Abstract
Meroterpenes are compounds of mixed biogenesis, isolated from plants, microorganisms and marine invertebrates. We have previously isolated and determined the structure for a series of meroterpenes extracted from the ascidian Aplidium aff. densum. Here, we demonstrate the chemical synthesis of three of them and their derivatives, and evaluate their biological activity on two bacterial strains, on sea urchin eggs, and on cancerous and healthy human cells.
Keywords: meroterpene, methoxyconidiol, epiconicol, didehydroconicol, antiproliferative activity
1. Introduction
Meroterpenes are compounds of mixed biosynthesis, mostly quinone or hydroquinones bonded with a terpenoid portion ranging from one to nine isoprene units. In the marine environment, these secondary metabolites are isolated mainly from: brown algae such as Cystoseira [1], marine microorganisms [2], soft corals [3], or marine invertebrates, such as sponges or ascidians [4,5]. Some prenylated hydroquinones inhibit proliferation of a panel of cancer cells [6–8]. Moreover, three new sesqui- and diterpene hydroquinone MK2 or PI3 kinase inhibitors have been described from demosponges [9,10].
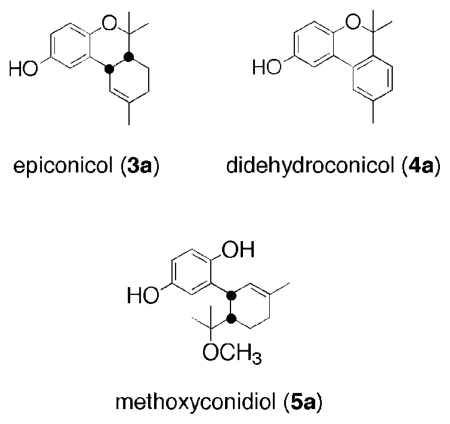
We recently extracted and elucidated the structure for a series of terpene hydroquinones from the ascidian Aplidium aff. densum [11]. Three of these secondary metabolites are shown in Figure 1.
Figure 1.
Structures of epiconicol (3a), didehydroconicol (4a) and methoxyconidiol (5a).
One of these compounds, methoxyconidiol, when tested on sea urchin embryos during cell division, disturbs mitotic spindle assembly leading to a cell cycle arrest during metaphase/anaphase transition [12]. These promising results led us to synthesize compound 5a, and the natural analogs 3a and 4a, as well as the methoxylated derivatives 3b–5b, in order to determine the role of the phenol functions.
The antiproliferative activity of these products was tested on bacteria (Micrococcus luteus and Escherichia coli), sea urchin eggs and normal and cancerous human cells. Considering the severe cell cycle disruption produced by methoxyconidiol treatment in sea urchin eggs, we directly evaluated the cytotoxicity of these new compounds on a panel of murine and human cells, both healthy and cancerous.
2. Results and Discussion
2.1. Chemistry
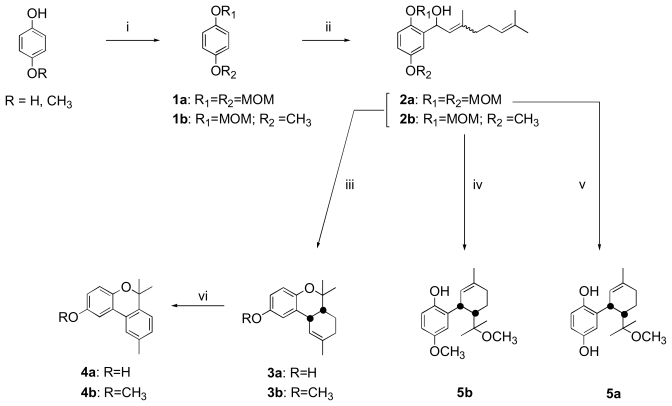
As previously described by Cruz-Almanza et al., the natural meroterpene 3a and the methoxy derivatives 3b–5b were synthesized starting from compounds 2a and 2b, respectively, using acidic conditions (Scheme 1) [13]. These two geranylhydroquinone derivatives used as key intermediates were prepared from methoxymethyl (MOM) protected hydroquinone 1a or MOM protected 4-methoxyphenol 1b by aromatic lithiation and subsequent treatment with citral. The new natural meroterpene 5a was synthesized by acidic treatment of 2a in methanol for 24 hours at 8 °C. Reaction time (24h) and constant temperature (8 °C) were found to be crucial in obtaining this compound; other conditions (room temperature with a shorter reaction time) led to the meroterpene 3a. The compound 5a was thus synthesized with a yield of 25%. It should be noted that the acidic treatment of 2a and 2b promoted dehydration followed by a rearrangement of the corresponding carbocation leading to meroterpenes 3a–b or 5a–b. This reaction was found to be diastereoselective with a cis/trans ratio greater than 4/1. The pair of cis isomers was purified, and finally, good yields of the new natural chromene 4a and its methoxy derivative 4b were obtained (70% yields) from 3a and 3b by dehydrogenation in the presence of sulfur at 250 °C. The 1H and 13C NMR spectra of compounds 3a, 4a and 5a were perfectly identical to those of the natural products epiconicol (3a), didehydroconicol (4a) and methoxyconidiol (5a) [11].
Scheme 1.
Reagents and conditions: (i) (a) NaH, THF, 1 h, rt, (b) MOMCl, THF, 3 h, rt; (ii) (a) nBuLi, THF, 2 h, rt, (b) citral, THF, 24 h, rt; (iii) HCl 6N, mixture 50: 50 THF/MeOH, 3 h, reflux; (iv) HCl 6N, MeOH, 3 h, rt; (v) HCl 12N, MeOH, 24 h, 8 °C; (vi) S, 4 h, 250 °C.
2.2. Biological activities
Some meroterpenes, of marine or plant origins, possess biological activities. Among them, cordiachromene A moderately inhibits the growth of Micrococus luteus [12], Staphyclococcus aureus and Streptococcus faecalis [14,15]. Hydroquinone sesquiterpenes are reported to be cytotoxic. Moreover, several meroterpenes isolated from terrestrial organisms inhibit the proliferation of a panel of cancer cells lines [9].
We evaluated the biological activities of compounds 3a–5a and their methoxy derivatives 3b–5b. Compounds 3b–5b were designed and synthesized because the introduction of a methoxy group (instead of a phenolic group) could influence their biological activities. Antibacterial assays were carried out on two bacterial strains and antiproliferative assays were carried out against sea urchin eggs and a panel of cancerous and healthy cells.
2.2.1. Antibacterial assays
The Microtiter Broth Dilution Method on gram negative (Escherichia coli) and gram positive (Micrococcus luteus) bacteria was used [16]. Meroterpenes dissolved in DMSO were added to bacteria suspensions (5:100 v:v) for 24 hours. The assay was carried out in triplicate. Bacterial growth was evaluated by absorbance measurement (630 nm). The minimum concentration that inhibits (MIC) bacterial growth was determined for each compound on each bacterial strain.
Four of the six meroterpenes had no effect on either of the two bacterial strains. Compounds 3a and 4a were weakly active. The MIC of epiconicol was 0.8 and 0.1 mM against E. coli and M. luteus respectively. Didehydroconicol inhibited the growth of M. luteus with a MIC of 0.5 mM. These two compounds possess phenolic group, and their substitution with a methoxy group (compounds 3b and 4b) led to totally inactive compounds.
2.2.2. Antiproliferative activities
The six meroterpenes were further tested on two models: sea urchin eggs as well as on healthy and tumorous human cells. We first assayed the capacity of each compound to inhibit sea urchin egg divisions (Paracentrotus lividus and Sphaerechinus granularis). Early sea urchin embryos used to be a model organism for antiproliferative screening and identification of cellular targets. In fact, several physiological features make this system ideal for conducting a wide range of biological tests. These include straight forward artificial spawning, in vitro fertilization, rapid synchronous development, embryo optical transparency, and well understood embryogenesis.
For cytotoxic assays on sea urchins, meroterpenes were dissolved in an egg suspension (described in the experimental section), and their ability to inhibit 50% of cell division (IC50) was estimated. Each experiment was carried out in triplicate and an average taken. Results are shown in table 1.
Table 1.
Antiproliferative activity of meroterpenes on sea urchin eggs (Paracentrotus lividus and Sphaerechinus granularis). An egg suspension was incubated with the six compounds in a 96-well plate. 85 minutes after fertilization (time when 100% division occurred in the control eggs), the number of dividing eggs was recorded and IC50 was calculated for each compound. Each experiment was carried out in triplicate.
| Compounds | IC50 (μM) | |
|---|---|---|
| P. lividus | S. granularis | |
| 3a | >25.0 | 9.8 ± 1.3 |
| 3b | >25.0 | >25.0 |
| 4a | 11.3 ± 0.8 | >25.0 |
| 4b | >25.0 | >25.0 |
| 5a | 4.3 ± 0.1 | 0.8 ± 0.0 |
| 5b | >25.0 | >25.0 |
Chromane (3a), chromene (4a) and hydroquinone (5a) inhibit egg division, methoxyconidiol (5a) being the most active. IC50 of methoxyconidiol was 0.80 μM on P. lividus eggs and 4.30 μM on S. granularis eggs, while epiconicol (3a) inhibited P. lividus eggs division with a IC50 value of 9.80 μM and the IC50 of didehydroconicol (4a) was 11.30 μM on P. lividus eggs and was inactive on S. granularis eggs. Methoxylated analogs are not active on sea urchin eggs. The substitution of a phenolic function with a methoxy group in these compounds produced a total loss of activity.
To provide information on their antiproliferative activity against solid cancers, the six meroterpenes were tested on three human carcinoma cell lines corresponding to cancers commonly observed in Western countries: MCF7 (breast), PA1 (ovary) and PC3 (prostate). To obtain additional information on their specificity for human cell lines and their specificity for cancer versus normal cell lines, each compound was tested versus L-929 murine immortalized cells and human normal fibroblasts. To mimic what happens in vivo, assays were carried out on exponentially growing cells cultured in the presence of 10% serum. The growth inhibitory effect was determined by measuring, after a 48 hour exposure to each chemical, the metabolic activity of treated and control cultures using the resazurin reduction test (RRT), which measures the amount of fluorescent resofurin produced by living cells. The results clearly demonstrated the non toxicity in all cell lines of the three methoxylated analogs (3b–5b), didehydroconicol (4a) and methoxyconidiol (5a) with an IC50 > 100 μM. A dose-dependent inhibition of cell proliferation was only observed for epiconicol (3a). The IC50 of this product varied with the cell lines from 37 to 69 μM. The toxicity of epiconicol observed in our model is in agreement with data obtained by Caroll and co-workers on a panel of cancer cells [17].
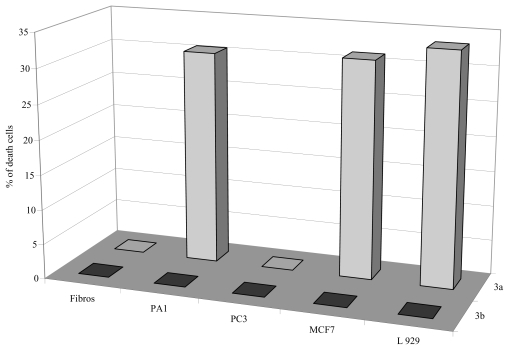
At 5 μM, epiconicol showed a weak but significant selectivity for PA1 and MCF7 carcinoma cells. Accordingly, 30% of the PA1 and MCF7 cultures were killed, while any antiproliferative activity was observed on primary fibroblasts or androgen-resistant prostate carcinoma cells (PC3). Data in Figure 2 showed that for epiconicol, the replacement of the hydroxyl group with a methoxy group suppressed the cytotoxicity on PA1, MCF7 and L929 cells and, consequently the selectivity for these cell lines.
Figure 2.
Effect of the compounds 3a and 3b on cell lines at 5 μM (PA1 ovarian, PC3 prostatical, MCF7 mammalian, human fibroblast and L929 murine fibroblasts). The antiproliferative effect was assessed by an evaluation of metabolical activity (RRT). Each experiment was carried out in triplicate.
The susceptibility of sea urchin eggs and human cells to methoxyconidiol was compared. Methoxyconidiol has the capacity to inhibit the proliferation of sea urchin eggs but not that of human cancer cell lines. This may be due either to a problem of penetration through human cell membranes, or to the rapid detoxification of methoxyconidiol by human cells inactivating the compound, or to the rapid bioconversion of methoxyconidiol by sea urchin eggs activating the compound.
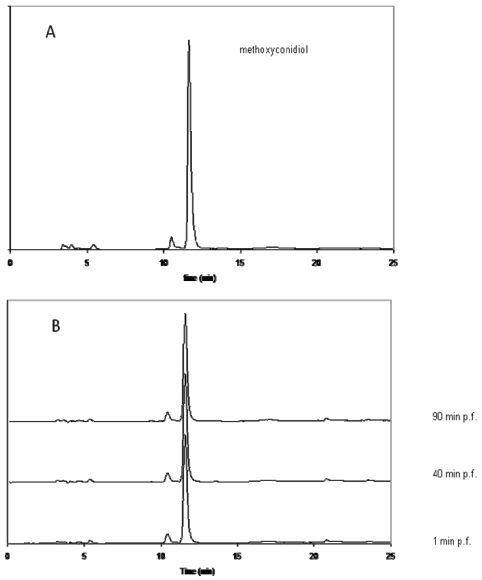
To test the latter hypothesis, S.s granularis eggs were treated with 5 μM of methoxyconidiol, a concentration that inhibits their division. Sea urchin eggs were incubated 1, 40 and 90 minutes post-fertilization (p.f.) [18] in the presence of methoxyconidiol. Cytoplasmic extracts of treated and control eggs were further prepared. Organic extracts of the cytoplasmic preparations were finally obtained and analyzed by HPLC (Figure 3).
Figure 3.
HPLC analysis of methoxyconidiol (System WATERS, column: synergy 4 μm RP max; elution water: methanol under a gradient; UV detection 285 nm; flow: 0.3 mL/min) A: control compound (methoxyconidiol) B: analysis after Solid Phase Extraction at 1, 40 and 90 minutes after fertilization (min p.f.).
Chromatograms were compared to the one obtained with a pure methoxyconidiol solution. A single peak at a characteristic retention time and UV spectrum (photodiode array detection) for methoxyconidiol (5a) was observed in cytoplasmic extracts from sea urchin eggs. The level of the xenobiotic compound did not decrease significantly during incubation. No other minor compound appeared after 40 or 90 minutes of incubation suggesting that the antiproliferative compound on sea urchins is methoxyconidiol and not a biotransformed analog. Furthermore, the presence of methoxyconidiol on the cytoplasm clearly indicates that the compound penetrates through sea urchin cell membranes. Methoxyconidiol was found ineffective on human cancer cells: this can be explained by a difference in membrane permeability or intracellular transport of compounds between sea urchin cells and human cells, a phenomena previously described for some cells [19,20].
3. Experimental Section
3.1. Chemistry
Commercial reagents were used without further purification. Melting point was determined on a Köfler melting point apparatus and is uncorrected. 1H NMR (400 MHz) and 13C (100 MHz) were recorded on a JEOL EX 400 spectrometer. Chemical shifts are expressed in ppm from the solvent chloroform (CDCl3) used as an internal standard: δH 7.24 (residual CHCl3), δC 77.0 for CDCl3. Mass spectrum was recorded on an Automass Benchtop Quadripole Mass Spectrometer. Column chromatography was performed on silica gel 60 merck (0.063–0.200 mm). The meroterpenes 3a and 3b–5b were synthesized as previously described [13].
Procedures for the preparation of compounds 4a–5a and 4b
Methoxyconidiol (5a)
12 N hydrochloric acid (0.5 mL) was added dropwise to a stirred solution of alcohol 2a (0.250 g, 0.7 mmol) in methanol (10 mL). The mixture was cooled at 8 °C for 24 h. The solution was diluted with a large excess of water and extracted with CH2Cl2. The combined organic layers were washed with water, dried (Na2SO4) and evaporated to produce a yellow oil, which was purified by the addition of a 8:2 mixture of ethyl acetate and heptane to furnish compound 5a as a white solid (48 mg, 25%). Mp 197 °C. 1H NMR and 13C NMR, see reference 11; MS (EI) m/z: 276.
Didehydroconicol (4a)
A mixture of compound 3a (0.154 g, 0.63 mmol) and sulfur (0.040 g, 1.26 mmol) was heated at 250 °C under a nitrogen atmosphere for 4h. The mixture was extracted with Et2O (2×10 mL). The solvent was evaporated and the residue was purified by column chromatography (heptane/ethyl acetate (8:2)) to furnish compound 4a as a colorless oil (105 mg, 70%). 1H NMR and 13C NMR, see reference 11; MS (EI) m/z: 240.
O-methyldidehydroconicol (4b)
This compound was prepared from 3b (0.140 g, 0.54 mmol) as described for 4a. The residue was purified by column chromatography (heptane/ethyl acetate (95:5)) to give 4b (96 mg) as a colorless oil with a yield of 70%. 1H NMR (CDCl3, 400 MHz): δ (ppm) 1.59 (s, 6H), 2.40 (s, 3H), 3.84 (s, 3H), 6.80 (dd, J = 9 Hz and J = 3 Hz), 6.87 (d, J = 9 Hz, 1H), 7.11–7.14 (m, 2H), 7.24 (d, J = 3 Hz, 1H), 7.49 (s, 1H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 21.4, 27.5, 55.9, 77.3, 108.0, 115.0, 118.7, 122.9, 123.22, 123.20, 128.6, 128.9, 137.2, 137.3, 146.9, 154.4. MS (EI): m/z: 254.
3.2. Biological activities
The antibacterial assay used was based on the previously described method published by the National Committee of Laboratory Safety and Standards [11].
3.2.1. Assays on sea urchin eggs
For antiproliferative assays on sea urchin eggs, we used a previously described method [12].
3.2.1.1. Extraction of methoxyconidiol from treated sea urchin eggs
3.2.1.1.1. Preparation of cytoplasmic extracts
Preparation of cytoplasmic extracts was based on the technique described by Collas and Poccia [18]. Gametes of P. lividus were collected in two separate beakers. Sperm was maintained undiluted on ice and eggs were stored at 19 °C until use. Sperm was diluted to 10% in Filtered Sea Water (FSW) and eggs were at 26 eggs/μL in FSW.
Fertilization was done in the proportion 1:1000 sperm:eggs (v:v). Elevation of the fertilization membrane was controlled under an inverted microscope (×10) and when 99% of the eggs were fertilized, methoxyconidiol dissolved in DMSO and FSW was added. A control without the addition of methoxyconidiol was carried out under the same conditions.
The cytoplasm of treated and control eggs was extracted just after fertilization, 40 and 90 minutes after fertilization. 10 mL of an homogeneous suspension of fertilized eggs was centrifuged at 2000 rpm for 5 min at 4 °C. 5 mL of Buffer A (10mM HEPES pH 8.0, 250 mM NaCl, 25 mM EGTA, 5 mM MgCl2, 110 mM glycine, 250 mM glycerol, 1 mM DTT and 1 mM PMSF) was added to the pellet and centrifuged again under the same conditions. Eggs were resuspended carefully in an ice-cold Buffer A at a volume ratio of 1:1 v:v and transferred into a 1.5 mL microcentrifuge tube on ice. To homogenize the solution, the eggs suspension was rapidly aspirated into a 3 mL syringe through a 22 gauge needle, expelled rapidly into the tube, re-aspirated and re-expelled quickly back into the tube. The lysate was centrifuged at 10,000 rpm for 10 min at 4 °C. An aliquot of the clear cytoplasm was taken, frozen in liquid nitrogen, and stored at −80 °C until chemical extraction.
3.2.1.1.2. Chemical extraction of methoxyconidiol and HPLC analysis
Cytoplasmic extracts of treated and control eggs were extracted with CH3OH and CH2Cl2 and analyzed by HPLC. Small columns of reverse (C18) phase were prepared and conditioned with methanol, methanol:water 1:1 (v:v) and water. Cytoplasmic extracts were eluted from the column with water to eliminate polar compounds and salts. Methoxyconidiol was eluted with methanol and further concentrated. This fraction was analyzed in HPLC (column: Phenomenex synergy 4μ max RP; elution with water and methanol under gradient, starting from 30% to 0% of water during 15 minutes; flow: 0.3 mL/min; detection photodiode array, UV at 285 nm).
3.2.1.2. Cell culture and cytotoxicity assay
Human primary fibroblasts were purchased from Biopredic International (Rennes, France). Immortalized murine L929 cells and human MCF7 breast adenocarcinoma, PA1 ovary teratocarcinoma and PC3 androgen-resistant prostate carcinoma cells were obtained from the European Collection of Cell Cultures (ECACC, Salibury, UK). All were cultured at 37 °C under 5% CO2 and maintained in Eagle’s minimum essential medium (Gibco-BRL, Paisley, Scotland) supplemented with 10% fetal calf serum, vitamins and antibiotics (Gibco™, Cergy-Pontoise, France) as described previously [21].
The cytotoxicity of each treatment was determined after a 48 h exposure to each chemical (6 concentrations in triplicate, each in DMSO 0.5%) by means of the resazurin reduction test (RRT) that measures the amount of fluorescent resorufin produced by living cells. Assays were carried out at 530/590 nm accordingly to the procedure described by Barthomeuf et al. [21]. IC50 values (mean ± S.D.) were calculated with the ALLFIT program and were expressed in μM.
4. Conclusions
Previous promising results [12] led us to synthesize six meroterpenes in order to evaluate their biological activities on two bacterial strains, on sea urchin eggs and on cancer cell lines. A first concern was to investigate the effect of a replacement of the phenolic hydroxyl by a methoxy group. In the present study, we conclude that this chemical modification reduced both the antibacterial and the antiproliferative activity of meroterpenes on sea urchin eggs and on normal, immortalized and cancer cell lines, highlighting the crucial role of the phenolic group at the C-4 position for the toxicity of meroterpenes. In contrast to previous findings on sea urchin eggs, methoxyconidiol was almost completely inactive on human serum-stimulated cells. We demonstrated that activity on sea urchin eggs was due to methoxyconidiol and not due to one of its degradation products. Furthermore, epiconicol, the most active compound on human cells, exhibited only medium toxicity on serumstimulated carcinoma cells. However, our data found evidence for a relative selectivity of this compound on serum-stimulated human MCF7 breast and PA1 ovary carcinoma cells.
Acknowledgements
We are grateful to Eric Debiton for his help in cells cultures. Financial support for this research (PhD Fellowship, Annabel SIMON-LEVERT and grant in help for scientific research) was provided by La Ligue Contre le Cancer (Comité des Pyrénées Orientales). This work is part of the ECIMAR (ANR-06-BDIV-001-04) project granted by the Agence Nationale de la Recherche (ANR).
References and Notes
- 1.Fadli M, Aracil JM, Jeanty G, Banaigs B, Francisco C. Novel meroterpenoids from Cystoseira mediterranea: Use of the crown-gall bioassay as a primary screen for lipophilic antineoplastic agents. J Nat Prod. 1991;54:261–264. doi: 10.1021/np50073a029. [DOI] [PubMed] [Google Scholar]
- 2.Cueto M, MacMillan JB, Jensen PR, Fenical W. Tropolactones A–D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry. 2006;67:1826–1831. doi: 10.1016/j.phytochem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Sheu JH, Su JH, Sung PJ, Wang GH, Dai CF. Novel meroditerpenoid-related metabolites from the formosan soft coral Nephthea chabrolii. J Nat Prod. 2004;67:2048–2052. doi: 10.1021/np0401314. [DOI] [PubMed] [Google Scholar]
- 4.Mitome H, Nagasawa T, Miyaoka H, Yamada Y, Van Soest RWM. Dactyloquinones C, D and E novel sesquiterpenoid quinones, from the Okinawan marine sponge Dactylospongia elegans. Tetrahedron Lett. 2002;58:1693–1996. [Google Scholar]
- 5.Garrido L, Zubia E, Ortega MJ, Salva J. New meroterpenoids from the ascidian Aplidium conicum. J Nat Prod. 2002;65:1328–1331. doi: 10.1021/np020176+. [DOI] [PubMed] [Google Scholar]
- 6.Shen YC, Chen CY, Kuo YH. New sesquiterpene hydroquinones from a taiwanese marine sponge Hippospongia metachromia. J Nat Prod. 2001;64:801–803. doi: 10.1021/np000610c. [DOI] [PubMed] [Google Scholar]
- 7.Aiello A, Fattorusso E, Luciano P, Menna M, Esposito G, Iuvone T, Pala D. Conicaquinones A and B, two novel cytotoxic terpene quinones from the Mediterranean ascidian Aplidium conicum. Eur J Org Chem. 2003;5:898–900. [Google Scholar]
- 8.de Menezes JE, Machado FEA, Lemos TLG, Silveira ER, Braz Filho R, Pessoa ODL. Sesquiterpenes and a Phenylpropanoid from Cordia trichotoma. Zeis Natur. 2004;59:19–22. doi: 10.1515/znc-2004-1-204. [DOI] [PubMed] [Google Scholar]
- 9.Marion F, Williams DE, Patrick BO, Hollander I, Mallon R, Kim SC, Roll DM, Feldberg L, Van Soest R, Andersen R. Liphagal, a selective inhibitor of PI3 kinase α isolated from the sponge Aka coralliphaga: Structure elucidation and biomimetic synthesis. J Org Lett. 2006;8:321–324. doi: 10.1021/ol052744t. [DOI] [PubMed] [Google Scholar]
- 10.Williams DE, Telliez JB, Liu J, Tahir A, van Soest R, Andersen RJ. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the Indonesian marine sponge Acanthodendrilla sp. J Nat Prod. 2004;67:2127–2129. doi: 10.1021/np049808d. [DOI] [PubMed] [Google Scholar]
- 11.Simon-Levert A, Arrault A, Bontemps-Subielos N, Canal C, Banaigs B. Meroterpenes from the ascidian Aplidium aff densum. J Nat Prod. 2005;68:1412–1415. doi: 10.1021/np050110p. [DOI] [PubMed] [Google Scholar]
- 12.Simon-Levert A, Aze A, Bontemps-Subielos N, Banaigs B, Genevière AM. Antimitotic activity of methoxyconidiol, a meroterpene isolated from an ascidian. Chem Biol Inter. 2007;168:106–116. doi: 10.1016/j.cbi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Almanza R, Pérez-Flores F, Lemini C. Synthesis of 2H-1-benzopyrans from aryllithium and α,β-unsaturated aldehydes. Heterocycles. 1994;37:759–774. [Google Scholar]
- 14.Benslimane AF, Pouchus YF, Le Boterf J, Verbist JF. Substances cytotoxiques et antibacteriennes de l’ascidie Aplidium antillense. J Nat Prod. 1988;51:582–583. doi: 10.1021/np50057a022. [DOI] [PubMed] [Google Scholar]
- 15.Benslimane AF, Pouchus YF, Verbist JF, Petit JY, Brion JD, Welin L. Antiinflammatory mechanism of cordiachromene A action. J Clin Pharmacol. 1995;35:298–301. doi: 10.1002/j.1552-4604.1995.tb04063.x. [DOI] [PubMed] [Google Scholar]
- 16.National Commitee for Clinical Laboratory Standards. NCCLS Document M7-A4. 4th ed. Vilanova, PA, USA: 1997. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobicaly Approved Standards. [Google Scholar]
- 17.Caroll AR, Bowden BF, Coll JC. Studies of Australian Ascidians III. A new THC derivatice from the ascidian Synocium castellatum. Aust J Chem. 1993;46:1079–1083. [Google Scholar]
- 18.Collas P, Poccia D. Methods for studying in vitro assembly of male pronuclei using oocyte extracts from marine invertebrates: Sea urchins and surf clams. Methods Cell Biol. 1998;53:417–452. doi: 10.1016/s0091-679x(08)60889-4. [DOI] [PubMed] [Google Scholar]
- 19.Semenova MN, Kiselyov A, Semenov VV. Sea urchin embryo as a model organism for the rapid functional screening of tubulin modulators. BioTechnniques. 2006;40:765–774. doi: 10.2144/000112193. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RS. Species-specific differences in toxicity of antimitotic agents toward cultured mammalian cells. J Natl Cancer Inst. 1985;74:159–164. [PubMed] [Google Scholar]
- 21.Barthomeuf C, Debiton E, Mshvildadze V, Kemertelidze E, Balansard G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002;68:672–675. doi: 10.1055/s-2002-33807. [DOI] [PubMed] [Google Scholar]