Abstract
Since the effect of smoking on plasma leptin has been divergent in clinical trials, which might have occurred due to selection of heterogeneous study populations, we investigated whether there is such an association in a group of healthy, non-obese, young male adults.
A total of 54 smokers (mean age: 21.18±1.62; body mass index (BMI): 19.60±0.85) and 26 non-smokers (mean age 21.69±3.0; BMI: 21.59±1.39) with similar daily physical activities and diet and without any documented disease were enrolled, and their plasma leptin levels were determined for the comparison between the two groups.
The mean BMI and plasma leptin of smokers were significantly lower than in non-smokers. Plasma leptin in the smokers group correlated inversely with BMI and the amount of daily smoking. Below BMI 20 kg/m2 and between 20.0 and 20.9 kg/m2 the plasma leptin levels in smokers were significantly lower when compared to non-smokers.
Plasma leptin is decreased in healthy, young non-obese male smokers independently of the amount of body fat. High amount of smoking is associated with lower serum leptin as well.
Keywords: Leptin, male, smoking
Introduction
Long-time smoking is characterized by reduced body-weight (1–3), while cessation of smoking usually leads to rapid weight-gain (4–7). The probable causes of weight-gain are increased food intake, decreased resting metabolic rate and physical activity, and increased lipoprotein lipase activity (7). Moreover, weight concerns affect the motivation to quit smoking (8). However, the molecular factors regulating food intake and body-weight in smokers have not yet been clearly identified. Leptin, a polypeptide hormone secreted by adipocytes, has been shown to influence body-weight mainly by reducing food intake and increasing energy expenditure through central and peripheral mechanisms (9–11). Leptin has a diurnal rhythm and a positive correlation with the degree of adiposity, body mass index (BMI), and circulating insulin levels (12–15). Gender differences also determine the plasma leptin levels (16).
Since there is a close association of body-weight with smoking and serum leptin, many authors have investigated the interaction between leptin and smoking. However, the effect of smoking on plasma leptin has been divergent in clinical trials. Some studies have mentioned decreased serum leptin in smokers (17–23) while others found no difference between smokers and non-smokers regarding circulating leptin (24–26). Since these controversial results might have occurred due to selection of study populations in those studies, which frequently included heterogeneous groups of individuals regarding age, gender, and BMI, we investigated the association between plasma leptin levels and smoking in a homogenous group of healthy, non-obese, young male adults with appropriate controls.
Methods
Study population
The study was conducted on 80 young, non-obese male adults (mean age: 21.35±2.16 years; BMI: 20.25±1.41) who were allocated into two groups according to their smoking habits as 54 smokers (mean age: 21.18±1.62; BMI: 19.60±0.85) and 26 non-smokers (mean age 21.69±3.0; BMI: 21.59±1.39). Being a smoker was defined as smoking cigarettes for at least 2 years, with more than 5 cigarettes daily. Only a ‘never-smoker’ was accepted as a non-smoker. No ex-smoker was included in any group. The participants of the study were the soldiers from a troop and had similar daily physical activities and diet for at least 6 months. None of the participants had any documented disease nor were taking any medication, including over-the-counter drugs. Physical activity levels and glucose tolerance status were similar for smokers and non-smokers.
The study was approved by the Ethics Committee of Gulhane Medical School. All subjects gave written informed consent before entrance in the study.
According to the daily numbers of cigarettes, the patients were grouped as heavy smokers (n=12) (more than 20 cigarettes a day), moderate smokers (n=12) (between 10 and 20 cigarettes a day), and mild smokers (n=30) (fewer than 10 cigarettes a day).
Anthropometric measurements
Height and weight were determined with the participant wearing underpants, without shoes. BMI was calculated as weight (kg)/height (m)2.
Analyses
Overnight fasting venous blood samples were collected at 08.00 for analysis of leptin in cold test tubes containing 0.084 mL Ethylene Diamine Tetraacetic Acid (EDTA) (0.34 mol/L). Leptin was measured by a radioimmunoassay using Human Leptin IRMA (DSL-23100 Leptin-Coated Tube Immunoradiometric Assay Kit) (Diagnostic Systems Laboratories Inc., Texas, USA). Serum was stored at -30°C until analyses, and all measurements were performed in duplicate.
Statistical analyses
All data are presented as mean±SD unless otherwise noted. Student's t test was used to compare the parameters of the two groups. The associations between serum leptin levels and BMI or daily cigarette number were investigated by Spearman's correlation analysis.
Results
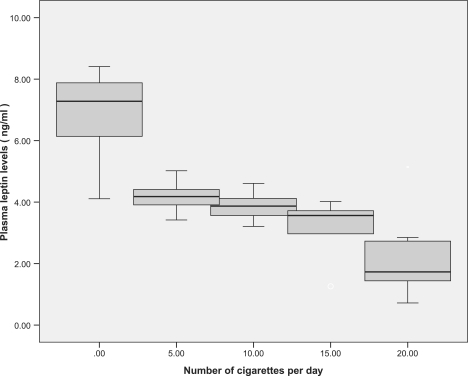
Age, blood pressure, total cholesterol, triglyceride, and fasting glucose levels were all similar in both the smoker and non-smoker groups. The mean BMI of smokers was lower than non-smokers (19.60±0.85 kg/m2 versus 21.59±1.39 kg/m2) (P <0.001). The plasma leptin levels in the smoker group were significantly lower than in non-smokers (3.47±1.1 ng/mL versus 6.63±1.5 ng/mL) (P =0.006). Plasma leptin in the smokers group correlated inversely with BMI (r=0.83, P <0.001) and the amount of daily smoking (r =-0.77, P <0.001) (Figure 1).
Figure 1.
Plasma leptin levels according to number of cigarettes per day.
The smokers and non-smokers were subanalyzed according to BMI values. Below 20 kg/m2 (n=22; 12 smokers, 10 non-smokers) and between 20.0 and 20.9 kg/m2 (n=30; 18 smokers, 12 non-smokers) of BMIs, the plasma leptin levels in smokers were significantly lower (P =0.009 and P =0.024, respectively) when compared to non-smokers with the same BMI values. Since there were only two individuals in the smoker group who had a BMI above 21, comparison of plasma leptin levels in the subjects having BMIs higher than this value was not performed.
Discussion
The present study, which was designed to search for any relation between smoking and plasma leptin levels in healthy and young non-obese males, indicates that smokers have significantly lower plasma leptin levels. Low leptin levels in smokers compared to non-smokers within the similar BMI interval suggest that smoking is associated with low plasma leptin independently of the body fat content. Moreover, there was an inverse association between the number of cigarettes smoked per day and the level of plasma leptin.
Leptin, which plays an important role in body-weight homeostasis through reducing food intake and increasing thermogenesis, has been studied in smokers widely. While a number of studies indicated that smoking does not affect plasma leptin levels (12,24–26), some others reported lower plasma leptin levels in smokers (17,18,20–23). More controversies exist considering the reports with increased plasma leptin in smoking people (27,28). Detailed analyses of these studies indicate selection of study population as the most probable reason for these conflicting results. For instance, Larsson et al. found no relation between smoking habits and plasma leptin levels in their study with postmenopausal, Caucasian, non-obese smoking women (26). Similar results were also reported in type 2 diabetic smoking Japanese males (25). On the other hand, Hodge et al. in a group of smokers in the South Pacific Islands (17) and Wei et al. in non-Hispanic whites and Mexican Americans (18) measured low plasma leptin levels. Though these results led to a question of an ethnicity-based association between plasma leptin levels and smoking, Donahue et al. reported in their large multiethnic study that ethnicity did not establish a direct relation between plasma leptin and life habits (20). According to their study, cigarette smoking was associated with lower plasma leptin levels in both men and women.
Another possible mechanism for the lower leptin in smokers could be related to the fact that smoking increases plasma catecholamines and free fatty acid concentrations, and lipolysis which may decrease leptin concentrations (29–32). Also, the nicotinic effect of smoking may alter the sensitivity of hypothalamic leptin receptors and modulate leptin synthesis and a reduction in body-weight (16,31,32).
The discrepant results could also be explained by other factors such as diet, exercise and other lifestyle habits, hormones, and inflammatory markers which may also regulate leptin secretion (29).
In a recent study, leptin levels were significantly higher in women compared with men, and for both sexes were positively correlated with both BMI and the well studied marker of inflammation Creactive protein (CRP) (33).
The link between smoking and insulin resistance syndrome was well identified (16,33–35). In addition, smoking can increase the release of catecholamines, cortisol, adrenocorticotrophic hormone, and growth hormone, and alter the sympathetic system activity (29,34). These effects may be responsible for the decrement of insulin-mediated glucose uptake in smokers (36,37). Correlation between fasting insulin and leptin levels was also previously reported (38). Thus, insulin resistance is likely to be associated with regulation of leptin metabolism in smokers. On the other hand, multiple regressions analysis of the study of Donahue et al. revealed that the effect of smoking on plasma leptin levels seems to be independent of fasting insulin levels (20). Since no overweight individual was included neither in the study nor the control group, and all subjects were young and fairly well exercised, we did not measure fasting plasma insulin levels.
In conclusion, the present study indicates that plasma leptin is decreased in healthy, young non-obese male smokers independently of the amount of body fat, and high amount of smoking is associated with lower serum leptin. Thus, smoking appears to be among the direct modulators of leptin metabolism.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Eisen SA, Lyons MJ, Goldberg J, True WR. The impact of cigarette and alcohol consumption on weight and obesity: an analysis of 1911 monozygotic male twin pairs. Arch Intern Med. 1993;153:2457–63. [PubMed] [Google Scholar]
- 2.Goss J, Grubbs L. Comparative analysis of body mass index, consumption of fruits and vegetables, smoking, and physical activity among Florida residents. J Community Health Nurs. 2005;22:37–46. doi: 10.1207/s15327655jchn2201_4. [DOI] [PubMed] [Google Scholar]
- 3.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236–43. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 4.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara CM, Kumar M, Nicklas B, McCrone S, Goldberg AP. Weight gain and adipose tissue metabolism after smoking cessation in women. Int J Obes Relat Metab Disord. 2001;25:1322–6. doi: 10.1038/sj.ijo.0801716. [DOI] [PubMed] [Google Scholar]
- 6.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 7.Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Anto JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–35. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- 8.Clark MM, Decker PA, Offord KP, Patten CA, Vickers KS, Croghan IT, et al. Weight concerns among male smokers. Addict Behav. 2004;29:1637–41. doi: 10.1016/j.addbeh.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Campfield LA, Smith FJ. The OB protein (leptin) pathway a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–32. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 10.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:455–62. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 11.Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes. 1996;104:293–300. doi: 10.1055/s-0029-1211457. [DOI] [PubMed] [Google Scholar]
- 12.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–13. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 13.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 14.Ahren B, Larsson H, Wilhelmsson C, Nasman B, Olsson T. Regulation of circulating leptin in humans. Endocrine. 1997;7:1–8. doi: 10.1007/BF02778056. [DOI] [PubMed] [Google Scholar]
- 15.Larsson H, Elmstahl S, Ahren B. Plasma leptin levels correlate to islet function independently of body fat in postmenopausal women. Diabetes. 1996;45:1580–4. doi: 10.2337/diab.45.11.1580. [DOI] [PubMed] [Google Scholar]
- 16.Klein LC, Corwin EJ, Ceballos RM. Leptin, hunger, and body weight: Influence of gender, tobacco smoking, and smoking abstinence. Addict Behav. 2004;29:921–7. doi: 10.1016/j.addbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Hodge AM, Westerman RA, de Courten MP, Collier GR, Zimmet PZ, Alberti KG. Is leptin sensitivity the link between smoking cessation and weight gain? Int J Obes Relat Metab Disord. 1997;21:50–3. doi: 10.1038/sj.ijo.0800362. [DOI] [PubMed] [Google Scholar]
- 18.Wei M, Stern MP, Haffner SM. Serum leptin levels in Mexican Americans and non-Hispanic whites: association with body mass index and cigarette smoking. Ann Epidemiol. 1997;7:81–6. doi: 10.1016/s1047-2797(96)00114-7. [DOI] [PubMed] [Google Scholar]
- 19.Mantzoros CS, Varvarigou A, Kaklamani VG, Beratis NG, Flier JS. Effect of birth weight and maternal smoking on cord blood leptin concentrations of full-term and pretermborns. J Clin Endocrinol Metab. 1997;82:2856–61. doi: 10.1210/jcem.82.9.4248. [DOI] [PubMed] [Google Scholar]
- 20.Donahue RP, Zimmet P, Bean JA, Decourten M, DeCarlo Donahue RA, Collier G, et al. Cigarette smoking, alcohol use, and physical activity in relation to serum leptin levels in a multi-ethnic population: the Miami Community Health Study. Ann Epidemiol. 1999;9:108–13. doi: 10.1016/s1047-2797(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 21.Perkins KA, Fonte C. Effects of smoking status and smoking cessation on leptin levels. Nicotine Tob Res. 2002;4:459–66. doi: 10.1080/1462220021000018434. [DOI] [PubMed] [Google Scholar]
- 22.Reseland JE, Mundal HH, Hollung K, Haugen F, Zahid N, Anderssen SA, et al. Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins Leukot Essent Fatty Acids. 2005;73:43–9. doi: 10.1016/j.plefa.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30:713–9. doi: 10.1038/sj.npp.1300597. [DOI] [PubMed] [Google Scholar]
- 24.Nystrom F, Ekman B, Osterlund M, Lindstrom T, Ohman KP, Arnqvist HJ. Serum leptin concentrations in a normal population and in GH deficiency: negative correlation with testosterone in men and effects of GH treatment. Clin Endocrinol (Oxf) 1997;47:191–8. doi: 10.1046/j.1365-2265.1997.2281039.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoshinari M, Wakisaka M, Fujishima M. Serum leptin levels in smokers with type 2 diabetes. Diabetes Care. 1998;21:516–7. doi: 10.2337/diacare.21.4.516. [DOI] [PubMed] [Google Scholar]
- 26.Larsson H, Ahren B. Smoking habits and circulating leptin in postmenopausal non-obese women. Diabetes Obes Metab. 1999;1:57–9. doi: 10.1046/j.1463-1326.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Eliasson B, Smith U. Leptin levels in smokers and long-term users of nicotine gum. Eur J Clin Invest. 1999;29:145–52. doi: 10.1046/j.1365-2362.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicklas BJ, Tomoyasu N, Muir J, Goldberg AP. Effects of cigarette smoking and its cessation on body weight and plasma leptin levels. Metabolism. 1999;48:804–8. doi: 10.1016/s0026-0495(99)90183-x. [DOI] [PubMed] [Google Scholar]
- 29.Smith U. Smoking elicits the insulin resistance syndrome: New aspects of the harmful effects of smoking. J Intern Med. 1995;237:435–7. doi: 10.1111/j.1365-2796.1995.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 30.Al Mutairi SS, Mojiminiyi OA, Shihab-Eldeen AA, Al Sharafi A, Abdella N. Effect of smoking habit on circulating adipokines in diabetic and non-diabetic subjects. Ann Nutr Metab. 2008;52:329–34. doi: 10.1159/000151487. [DOI] [PubMed] [Google Scholar]
- 31.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–7. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Hansen MJ, Jones JE, Vlahos R, Bozinovski S, Anderson GP, et al. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am J Respir Crit Care Med. 2006;173:1248–54. doi: 10.1164/rccm.200506-977OC. [DOI] [PubMed] [Google Scholar]
- 33.Corwin EJ, McCoy CS, Whetzel CA, Ceballos RM, Klein LC. Risk indicators of metabolic syndrome in young adults: a preliminary investigation on the influence of tobacco smoke exposure and gender. Heart Lung. 2006;35:119–29. doi: 10.1016/j.hrtlng.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, et al. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90:248–53. doi: 10.1161/01.cir.90.1.248. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988;319:1318–30. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 36.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance a potential link with the insulin resistance syndrome. J Intern Med. 1993;233:327–32. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 37.Epifano L, Di Vincenzo A, Fanelli C, Porcellati F, Perriello G, De Feo P, et al. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus. Eur J Clin Pharmacol. 1992;43:257–63. doi: 10.1007/BF02333019. [DOI] [PubMed] [Google Scholar]
- 38.Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, Brabant G. UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab. 1996;82:654–7. doi: 10.1210/jcem.82.2.3744. [DOI] [PubMed] [Google Scholar]