Abstract
AIM: To evaluate if canine models are appropriate for teaching endoscopy fellows the techniques of endoscopic submucosal dissection (ESD).
METHODS: ESD was performed in 10 canine models under general anesthesia, on artificial lesions of the esophagus or stomach marked with coagulation points. After ESD, each canine model was euthanized and surgical resection of the esophagus or stomach was carried out according to “The Principles of Humane Experimental Technique, Russel and Burch”. The ESD specimens were fixed with needles on cork submerged in a formol solution with the esophagus or stomach, and delivered to the pathology department to be analyzed.
RESULTS: ESD was completed without complications using the Hook-knife in five esophageal areas, with a procedural duration of 124 ± 19 min, a length of 27.4 ± 2.6 mm and a width of 21 ± 2.4 mm. ESD was also completed without complications using the IT-knife2 in five gastric areas, with a procedural duration of 92.6 ± 19 min, a length of 32 ± 2.5 mm and a width of 18 ± 3.7 mm.
CONCLUSION: ESD is feasible in the normal esophagus and stomach of canine models, which are appropriate for teaching this technique.
Keywords: Endoscopic mucosal resection, Endoscopic submucosal dissection, Stomach neoplasms, Training
INTRODUCTION
The incidence of esophageal adenocarcinoma is currently rising in Western countries and Latin America. Also, gastric cancer is the second leading cause of cancer-related death worldwide. Endoscopic submucosal dissection (ESD) has provided a new alternative for minimally invasive treatment of gastrointestinal (GI) early-stage cancer[1-10].
With the purpose of preserving gastrointestinal function and obtaining specimens for precise histological evaluation, ESD[11,12] has been developed for lesions ≥ 2 cm that are not amenable for endoscopic mucosal resection (EMR) because of their size[1,13,14]. Adequate training is essential, because complications[11,12] such as perforation are more likely with ESD than with EMR[2,4,15-18]. In selected cases, ESD may replace surgery and provide clean margins for accurate histological diagnosis of the lesion borders and a complete curative treatment unlike other techniques such as piecemeal EMR[2,4,15,16], cryotherapy, laser, argon plasma and photodynamic therapy because of their local recurrence rates.
There are few training centers around the world in which an endoscopy fellow can be trained in the ESD technique. There is probably only a formal ESD training program in Asian countries[4-7,12,16], Germany[8,9], recently in Portugal[10] and at one site in Colombia. Animal models have been used to test ESD devices and new technology in this field, but a formal training program is still necessary to teach this technique in western countries and Latin America. For this reason, animal models are an invaluable learning resource.
Our aim was to evaluate if canine models are appropriate to teach endoscopy fellows the ESD technique.
MATERIALS AND METHODS
Ethics
This study was carried out in accordance with “The Principles of Humane Experimental Technique, Russel and Burch”[19]. After receiving approval for the protocol from the ethics and animal research committees at our institution (GAS-14-09/09-1), the procedures were conducted on 10 mongrel dogs that weighed 18-20 kg that had previously passed the quarantine period. All the procedures were done by a staff member of the Gastroenterology Department, who was trained at the Fujigaoka Hospital of the Showa University, Yokohama, Japan.
Cutting devices
Hook knife: The hook-type knife KD-620LR (Olympus Optical, Tokyo, Japan) was used to perform ESD in the esophagus[6] of the canine models. It was right-angled 1 mm at the tip. Safety is improved compared with the use of a needle knife, because the submucosal tissue is hooked and pulled before incision. Safety is further improved when used in conjunction with a transparent soft cap D-201-11804 (Olympus Optical) because the tissue can then be pulled inside the hood (Figure 1E). The knife had a rotating function so that the operator could select the optimal direction of the hook.
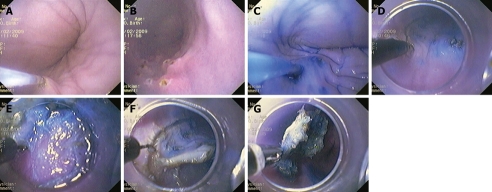
Figure 1.
Procedure. A, B: Artificial lesion marked with coagulation points; C: Injection of normal saline with epinephrine and indigo carmine; D: Knife cutting of a circumference around the lesion; E: Transparent softcap provides lesion counter-traction; F: Soft cap attached to the tip of the endoscope during endoscopic submucosal dissection (ESD); G: Grasping forceps during retrieval of ESD specimens.
Modified insulated-tipped knife 2: The modified insulated-tipped diathermic knife2 (IT-knife2) (KD-611L; Olympus Optical) was used to perform ESD in the stomach[14] of the canine models. This knife consisted of a small ceramic ball attached to the tip of the blade, which functioned as an insulator so that the incision and dissection of the mucosa and submucosa could be performed safely.
A specialized feature of the modified IT-knife2[6], which differs from the original IT-knife and other incision instruments, is that the portion between the insulator tip and the sheath, on the base of the ceramic ball, has a combination of the original IT-knife and the triangular knife that is used for removing the tissue with the electrodes on the distal side of the blade. This feature allows a less horizontal cutting position, which was necessary with the previous knife model.
Injection solution for mucosal and submucosal elevation
For all the canine models, we used normal saline solution with diluted epinephrine (1:10 000) and indigo carmine[3,4,15], which were injected to elevate the lesion and separate the submucosal layer from the muscular layer, as required during all the steps of the ESD procedure, at a volume of 10-30 mL, depending on the size of the lesion. Also, this solution was used for small bleeding sites during ESD[11,12], or when the cushion of the submucosal layer was insufficient for good dissection. Too much infiltration can obstruct good vision of the circumferential cutting, which interferes with the final cut of the lesion.
Electrosurgical HF-generator
The new electrosurgical HF-generator (ESG-100; Olympus Optical) allowed us to set the power for preprogrammed coagulation and cutting modes.
Endoscopic unit and video-endoscope
All the procedures were done with a CV-145 endoscopy unit (Olympus Optical) and a GIF-Q1145 video-endoscope (Olympus Optical).
ESD procedure
The initial step was to produce an artificial lesion marked with coagulation points (Figure 1A and B) in the esophagus or stomach of 10 canine models (one at a time), under general anesthesia. The settings for marking the points with the precutting knife (KD-10Q-1; Olympus Optical) for the artificial lesions were: power, 20, and force coag2 mode. After marking, the above-mentioned solution was injected so that the peak of the lifting was outside of the markings (Figure 1C). ESD was performed with the Hook-knife in the esophagus or the IT-knife2 in the stomach. After that, we cut a circumference around the lesion with the knife (Figure 1D). We then used another injection to elevate the submucosal layer of the center of the lesion. The settings for the mucosal cutting of the circumference were power at 80 in pulse cut slow mode. Afterwards, we performed submucosal dissection using a soft cap D-201-11804 attached to the tip of the endoscope (Figure 1E) using the same settings as in the circumferential cut. This transparent soft cap provided lesion counter-traction (Figure 1F), which is similar to a surgeon’s left hand, which made it easy to dissect the submucosal layer. The grasping forceps (FG-49L-1; Olympus Optical) were used to retrieve the ESD specimens (Figure 1G).
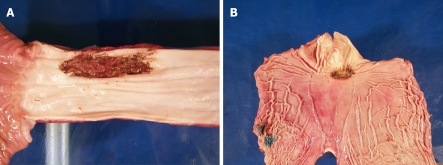
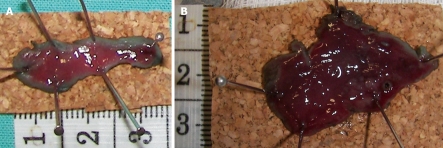
After ESD, each dog was euthanized and surgical resection of the esophagus or stomach (Figure 2A and B) was carried out according to “The Principles of Humane Experimental Technique, Russel and Burch”[19]. The ESD specimens were fixed with needles on a piece of cork (Figure 3A and B) submerged in formol solution, together with the resected esophagus or stomach, and delivered to the pathology department for investigation.
Figure 2.
Photographs of the retrieved surgical specimens. Esophagus (A) and stomach (B) after ESD.
Figure 3.
ESD specimens (A and B) fixed with needles in cork.
RESULTS
The ESD procedure was completed without complications using the Hook-knife in five esophageal areas, with a procedural duration of 124 ± 19 min, a length of 27.4 ± 2.6 mm, and a width of 21 ± 2.4 mm. The ESD procedure was also completed without complications using the IT-knife2 in five gastric areas, with a procedural duration of 92.6 ± 19 min, a length of 32 ± 2.5 mm, and a width of 18 ± 3.7 mm.
There was no perforation in either group. This fact was corroborated by an expert pathologist[8] after histological and macroscopic study of the specimens.
Descriptive statistics of the pilot study are shown in Table 1. The size of each specimen, the procedural duration, the site of ESD, and its complications are shown in Table 2 for the esophagus using a Hook-knife and in Table 2 for the stomach using an IT-knife2.
Table 1.
Descriptive statistics of the endoscopic submucosal dissection (ESD) pilot study
| Group | Values | Procedural duration (min) | Specimen length (mm) | Specimen width (mm) |
| Hook-knife (Esophagus) | n = 5 | |||
| mean | 124’ | 27.400 | 21.000 | |
| SD | 42’ 778” | 5.814 | 5.447 | |
| SE | 19’ 131” | 2.600 | 2.449 | |
| Range | 90’ | 13.000 | 15.000 | |
| IT-knife2 (Stomach) | n = 5 | |||
| mean | 92’ 60” | 32.000 | 18.000 | |
| SD | 43’ 518” | 5.701 | 8.367 | |
| SE | 19’ 462” | 2.550 | 3.742 | |
| Range | 107’ | 15.000 | 20.000 | |
| Total (Esophagus and stomach) | n = 10 | |||
| mean | 108’ 30” | 29.700 | 19.500 | |
| SD | 43’ 919” | 5.945 | 6.852 | |
| SE | 13’ 888” | 1.880 | 2.167 | |
| Range | 137’ | 23.000 | 20.000 |
Table 2.
ESD in the esophagus using the Hook-knife (KD-620LR) and in the stomach using the IT-knife2 (KD-611L)
| Size of specimen | Procedural duration (min) | Site of ESD | Complications |
| Hook-knife (Esophagus) | |||
| 30 mm × 20 mm | 180 | Lower | None |
| 30 mm × 20 mm | 160 | Lower | None |
| 30 mm × 20 mm | 100 | Lower | None |
| 17 mm × 15 mm | 90 | Upper | None |
| 30 mm × 30 mm | 90 | Mid | None |
| IT-knife2 (Stomach) | |||
| 25 mm × 10 mm | 150 | Lesser curvature of antrum | None |
| 35 mm × 30 mm | 120 | Antrum posterior wall | None |
| 30 mm × 20 mm | 90 | Lesser curvature of antrum | None |
| 40 mm × 10 mm | 60 | Lesser curvature of antrum | None |
| 30 mm × 20 mm | 43 | Upper greater curvature | None |
DISCUSSION
ESD has provided a new alternative for minimally invasive treatment of gastrointestinal early-stage[13] (T1mN0) cancer lesions > 2 cm, with minimal risk of deeper wall-layer involvement or lymph node metastases, as confirmed by endoscopic ultrasonography (EUS)[1-7]. This technique provides a curative alternative that preserves gastrointestinal function and offers an accurate histological T stage that replaces, in selected cases, surgery or other therapeutic attempts like piecemeal EMR[2,4,15,16], cryotherapy, laser, argon plasma and photodynamic therapy because of their local recurrence rates.
Multiple factors make esophageal, small intestine and colon ESD more difficult compared with gastric ESD, including difficulties in maintaining the position of the endoscope, the thinness of their walls, the luminal angulations, and peristalsis. To avoid complications such as perforation during the ESD procedure requires adequate training[4-10,12,16], and for this reason, animal models are invaluable as a learning resource.
Injection of indigo carmine[3,4,15] and the consequent blue staining of the submucosal tissue (Figure 1B) was used to identify the submucosal layer and the deep margin during the resection process.
Although there are several injection solutions commercially available in Japan for the submucosal cushion (Artz, 3.8% NaCl[15], Suveniel, 20% glucose, and glyceol), we decided to standardize the use of normal saline with epinephrine and indigo carmine for all the ESD procedures, and reserve for difficult cases the use of Suprahyal (Asofarma de Mexico S.A. de C.V., Mexico) and sodium hyaluronate[17] (Meiji Seika Kaisha LTD, Tokyo, Japan).
Also, the solution of normal saline, epinephrine and indigo carmine was used in cases with small bleeding sites during ESD. Fortunately, we did not have any bleeding complications[11,12] during ESD. It is also very important to use hemostatic forceps as the submucosal dissection progresses for all the visible vessels below the lesion, to prevent bleeding obstructing vision of the cutting direction and post-ESD bleeding.
The electrosurgical HF-generator allowed us to set the power for preprogrammed coagulation and cutting modes, which proved to be very important in performing the ESD without bleeding or perforation.
The right amount of infiltration always allows visualization of the circumference of the lesion for the final cut. Also, it is important to keep in mind how the muscular layer runs and to observe accurately the accumulation of liquids, to plan the cutting direction so that gravity can assist with the total dissection of the lesion.
Histopathological examination of the ESD specimens by an expert pathologist is essential to confirm clean margins, and complete removal of the mucosa and submucosa in all cases.
The limitations of this research were that it involved artificial non-neoplastic lesions and a small number of specimens in a pilot study.
ESD is a feasible technique in the normal esophagus and stomach of dogs and the use of these models is appropriate for teaching this technique.
ESD is a procedure that requires not only endoscopic skills, but also a good understanding of the endoscopy devices, techniques and technology to identify and treat EGC lesions. The minimum proficiency required by an endoscopy fellow to start ESD training is achieved after the appropriate knowledge is acquired. To begin with, the trainee assists an expert in the field, and eventually, he/she is assisted by an expert. Team work and coordination between the endoscopist and their assistants is also essential to perform ESD successfully.
Further studies and practice are needed to improve performance in canine models and to evaluate when the learning curve has been completed and a trainee is ready to perform ESD in patients. Also, in western countries and Latin America, multicenter studies are necessary before this technique can become a routine procedure.
The implementation of an ESD course at a WGO training center[1] will not only help to open numerous research areas that could contribute to the treatment of early GI cancer lesions, but will also help the worldwide dissemination of these techniques.
COMMENTS
Background
The incidence of esophageal adenocarcinoma is currently rising in Western countries and Latin America. Also, gastric cancer is the second leading cause of cancer-related death worldwide. Endoscopic submucosal dissection (ESD) has provided a new alternative for minimally invasive treatment of gastrointestinal early-stage cancer, with the preservation of gastrointestinal (GI) function. Because of possible complications, such as perforation, adequate training is necessary to perform ESD successfully.
Research frontiers
Complete curative treatment of early GI cancer with ESD has been demonstrated in the Asian countries. However, after many years of investigation in this field, there are still a lack of multicenter studies that have incorporated this technique into the everyday practice in Western countries and Latin America. In the present study, the authors demonstrated the feasibility of using animal models as a learning resource to teach the ESD technique.
Innovations and breakthroughs
There are few training centers around the world at which an endoscopy fellow can be trained in the ESD technique. There is probably only a formal ESD training program in Asian countries, Germany, recently in Portugal, and at one site in Colombia. Completion of the learning curve and implementation of training in the ESD technique at a health institute and World Gastroenterology Organisation training center that annually receives trainees from all over Mexico and Central and South America will not only help to open numerous research areas that could contribute to the treatment of early GI cancer, but will also help the worldwide dissemination of these techniques.
Applications
Until now, ESD has been considered technically difficult, hazardous and time consuming. However, new technology is allowing us to overcome these drawbacks. Animal models have been used to test ESD devices and new technology but a formal training program is still necessary to teach this technique in western countries and Latin America. For this reason, animal models are an invaluable learning resource.
Terminology
ESD is a technique that allows en-bloc resection of early GI cancers > 2 cm, with minimal risk of deeper wall-layer involvement or lymph node metastases, as confirmed by endoscopic ultrasonography. In selected cases, ESD may replace surgery and provide clean margins for accurate histological diagnosis of the lesion borders and complete curative treatment.
Peer review
This is an interesting investigation that presents the feasibility of using canine models for ESD training.
Footnotes
Peer reviewers: Hoon Jai Chun, MD, PhD, AGAF, Professor, Department of Internal Medicine, Institute of Digestive Disease and Nutrition, Korea University College of Medicine, 126-1, Anam-dong 5-ga, Seongbuk-gu, Seoul 136-705, South Korea; Jae J Kim, MD, PhD, Associate Professor, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50, Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea; Nageshwar D Reddy, Professor, Asian Institute of Gastroenterology, 6-3-652, Somajiguda, Hyderabad - 500 082, India
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Japanese Gastric Cancer Association. Gastric Cancer Treatment Guideline, 2nd ed (in Japanese) Kyoto: Japanese Gastric Cancer Association; 2004. [Google Scholar]
- 2.Hoteya S, Yahagi N, Iizuka T, Kikuchi D, Kawano K, Noguchi T, Mizuno H, Hashimoto M. [Endoscopic resection for early gastric cancers by EMR/ESD] Gan To Kagaku Ryoho. 2007;34:16–20. [PubMed] [Google Scholar]
- 3.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 4.Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Uraoka T, Kawahara Y, Kato J, Saito Y, Yamamoto K. Endoscopic submucosal dissection in the colorectum: present status and future prospects. Dig Endosc. 2009;21 Suppl 1:S13–S16. doi: 10.1111/j.1443-1661.2009.00863.x. [DOI] [PubMed] [Google Scholar]
- 6.Seol SY. Current techniques and devices for safe and convenient endoscopic submucosal dissection (ESD) and Korean experience of ESD. Digestive Endoscopy. 2008;20:107–144. [Google Scholar]
- 7.Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc. 2009;23:1546–1551. doi: 10.1007/s00464-009-0395-5. [DOI] [PubMed] [Google Scholar]
- 8.Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic submucosal dissection of early cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2009;7:149–155. doi: 10.1016/j.cgh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Neuhaus H. Endoscopic submucosal dissection in the upper gastrointestinal tract: present and future view of Europe. Dig Endosc. 2009;21 Suppl 1:S4–S6. doi: 10.1111/j.1443-1661.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 10.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Shiba M, Higuchi K, Kadouchi K, Montani A, Yamamori K, Okazaki H, Taguchi M, Wada T, Itani A, Watanabe T, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335–7339. doi: 10.3748/wjg.v11.i46.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 13.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 14.Tada M, Shimada M, Murakami F, Shimada M, Mizumachi M, Arima T, Yanai H, Oka S, Shigeeda M, Ogino M, et al. Development of the strip-off biopsy (in Japanese with English abstract) Gastroenterol Endosc. 1984;26:833–839. [Google Scholar]
- 15.Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 16.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507–512. doi: 10.1067/mge.2002.128108. [DOI] [PubMed] [Google Scholar]
- 18.Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866–867. doi: 10.1016/j.gie.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Russel WMS, Burch RL. The principles of humane experimental technique. London: UWAF; 1959. [Google Scholar]