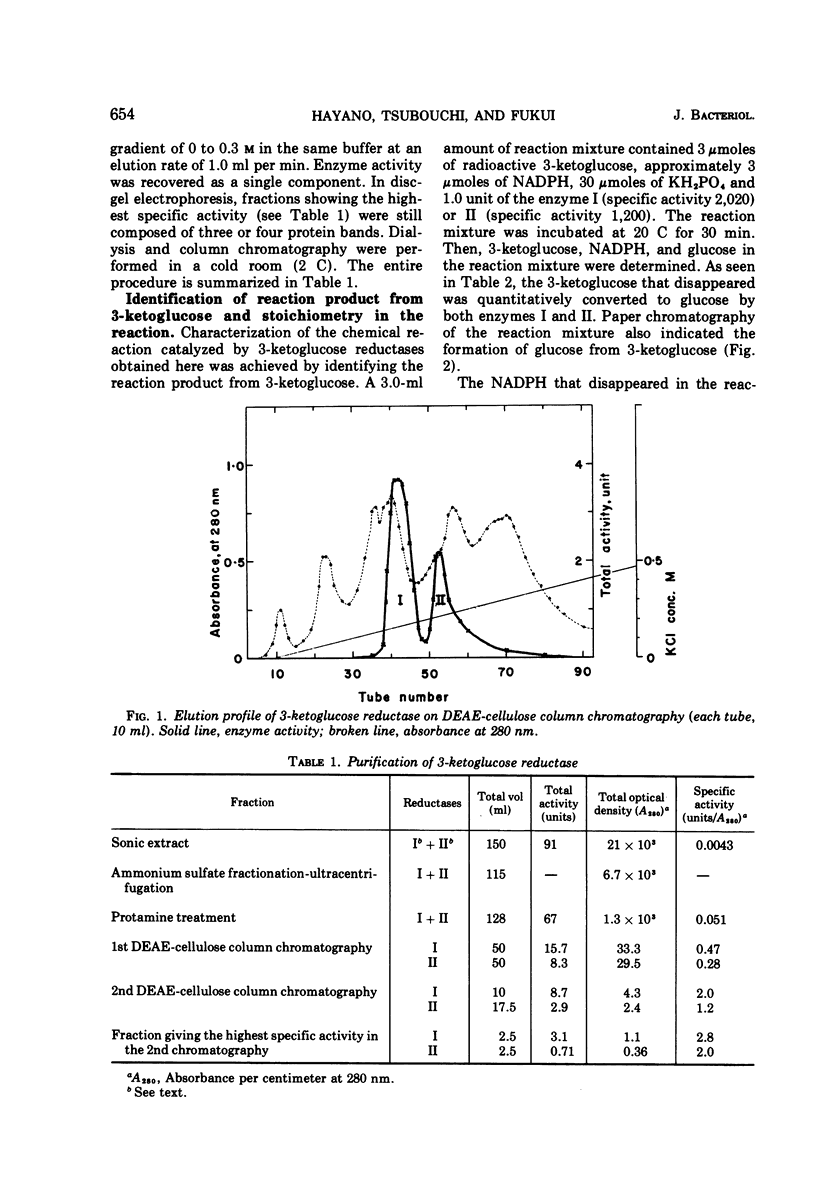
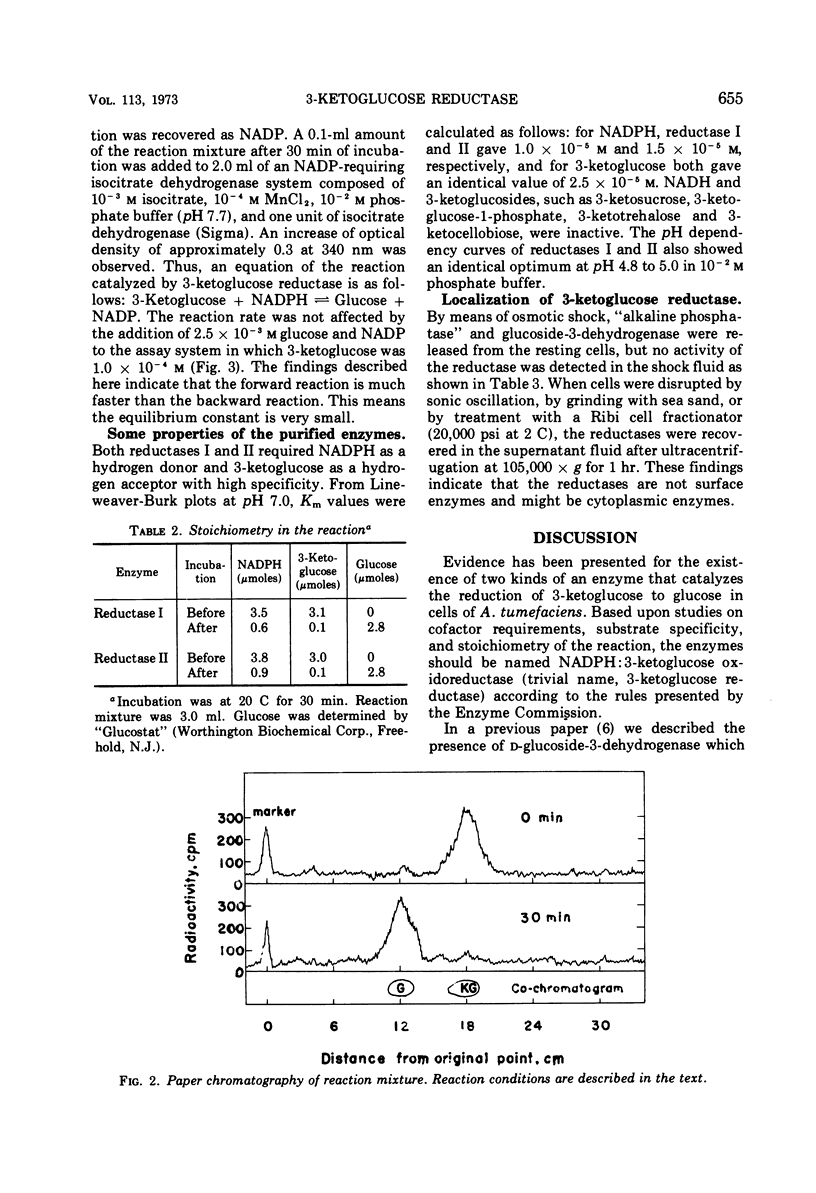
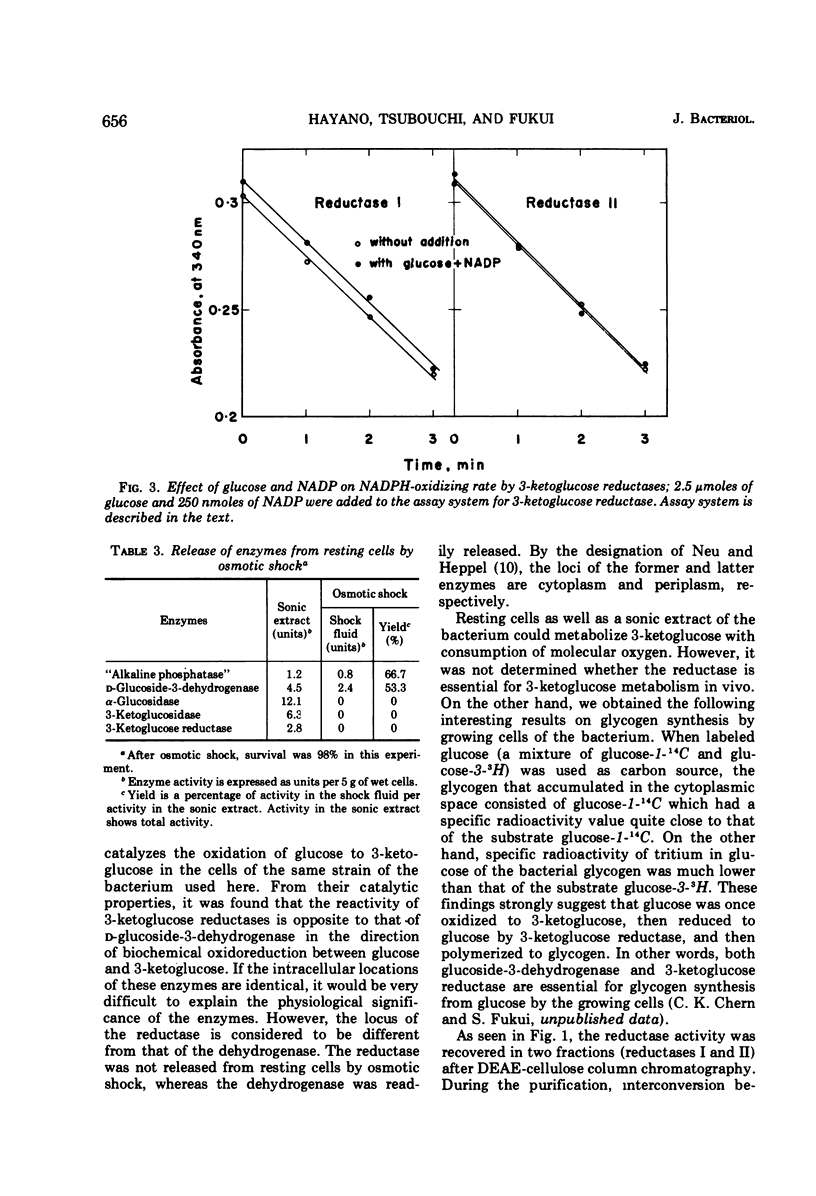
Two kinds of 3-ketoglucose-reducing enzyme were partially purified from the sonic extract of Agrobacterium tumefaciens IAM 1525 grown on a sucrose-containing medium. Both enzymes have a specific requirement for reduced nicotinamide adenine dinucleotide phosphate (NADPH) as a hydrogen donor and catalyze the reduction of 3-ketoglucose to glucose but do not reduce 3-ketoglucosides such as 3-ketosucrose, 3-ketoglucose-1-phosphate, 3-ketotrehalose, and 3-ketocellobiose. From the requirement and substrate specificity of the enzymes, the name NADPH:3-ketoglucose oxidoreductase (trivial name, 3-ketoglucose reductase) was proposed. By diethylaminoethyl-cellulose column chromatography, two reductases were separated, and the early and late eluted enzymes were designated reductase I and II, respectively. Km values of reductase I and II were as follows: for 3-ketoglucose both had an identical value of 2.5 × 10−5m, and for NADPH the values were 1.0 × 10−5m and 1.5 × 10−5m, respectively. Optimal pH values were also identical: pH 4.8 to 5.0 in 10−2m phosphate buffer. Intracellular localization of the enzymes is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FUKUI S., HOCHSTER R. M. CONVERSION OF DISACCHARIDES TO THE CORRESPONDING GLYCOSIDE-3-ULOSES BY INTACT CELLS OF AGROBACTERIUM TUMEFACIENS. Can J Biochem Physiol. 1963 Nov;41:2363–2371. [PubMed] [Google Scholar]
- Fukui S. Conversion of glucose-1-phosphate to 3-keto-glucose-1-phosphate by cells of Agrobacterium tumefaciens. J Bacteriol. 1969 Feb;97(2):793–798. doi: 10.1128/jb.97.2.793-798.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano K., Fukui S. Alpha-3-ketoglucosidase of Agrobacterium tumefaciens. J Bacteriol. 1970 Mar;101(3):692–697. doi: 10.1128/jb.101.3.692-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano K., Fukui S. Biochemical conversion of cellobiose to 3-ketocellobiose. J Biochem. 1968 Dec;64(6):901–903. doi: 10.1093/oxfordjournals.jbchem.a128973. [DOI] [PubMed] [Google Scholar]
- Hayano K., Fukui S. Purification and properties of 3-ketosucrose-forming enzyme from the cells of Agrobacterium tumefaciens. J Biol Chem. 1967 Aug 25;242(16):3655–3672. [PubMed] [Google Scholar]
- Hirata H., Fukui S. Cytochrome C552 in Agrobacterium tumefaciens. J Biochem. 1968 Jun;63(6):780–788. doi: 10.1093/oxfordjournals.jbchem.a128843. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]



