Abstract
Purpose
The goal of this study is to assess the interactions among alcohol consumption, cigarette smoking, and aspartate aminotransferase (AST) / alanine aminotransferase (ALT) ratios on esophageal cancer.
Materials and Methods
Alcohol and the risk of incidence and death from esophageal cancer were examined in a 14-year prospective cohort study of 782,632 Korean men, 30 to 93 years of age, who received health insurance from the National Health Insurance Corporation and had a medical evaluation from 1992 to 1995.
Results
Smoking, alcohol intake, and AST/ALT ratios were associated with the increased risk of esophageal cancer in a dose-dependent manner independent of each other. Smoking was associated with an increased risk of incidence [Hazard ratio (HR) = 2.2, 95% CI = 1.8 to 2.5] and mortality (HR = 2.5, 2.0 to 3.1). Combined HR of incidence for alcohol consumption (> 25 g/day) and smoking was 4.5 (3.8-5.5); for alcohol (> 25 g/day) and the AST/ALT ratio (≥ 2.0), it was 5.8 (4.6-7.2); for smoking and the AST/ALT ratio (≥ 2.0), it was 6.3 (5.1-7.5). Similar results were seen for mortality from esophageal cancer. Subjects who drank ≥ 25 g/day with an AST/ALT ratio ≥ 2 had a higher risk of esophageal cancer incidence (HR = 6.5, 4.8 to 8.7) compared with those who drank ≥ 25 g/day with an AST/ALT ratio < 2 (HR = 2.2, 1.9 to 2.6).
Conclusion
Alcohol, smoking, and the AST/ALT ratio are independently associated with increased risk of esophageal cancer but did not interact synergistically. The combination of the AST/ALT ratio with a questionnaire for alcohol consumption may increase the effectiveness for determining the risk of esophageal cancer.
Keywords: Alcohol, smoking, alanine transaminase, aspartate aminotransferases, esophageal neoplasms
INTRODUCTION
Cancer of the esophagus was the eighth leading cause of cancer related death among men and women in Korea in 2006. Squamous cell carcinoma (SCC) is the dominant histological type (≥ 95%) for esophageal cancer in Korea.1
Smoking tobacco and consuming alcohol are two factors strongly associated with a risk of developing esophageal SCC and to a lesser degree esophageal adenocarcinoma.2,3 Esophageal cancer is one of the most common cancers worldwide. These risk factors have also been reported to interact in a multiplicative way in the etiology of this neoplasm.2,4,5 However, the majority of the reviewed epidemiological studies were case-control or small scaled cohort studies with a limitation for the examination of the interaction between the two substances: tobacco and alcohol.5-7
Even though alcohol consumption was the strongest risk factor for esophageal cancer, it was difficult to compare the risk magnitude for alcohol consumption across studies due to the variety of alcohol consumption behaviors throughout various countries. The ratio of serum aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is a common biomarker for alcohol consumption.8 Therefore, we assessed the value of the AST/ALT ratio to predict the risk of esophageal cancer even though there were relatively few reports on the association between AST/ALT ratios and the risk of esophageal cancer.
Previous studies reported that smoking and alcohol usage increased the risk of esophageal cancer multiplicatively.6,7,9 However, longitudinal studies which evaluated the combined effects of not only alcohol and smoking, but also the AST/ALT ratio, were rare.
Therefore, the aim of this study was to determine the relationship between alcohol consumption, smoking, and the AST/ALT ratio, as well as the incidence and mortality rate for esophageal cancer. Although some epidemiologic studies have addressed these and other risk factors, the reviewed studies had limitations, such as: 1) not exploring the synergistic effects when being exposed to multiple risk factors including the AST/ALT ratio, 2) the sample population size, and 3) exposure data. Elucidating the synergistic effect between the risk factors (smoking, alcohol consumption, and AST/ALT ratio) could result in substantial benefits for public health and have clinical relevance for practitioners. Individuals at extremely high risk could be readily identified and counseled.
We have conducted a prospective cohort study for the causes of cancer in a cohort of Koreans (the Korean Cancer Prevention Study) insured by the National Health Insurance Corporation.10 The cohort is large with over 1.3 million participants - each was given information on smoking, alcohol consumption, and aminotransferase levels. Follow-ups, accomplished through record linkage at the national level, was completed for all participants except for emigrants. In this manuscript, we describe the risk for esophageal cancer in relation to smoking, alcohol consumption, and the AST/ALT ratio during a 14-year follow-up where there were 1,464 incidences of esophageal cancer and 1,034 deaths from esophageal cancer.
MATERIALS AND METHODS
Study participants
The Korean Cancer Prevention Study is a prospective cohort study designed to assess risk factors for mortality, incidence, and hospital admission from cancer with a 14-year follow-up. Information concerning the development of this cohort from participants in the Korea Medical Insurance Corporation has been provided elsewhere.10,11 In brief, the cohort: 1) was derived from government employees, teachers, and their respective dependents, 2) was insured by the Korea Medical Insurance Corporation from 1992 to 1995, 3) had at least one medical examination, and 4) completed a questionnaire during that time. In Korea, all insured workers are required to participate in biennial medical examinations. In 1992, 94% of insured workers completed these biennial examinations, while 95% completed them in 1994. Thirty-seven percent in 1993 and 24% in 1995 of the insured workers' dependents completed biennial medical examinations.10
The Korean Cancer Prevention Study cohort includes 1,329,525 Koreans (846,907 men and 482,618 women) from 30 to 95 years of age who met the above selection criteria. Of the study participants, 784,870 (59.0%) were enrolled in 1992, 367,903 (27.7%) in 1993, 98,417 (7.4%) in 1994, and 78,335 (5.9%) in 1995. Of the 1,329,525 participants, we excluded 4,498 who reported a history of cancer (any type) at enrollment, 875 who died before the start of follow-up, and 53,810 who tested positive for the hepatitis B surface antigen (HBsAg) among those with HBsAg data (n = 611,034). We further excluded the following numbers of participants due to missing information: 9,411 on alcohol consumption and 17,139 on alanine aminotransferase or aspartate aminotransferase levels. In addition, 2,585 subjects with a low body mass index [body mass index (BMI) less than 16] (the weight in kilograms divided by the square of the height in meter) or short stature (1.3 m or less) were excluded. Finally, this study was limited to men due to the small number of women participants having esophageal cancer. Therefore, the final sample size included 782,632 male subjects.
Data collection
The biennial examinations followed a standard procedure and were conducted by medical staff at local hospitals. In the 1992, 1993, 1994 and 1995 questionnaires, participants were asked to describe their smoking habits along with other health habits including alcohol consumption. The completed questionnaires were reviewed by trained staff and then entered into a database. The data was further checked during analysis.
Based on responses from the questionnaire during the baseline examination, participants were classified as "current smokers" if they reported smoking for at least one year at the time the questionnaire was filled out, "never smoker" if they had never smoked, and "ex-smoker" if they had smoked but quit.
Alcohol consumption was categorized as follows: 1-24 g, 25-49 g, 50-99 g, and 100 g per day or more to characterize light, moderate, or heavy drinking. Total daily alcohol consumption was expressed as the number of glasses per week, expressed in relation to Korea's most popular alcoholic beverage, 'Soju'. One glass of 'Soju' contains about 12 g of ethanol.
Obesity was also examined as a risk factor. We used the WHO standard BMI cut-off points for Asians: < 18.5, 18.5-22.9, 23.0-24.9, ≥ 25.0 kg/m2.12
Fasting serum glucose and liver function tests such as alanine aminotransferase (alanine transaminase), aspartate aminotransferase, and HBsAg were measured. Serum HBsAg was tested by radioimmunoassay or reversed passive hemagglutination in hospital laboratories.
The median follow-up period was 14 years from January 1, 1993 to December 31, 2006. The exact dates of the survey form completion was not recorded. Consequently, follow-up accrual began on January 1 following the year in which the survey form was completed. Persons who completed a survey but died in the same calendar year were excluded.
Because the study involved routinely collected medical data, participant consent was not specifically obtained. The study was approved by the Institutional Review Boards of Yonsei University and the Johns Hopkins Bloomberg School of Public Health.
Cancer outcomes
The principal outcome variables were incident cases and mortality from esophageal cancer. Outcomes for incidents were based on national cancer registry data and hospitalization records. Although Korea has a national cancer registry, reporting was not complete during the follow-up; consequently, hospital admission files were used to identify a first admission event for esophageal cancer. An incident cancer case was coded as occurring based on either a positive report from the national cancer registry or on a hospital admission for a cancer diagnosis. Outcomes for mortality were ascertained from death certificates. A computerized search of the death certificate data from the National Statistical Office in Korea was performed using the unique identification number assigned at birth. Causes of death were assigned at the hospitals by trained abstractors. Esophageal cancer was classified according to the 10th revision of the International Classification of Disease: Malignant neoplasm of esophagus (ICD code 15).
Statistical analysis
Age-adjusted death rates were calculated for each category of smoking, alcohol consumption, and aminotransferase levels and directly standardized to the age distribution of the Korean national population in 1995. We also used Cox proportional hazards modeling to compute hazard ratios (HR), 95% confidence intervals (CIs), and to adjust for other potential risk factors.13 The proportionality assumption was verified by inspection of the hazard plots. Because the incidence of esophageal cancer varies steeply with age, we used linear and quadratic trends for age. Indicator variables were used for smoking and alcohol consumption. To calculate the population-attributable risk for high levels of AST/ALT ratio, cigarette smoking, and any alcohol use, we used Levin's formula14 with a generalization to the two strata of smoking status.15 We used the adjusted HR values to calculate the population-attributable risk. All statistical tests were two-sided and statistical significance was determined at p < .05.
RESULTS
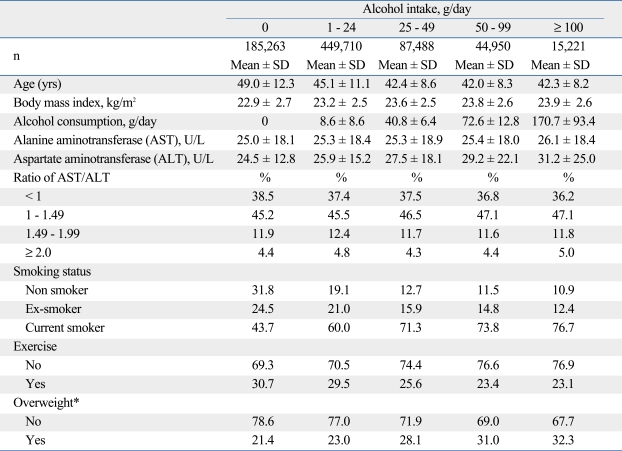
The characteristics of the study population are shown in Table 1. The mean age in non drinkers (49.0 years) was higher than those for drinkers (ranges 42.3 to 45.1 years). BMI did not differ according to alcohol intake per day. The means for baseline AST and ALT in this study population were associated with increased amounts of alcohol intake per day. People who drank ≥ 100 g/day had a higher AST/ALT ratio, higher prevalence of being a current smoker, a greater chance of being overweight, and were more likely to do less exercise than the other groups.
Table 1.
General Characteristics of the Study Population in Korean Men
SD, standard deviation.
*Overweight was defined as body mass index ≥ 25 kg/m2.
During the 14 years of follow-up, 1,383 incident cases of esophageal cancer occurred among men with a total of 996 esophageal cancer deaths observed in this population (Table 2). During this period, the incidence of esophageal cancer was 6.2 per 100,000 person-years for non-drinkers and 17.9 for drinkers. Smoking, alcohol intake, and the AST/ALT ratio were associated with the increased risk of esophageal cancer in a dose-dependent manner independently of each other.
Table 2.
Age-Adjusted Rate and Hazard Ratios (HR) and 95% Confidence Intervals (CI) for Esophageal Cancer among Korean Men
*per 100,000 person year.
†Cox model included for age, age2, alcohol intake, aspartate aminotransferase (GOT), body mass index, and exercise.
Compared to non-drinkers, drinkers had significantly elevated HRs of incidence and mortality (2.4 and 2.1, respectively) for esophageal cancer. Higher amounts of alcohol intake (> 100 g/d) were also statistically significant and increased both esophageal cancer incidence (HR = 4.1, 95% CI = 2.9-5.8) and morbidity (HR = 3.4, 95% CI = 2.2-5.3).
Compared with non-smokers, current smokers had significantly elevated HRs of incidence and mortality (2.2 and 2.5, respectively) for esophageal cancer. There was a linear relationship between increasing levels of smoking and the HR for esophageal cancer. We also observed a linear trend for the HR of esophageal cancer with increasing levels of AST, ALT, and the AST/ALT ratio (Table 3). People with an AST/ALT ratio ≥ 2.0 were at a high risk of morbidity (HR = 3.2, 95% CI = 2.7-3.9) and mortality (HR = 3.4, 95% CI = 2.8-4.2) compared to those who had an AST/ALT ratio < 2.0.
Table 3.
Age-Adjusted Rate and Hazard Ratios (HR) and 95% Confidence Intervals (CI) for Esophageal Cancer among Korean Men
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*PY, person-year.
†adjusted for age, diabetes status, body mass index, exercise and alcohol use.
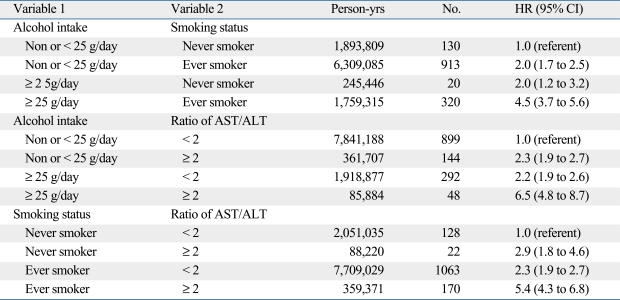
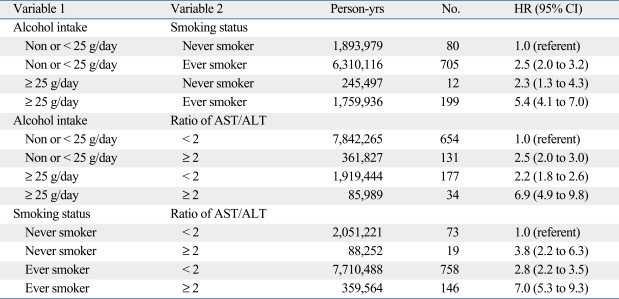
We explored patterns of effect modification among alcohol intake, smoking, and AST/ALT ratios using the Cox proportional hazards model. Table 4 and 5 show the HR estimates for strata defined by pairs of three variables. The highest risk of esophageal cancer incidence was observed among the following groups: 1) ever-smokers who had an alcohol intake ≥ 25 g/day (HR = 4.5, 95% CI = 3.7-5.6); 2) alcohol intake ≥ 25 g/day who had an AST/ALT ratio ≥ 2.0 (HR = 6.5, 95% CI = 4.8-8.7); and 3) ever-smokers who had an AST/ALR ratio ≥ 2.0 (HR = 5.4, 95% CI = 4.3-6.8). Alcohol consumption, cigarette smoking, and AST/ALT ratios were independent risk factors for esophageal cancer in men but no synergistic interactions were found among these risk factors. Similar results were found for mortality from esophageal cancer in men (Table 5).
Table 4.
Hazard Ratio (HR) of Incidence for Esophageal Cancer among Men in the Korean Cancer Prevention Study (1993 to 2006)*
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*Hazard ratios (HRs) and 95% confidence intervals (CIs) from multivariable Cox proportional models after adjusting for age, age2, smoking, alcohol intake, and body mass index. All statistical tests were two-sided.
Table 5.
Hazard Ratio (HR) of Mortality for Esophageal Cancer among Men in the Korean Cancer Prevention Study (1993 to 2006)*
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*Hazard ratios (HRs) and 95% confidence intervals (CIs) from multivariable Cox proportional models after adjusting for age, age2, smoking, alcohol intake, and body mass index. All statistical tests were two-sided.
In this study population, we estimated the population-attributable risk for any alcohol use and for cigarette smoking as 47.6% (95% CI = 37.3 to 57.3%) and 49.8% (95% CI = 37.4 to 60.1%) for esophageal cancer death, respectively.
DISCUSSION
In this large prospective cohort study of Korean men and women, we found that alcohol drinking, cigarette smoking, and AST/ALT ratios are independent risk factors for esophageal cancer in men. Additive, not multiplicative, interaction was observed among these three risk factors.
Cigarette smoking occurred in about 79% of Korean men in 1980 and still remained high in Korea until 2000. The prevalence of alcohol consumption (any type) was more than 80% in Korea. This indicates a strong association between the two life styles. The reason being is that over 90% of adult Korean male current smokers also consumed alcohol beverages.16
Previous studies have shown that smoking tobacco and drinking alcohol interacts multiplicatively contributes to an increased risk of esophageal cancer.3-5,7 However, most epidemiological studies were case - control studies or small scaled cohort studies with limitations of examining the interaction between the two substances. In a recent prospective study, the effect of alcohol was not modified by smoking. According to their report, the power to calculate the effect of interaction was limited due to the small number of malignant cases.9 Similarly, our study provided evidence that cigarette smoking and alcohol consumption does not have a multiplicative interaction effect on the risk for esophageal cancer. Further research is needed to confirm and understand the differences between these studies in to order to ascertain any synergistic effects on esophageal cancer risk.
Several epidemiological studies have also found that the amount of alcohol consumed and the amount of smoking by an individual are strongly associated with elevated risk of esophageal cancer.7,17 Generally, smoking tobacco and drinking alcohol are two habits strongly associated with an increased risk of esophageal SCC and to a lesser degree, esophageal adenocarcinoma.2,3 SCC is the dominant histological type (95%) for esophageal cancer in Korea.1 In conjunction with findings from earlier research groups,18 our study shows that low and moderate alcohol intake (1-24 g/day) was also associated with an increased risk of esophageal cancer (HR = 2.2 for incidence and HR = 1.9 for mortality from esophageal cancer). There are many reports highlighting the fact that heavy alcohol drinking is associated with an elevated risk of esophageal cancer. However, their data inconsistently claims that a higher risk is associated with light to moderate drinking.18 Epidemiological reports from South India,19 the US,20 Northern Italy,21 and Hong Kong22 concordantly reported that alcohol intake below 140 g/week is linked to a slight increase or even decrease in risk for esophageal cancer. However, a meta-analysis found that consuming 25 g of alcohol daily carried a significantly elevated risk, or 1.5 fold.23
Even though alcohol consumption was the strongest risk factor for esophageal cancer, it was not easy to compare the magnitude of risk for alcohol consumption across studies because the types of alcohol consumption and behavior were quite different across various countries. In our study, both AST and ALT levels alone, and AST/ALT ratios were associated with elevated risks of esophageal cancer. We also examined the synergistic effects of alcohol, smoking, and AST/ALT ratios. The results were not surprising because these serum markers are some of the most common biomarkers for alcohol consumption. However, relatively few studies have looked at the association between biomarkers for alcohol consumption and the risk of esophageal cancer. In our study, people who drank ≥ 25 g/day and had an AST/ALT ratio of ≥ 2 had a much higher risk of esophageal cancer compared with people who only drank ≥ 25 g/day. These results indicate that using both biomarkers to measure alcohol intake and a questionnaire that measures alcohol consumption can be an effective marker for determining the risk of esophageal cancer. Ethanol is a mitochondrial toxin and may cause AST to be greater than ALT. Thus, in patients with liver disease, an AST/ALT ratio over 2.0 may be suggestive of alcoholic etiology.24
The exact mechanism behind AST or AST/ALT ratios is unclear and is it not known how they induce esophageal cancer. One possible explanation is that aminotransferase levels partially reflect the amount of alcohol intake. However, ethanol is not a carcinogen; under certain experimental conditions, though, it is a cocarcinogen and/or a tumor promoter. The metabolism of ethanol leads to the generation of acetaldehyde (AA) and free radicals. Accumulated evidence suggests that AA is predominantly responsible for alcohol associated carcinogenesis.24 Another possible mechanism could be that alcohol acts as a solvent that enhances the penetration of carcinogenic compounds into the mucosa. Ethanol may facilitate the uptake of environmental carcinogens, especially those from tobacco smoke, through cell membranes that are damaged and changed at the molecular level by the direct effect of alcohol.25
A particular strength of this study was the large number of participants, which allowed for the exploration of interactions among the previously mentioned, well-established risk factors.
The potential limitations of our study arose primarily from the use of certain data, mainly those collected through insurance plans. The questionnaires, for example, only provided self-reported smoking and alcohol use without additional verification. Additionally, causes-of-death that are taken from death certificates are subject to misclassification. Data on the histological type for all subjects were not available. But SCC is the dominant histological type (95%) for esophageal cancer in Korea.1
While false-positives undoubtedly occur, we do not think that our findings can be explained by inadequate specificity of death certificate classification. We anticipate a general bias towards the null due to misclassification unless it was differentiated by exposure. Given the high mortality rate of persons with esophageal cancer and the high rate of coding of death in incident esophageal cancer cases, we do not consider this misclassification of outcome an issue in interpreting our findings. Additionally, our study cohort is not representative of all Koreans because it includes employed persons and their families and, consequently, may under-represent heavy users of alcohol and tobacco. However, a follow-up should be nearly complete due to our use of unique personal identifiers within national databases.
Low intake of fresh fruits and vegetables26 or genetic variance on genes such as ALDH2 or CYP1A127 could not be included in the analysis due to lack of information.
In conclusion, alcohol, smoking, and AST/ALT ratios are independently associated with increased risk of esophageal cancer but they do not interact synergistically. Our results indicate that consuming alcohol and smoking cigarettes have an independent, causal role in the etiology of esophageal cancer. The combination of using AST/ALT ratios with questionnaires for alcohol may increase the effectiveness of determining esophageal cancer risk.
ACKNOWLEDGEMENTS
The authors would thank the staff of the Korean National Health Insurance Corporation. This study was supported by a grant of the Seoul R&BD program, Republic of Korea (10526) and this study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0920330). The authors would also like to thank Jaeseong Jo for editorial assistance.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.2006 Cancer mortality in Korea. National Cancer Information Center, Korean National Cancer Center. Available from: URL: http://www.cancer.go.kr/cms/statics/mortality/index.html.
- 2.International Agency for Research on Cancer (IARC) Alcohol Drinking. Vol 44. Lyon, France: IARC Press; 1988. [Google Scholar]
- 3.International Agency for Research on Cancer (IARC) Tobacco smoke and involuntary smoking. Vol. 83. Lyon, France: International Agency for Research on Cancer; 2004. IARC monograph 83. [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to human. Tobacco smoking. Lyon, France: IARC Press; 1986. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Lee JM, Wu DC, Hsu HK, Kao EL, Huang HL, et al. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int J Cancer. 2005;113:475–482. doi: 10.1002/ijc.20619. [DOI] [PubMed] [Google Scholar]
- 6.Wu IC, Lu CY, Kuo FC, Tsai SM, Lee KW, Kuo WR, et al. Interaction between cigarette, alcohol and betel nut use on esophageal cancer risk in Taiwan. Eur J Clin Invest. 2006;36:236–241. doi: 10.1111/j.1365-2362.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 7.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Hannuksela ML, Liisanantti MK, Nissinen AE, Savolainen MJ. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953–961. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- 9.Weikert C, Dietrich T, Boeing H, Bergmann MM, Boutron-Ruault MC, Clavel-Chapelon F, et al. Lifetime and baseline alcohol intake and risk of cancer of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer. 2009;125:406–412. doi: 10.1002/ijc.24393. [DOI] [PubMed] [Google Scholar]
- 10.Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341–348. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 11.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. The Asia-Pacific Perspective: Redefining obesity and its treatment. Sydney: Health Communication; 2000. [Google Scholar]
- 13.Cox DR. Regression models and life-tables. J Roy Stat Soc. 1972;34:187–202. [Google Scholar]
- 14.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 15.National Center for Chronic Disease Prevention and Health Promotion. Smoking-attributable mortality, morbidity and economic costs (SAMMEC) Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 16.The Korean National Health and Nutritional Survey 2005. Seoul: Korean Ministry of Health; 2005. [Google Scholar]
- 17.Zambon P, Talamini R, La Vecchia C, Dal Maso L, Negri E, Tognazzo S, et al. Smoking, type of alcoholic beverage and squamous-cell oesophageal cancer in northern Italy. Int J Cancer. 2000;86:144–149. doi: 10.1002/(sici)1097-0215(20000401)86:1<144::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Wu DC, Lee JM, Wu IC, Goan YG, Kao EL, et al. Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur J Cancer. 2007;43:1188–1199. doi: 10.1016/j.ejca.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Znaor A, Brennan P, Gajalakshmi V, Mathew A, Shanta V, Varghese C, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681–686. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]
- 20.Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–1284. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi S, Bidoli E, Negri E, Barbone F, La Vecchia C. Alcohol and cancers of the upper aerodigestive tract in men and women. Cancer Epidemiol Biomarkers Prev. 1994;3:299–304. [PubMed] [Google Scholar]
- 22.Cheng KK, Duffy SW, Day NE, Lam TH, Chung SF, Badrinath P. Stopping drinking and risk of oesophageal cancer. BMJ. 1995;310:1094–1097. doi: 10.1136/bmj.310.6987.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hietala J, Puukka K, Koivisto H, Anttila P, Niemelä O. Serum gamme-glutamyl transferase in alcoholics, moderate drinkers and abstainers: effects on gt reference intervals at population level. Alcohol Alcohol. 2005;40:511–514. doi: 10.1093/alcalc/agh201. [DOI] [PubMed] [Google Scholar]
- 25.Pöschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 26.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121:2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 27.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]