Abstract
Actinomycetes are prolific producers of pharmacologically important compounds accounting for about 70% of the naturally derived antibiotics that are currently in clinical use. In this study, we report on the isolation of Streptomyces sp. strains from Mediterranean sponges, on their secondary metabolite production and on their screening for anti-infective activities. Bioassay-guided isolation and purification yielded three previously known compounds namely, cyclic depsipeptide valinomycin, indolocarbazole alkaloid staurosporine and butenolide. This is the first report of the isolation of valinomycin from a marine source. These compounds exhibited novel anti-parasitic activities specifically against Leishmania major (valinomycin IC50 < 0.11 μM; staurosporine IC50 5.30 μM) and Trypanosoma brucei brucei (valinomycin IC50 0.0032 μM; staurosporine IC50 0.022 μM; butenolide IC50 31.77 μM). These results underscore the potential of marine actinomycetes to produce bioactive compounds as well as the re-evaluation of previously known compounds for novel anti-infective activities.
Keywords: marine sponges, Streptomyces, valinomycin, staurosporine, butenolide, anti-parasitic
1. Introduction
The class Actinobacteria, specifically bacteria belonging to the order Actinomycetales, are common soil inhabitants that have the unprecedented ability to produce a wide range of secondary metabolites. Among the more than 140 described actinomycete genera, only a few are responsible for the majority of over 20,000 microbial natural products identified so far. In particular, the genus Streptomyces accounts for about 80% of the actinomycete natural products reported to date [1,2]. Given the unparalleled potential of actinomycetes and specifically streptomycetes in this regard, significant effort has been directed towards the isolation of these bacteria from various sources for drug screening programs. The majority of the actinomycetes were previously isolated from terrestrial soils and from marine sediments [3–5] and quite recently also from marine sponges [6–10] and cone snails [11]. The discovery of numerous marine actinomycete taxa and their bioactive secondary metabolites dispel the notion that actinomycetes are merely dormant spores that have been washed off from the shores [1,3,12,13].
The major goal of our research is to discover novel anti-infective agents such as those against the parasites Leishmania major and Trypanosoma brucei that cause leishmaniasis and African sleeping sickness, respectively. These parasites currently affect around 12 million people living in tropical and subtropical areas [14]. The alarming death rate caused by these parasites and the emergence of antibiotic resistance underline the need for new and effective drugs. Our research program focuses on the discovery of anti-infective agents from marine sponges and their associated microorganisms. In the course of our study, we have taxonomically described two new actinomycete species [15,16] isolated from marine sponges as well as novel compounds [17]. During our screening efforts for bioactive natural products from marine sponge-associated actinomycetes, we have encountered some previously known compounds but with yet unprecedented biological activities. We report here the isolation and characterization of these compounds from actinomycetes associated with Mediterranean sponges with novel anti-parasitic activities.
2. Results and Discussion
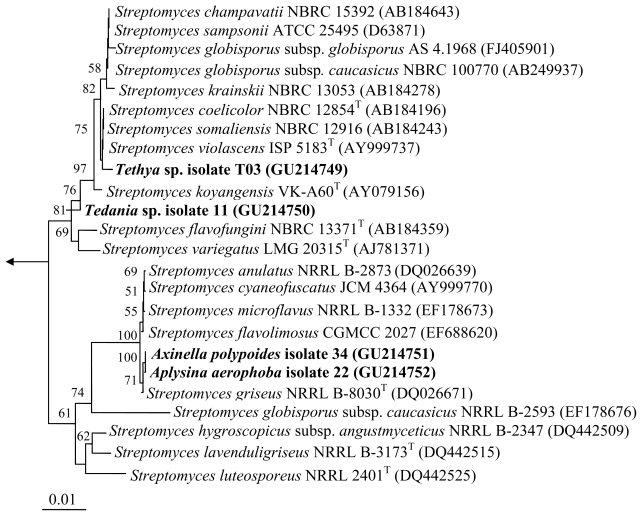
Actinomycetes associated with the following sponges: Aplysina aerophoba, Axinella polypoides, Tedania sp. and Tethya sp. collected by SCUBA diving offshore Rovinj, Croatia (45°05′N, 13°38′E) in May 2006 were cultivated as described previously [15,16,18]. 16S rRNA gene sequencing revealed the affiliation of four strains, namely isolate 11 (GU214750), isolate 34 (GU214751), isolate 22 (GU214752) and isolate TO3 (GU214749) to the genus Streptomyces (Figure 1). They exhibited 99.7–99.9% sequence similarities to validly described species of the genus Streptomyces.
Figure 1.
Neighbor-joining tree of the strains and representative species of the genus Streptomyces based on nearly complete 16S rRNA gene sequences. Numbers at the nodes indicate the levels of bootstrap support based on 1000 resampled data sets. Only values greater than 50% are shown. The arrow points to the outgroup consisting of six species belonging to Enterobacteriaceae and Pasteurellaceae. The scale bar indicates 0.01 substitution per nucleotide position.
The strains 22 and 34 collected from two different sponge species, Axinella polypoides and Aplysina aerophoba, respectively exhibited 99.9% 16S rRNA gene sequence similarities, with only one nucleotide difference. This suggests that these isolates are most probably the same strain and their isolation from different hosts indicates that these bacteria could be transient organisms coming from the surrounding seawater that were merely present within the sponge during collection.
The strains 11, 22, 34 and T03 were each grown on 100 M1 [4] agar plates and incubated at 30 °C for seven days. Mycelial mass together with the agar were cut into small pieces and macerated overnight with 200 mL of ethyl acetate. The resulting solution was filtered using Whatman filter paper and the same maceration step with ethyl acetate was repeated. Both filtrates were combined and subsequently dried by rotary evaporation. The crude extracts were subjected to pre-fractionation with Diaion HP-20ss resin (Mitsubishi Chemical Corporation, Japan) using a gradient of water/isopropanol (100%, 75%:25%, 50%:50%, 25%:75%) followed by 100% MeOH. The fractions were subsequently purified by RP-HPLC (Agilent 1100, Agilent Technologies, USA). High resolution ESIMS analyses were performed on a Micromass Q-Tof micro mass spectrometer. NMR spectra were obtained on Varian INOVA 500 (1H 500 MHz, 13C 125 MHz) and Varian INOVA 600 (1H 600 MHz, 13C 150 MHz) NMR spectrometers with a 3 mm Nalorac MDBG probe and a 5 mm cold probe, respectively.
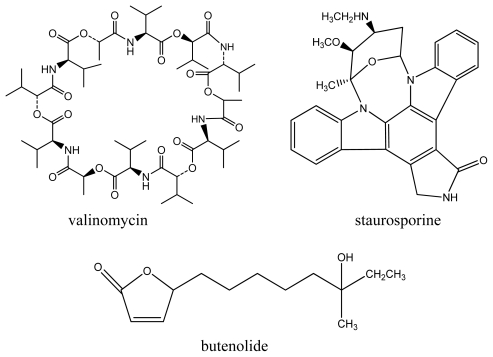
High-resolution mass spectrometry of the purified compound both from Streptomyces sp. strains 22 and 34 established a molecular formula of C54H90N6NaO18 (m/z 1133.6385 for [M + Na]+, calculated 1133.6394) [19]. A combination of NMR and MS-MS fragmentation suggested the presence of one α-hydroxyisovaleryl unit (Hiv), a lactoyl group (Lac), and two valines (Val), thus confirming the identity of the compound as valinomycin (Figure 2) (see Supplementary Information for NMR data). The isolation of the same compound from these strains is not surprising since both exhibited very high 16S rRNA gene sequence similarities. This cyclodepsipeptide has been recovered from various soil-derived actinomycetes, Streptomyces fulvissimus, Streptomyces roseochromogenes and Streptomyces griseus var. flexipartum [20]. To date, this is the first report of valinomycin isolated from a marine organism. This cyclic depsipeptide consists of polar groups oriented toward the central cavity, whereas the rest of the molecule is relatively nonpolar thus behaving like an ionophore that modulates transport of ions such as potassium across biological membranes. In this study, valinomycin exhibited significant inhibitory activities against the parasites Leishmania major (IC50 < 0.11 μM) and Trypanosoma brucei brucei (IC50 0.0032 μM) [21,22]. Previous studies have shown other biological activities of valinomycin in insecticidal, nematocidal and antifungal assays [23].
Figure 2.
Compounds isolated from Streptomyces sp. strains.
The compound staurosporine (Figure 2) was isolated from Streptomyces sp. strain 11 with a molecular formula of C11H18N2NaO2 (m/z 233.1262 for [M + Na]+, calculated 233.1266) [24]. The structure was confirmed by comparison of NMR analysis (see Supplementary Information) with published spectral data of the compound [25]. This indolocarbazole alkaloid was previously isolated from various terrestrial Streptomyces sp. strains. Interestingly, staurosporine and its derivatives have also been isolated from the marine ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. [26]. Furthermore, staurosporine and its derivatives have aroused considerable interest as these compounds exhibit strong inhibitory activities against protein kinase C as well as inhibition of platelet aggregation, blocking of growth phases in cancer cells and reversal of multidrug resistance [27]. In this study, stauroporine exhibited significant anti-parasitic activity against Leishmania major (IC50 5.30 μM) and Trypanosoma brucei brucei (IC50 0.022 μM) which has not been previously reported in literature.
The third compound, butenolide (Figure 2), was isolated from Streptomyces sp. strain T03 with a molecular formula of C13H22O3Na (m/z 249.1447 for [M + Na]+, calculated 249.1467) exhibiting identical spectral data with published literature [28] (see Supplementary Information). This lactone-containing metabolite has also been previously isolated from a marine sediment-derived Streptomyces sp. strain M027750 [28]. Butenolides are a family of α,β-unsaturated lactones commonly produced by fungi, bacteria and gorgonians (colonial soft corals). Their saturated analogs act as signaling substances in bacteria, enhance spore formation of Streptomyces sp. as well as induce metabolite formation [29]. In this study, butenolide was found to exhibit anti-Trypanosoma activity (IC50 0.022 μM).
The compounds valinomycin and staurosporine were found to exhibit general cytotoxicity against 293T kidney epithelial cells (valinomycin IC50 11.24 μM; staurosporine IC50 1.30 μM) and J774.1 macrophages (valinomycin IC50 < 0.10 μM; staurosporine IC50 < 0.13 μM) while butenolide was not found to exhibit cytotoxicity against these cell lines [30]. Nevertheless, these compounds have been shown to exhibit significant anti-parasitic activities (Table 1) which has not been previously reported. Structure modification of these compounds with the aim to decrease cytotoxicity is therefore a worthwhile endeavour. These results highlight the potential of actinomycetes associated with marine sponges to produce bioactive compounds. Furthermore, the re-isolation of previously known compounds is still considered a worthwhile pursuit particularly for finding new pharmacological uses such as anti-infectives. The emergence of antibiotic resistance and the alarming death rate caused by infectious diseases necessitates the need for re-evaluating the current multitude of compounds that have been discovered over the past years.
Table 1.
Anti-parasitic activities of the compounds (IC50, μM).
| Compound | Leishmania major | Trypanosoma brucei brucei (48 h) | Trypanosoma brucei brucei (72 h) |
|---|---|---|---|
| valinomycin | <0.11 | 0.0032 | 0.0036 |
| staurosporine | 5.30 | 0.022 | 0.035 |
| butenolide | >100 | 31.77 | 33.08 |
Acknowledgments
We gratefully acknowledge A. Nätscher for technical assistance, J. Kamke for help with phylogenetic tree construction, H. Bruhn, T. Ölschläger, A. Stich and co-workers of the SFB 630 TP Z1 for the anti-infective screening assays (University of Würzburg), R. Jadulco-Koch and M.K Harper for fruitful discussions (University of Utah). Financial support was provided by the Deutsche Forschungsgemeinschaft SFB 630 TP A5 to U. Hentschel and NIH Grant CA36622 to C.M. Ireland.
Footnotes
Samples Availability: Available from the authors.
References and Notes
- 1.Jensen PR, Mincer TJ, Williams PG, Fenical W. Marine actinomycete diversity and natural product discovery. Anton Leeuwenhoek. 2005;87:43–48. doi: 10.1007/s10482-004-6540-1. [DOI] [PubMed] [Google Scholar]
- 2.Bull AT, Stach JE. Marine actinobacteria: New opportunities for natural product search and discovery. Trends Microbiol. 2007;15:491–499. doi: 10.1016/j.tim.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Fenical W, Jensen PR. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 4.Mincer TJ, Jensen PR, Kauffman CA, Fenical W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol. 2002;68:5005–5011. doi: 10.1128/AEM.68.10.5005-5011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldonado LA, Stach JE, Pathom-aree W, Ward AC, Bull AT, Goodfellow M. Diversity of culturable actinobacteria in geographically widespread marine sediments. Anton Leeuwenhoek. 2005;87:11–18. doi: 10.1007/s10482-004-6525-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim TK, Garson MJ, Fuerst JA. Marine actinomycetes related to the “Salinospora” group from the Great Barrier Reef sponge Pseudoceratina clavata. Environ Microbiol. 2005;7:509–518. doi: 10.1111/j.1462-2920.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 7.Webster NS, Wilson KJ, Blackall LL, Hill RT. Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl Environ Microbiol. 2001;67:434–444. doi: 10.1128/AEM.67.1.434-444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montalvo NF, Mohamed NM, Enticknap JJ, Hill RT. Novel actinobacteria from marine sponges. Anton Leeuwenhoek. 2005;87:29–36. doi: 10.1007/s10482-004-6536-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Lee YK, Zhang W, Lee HK. Culturable actinobacteria from the marine sponge Hymeniacidon perleve: Isolation and phylogenetic diversity by 16S rRNA gene-RFLP analysis. Anton Leeuwenhoek. 2006;90:159–169. doi: 10.1007/s10482-006-9070-1. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S, Sun W, Chen M, Dai S, Zhang L, Liu Y, Lee KJ, Li X. Diversity of culturable actinobacteria isolated from marine sponge Haliclona sp. Anton Leeuwenhoek. 2007;92:405–416. doi: 10.1007/s10482-007-9169-z. [DOI] [PubMed] [Google Scholar]
- 11.Peraud O, Biggs JS, Hughen RW, Light AR, Concepcion GP, Olivera BM, Schmidt EW. Microhabitats within venomous cone snails contain diverse actinobacteria. Appl Environ Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam KS. Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol. 2006;9:245–251. doi: 10.1016/j.mib.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Fenical W. Marine pharmaceuticals: past, present and future. Oceanography. 2006;19:110–119. [Google Scholar]
- 14.Natera S, Machuca C, Padron-Nieves M, Romero A, Diaz E, Ponte-Sucre A. Leishmania spp.: Proficiency of drug-resistant parasites. Int J Antimicrob Agents. 2007;29:637–642. doi: 10.1016/j.ijantimicag.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Pimentel-Elardo SM, Scheuermayer M, Kozytska S, Hentschel U. Streptomyces axinellae sp. nov., isolated from the Mediterranean sponge Axinella polypoides (Porifera) Int J Syst Evol Microbiol. 2009;59:1433–1437. doi: 10.1099/ijs.0.007856-0. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel-Elardo SM, Tiro LP, Grozdanov L, Hentschel U. Saccharopolyspora cebuensis sp. nov., a novel actinomycete isolated from a Philippine sponge (Porifera) Int J Syst Evol Microbiol. 2008;58:628–632. doi: 10.1099/ijs.0.64971-0. [DOI] [PubMed] [Google Scholar]
- 17.Pimentel-Elardo S, Gulder TAM, Hentschel U, Bringmann G. Cebulactams A1 and A2, new macrolactams isolated from Saccharopolyspora cebuensis, the first obligate-marine strain of the genus Saccharopolyspora. Tetrahedron Lett. 2008;49:6889–6892. [Google Scholar]
- 18.Pimentel-Elardo S, Wehrl M, Friedrich A, Jensen PR, Hentschel U. Isolation of planctomycetes from Aplysina sponges. Aquat Microb Ecol. 2003;33:239–245. [Google Scholar]
- 19.The isolation of valinomycin was carried out by semi-preparative HPLC (Phenomenex Luna SemiPrep RP18e 10 × 250 mm) using H2O (A) and CH3OH (B) as the solvents and the following gradient: flow 4.5 mL/min; 0–5 min 90% B, 11–15 min 100% B yielding 8.4 mg of the compound (Rt = 13.085 min).
- 20.Brockmann H, Schmidt-Kastner G. Valinomycin I, XXVII. Mitteilung über Antibiotika aus Actinomyceten. Chem Ber. 1955;88:57–61. [Google Scholar]
- 21.Leishmania major promastigotes were seeded at a cell density of 1 × 107 cells/mL into 96-well plates in complete medium (RPMI with NaHCO3, 10% FCS, 2 mM glutamine, 10 mM Hepes pH 7.2, 100 U/mL penicillin, 50 μg/mL gentamicin, 50 mM 2-mercaptoethanol) without phenol red (200 mL), in the absence or presence of different concentrations of the compounds. These were then incubated for 24 h at 26 °C, 5% CO2 and 95% humidity. Following the addition of 20 mL of Alamar Blue, the plates were incubated again and the optical densities (ODs) measured 24 h and 48 h later with an enzyme-linked immunosorbent assay (ELISA) reader (Multiskan Ascent, Germany) using a test wavelength of 540 nm and a reference wavelength of 630 nm. Absorbance in the absence of compounds was set as 100% of growth. Amphotericin B was used as a reference compound and positive control. The effects of cell density, incubation time and the concentration of DMSO were examined in control experiments. The final concentration of DMSO in the medium never exceeded 1% vol/vol and had no effect on the proliferation of extracellular or intracellular parasites. For each experiment, each drug concentration was assayed in duplicate wells [31]
- 22.Trypomastigote forms of Trypanosoma brucei brucei laboratory strain TC 221 were cultured in complete Baltz medium [80 mL Baltz medium basic solution, 0.8 mL 2 mercaptoethanol stock solution (20 mM), 0.8 mL penicillin/streptomycin (10,000 U/mL), 16 mL FCS (inactivated for 30 min at 56 °C). Baltz medium basic solution is composed of the following: 500 mL MEM with Earle’s salts and L-glutamine, 3 g Hepes, 0.5 g monohydrate glucose, 0.110 g sodium pyruvate, 0.007 g hypoxanthine, 0.002 g thymidine, 0.0107 g adenosine, 0.0141 g bathocuproine disulfonic acid disodium salt, 0.146 g glutamine, 5 mL sterile non-essential amino acid concentrate (100×, pH 7.5). A defined number of parasites (104 trypanosomes per mL) were exposed in test chambers of 96-well plates to various concentrations of the test substances (previously dissolved in DMSO) to make a final volume of 200 μL in duplicates. Positive (trypanosomes in culture medium) and negative controls (test substance without trypanosomes) were run simultaneously with each plate. The plates were then incubated at 37 °C in an atmosphere of 5% CO2 for a total time period of 72 h. After 24 h, 20 μL of Alamar Blue was added. The activity of the test substances was measured by light absorption using MR 700 Microplate Reader at a wavelength of 550 nm with a reference wavelength of 630 nm. The first reading was done at 48 h and subsequently at 72 h. The effect of the test substances was quantified in IC50 values by linear interpolation of three independent measurements [32].
- 23.Heisey R, Huang J, Mishka SK, Keller JE, Miller JR, Putnam AR, D’Silva TD. Production of valinomycin, an insecticidal antibiotic, by Streptomyces griseus var. flexipartum var. nov. J Agric Food Chem. 1988;36:1283–1286. [Google Scholar]
- 24.The isolation of staurosporine was carried out by semi-preparative HPLC (Phenomenex Luna SemiPrep RP18e 10 × 250 mm) using H2O + 0.1% TFA (A) and CH3OH (B) as the solvents and the following gradient: flow 4.5 mL/min; 0–5 min 70% B, 10 min 80% B, 20–25 min 100% B to yield 1.4 mg of the compound (Rt = 4.162 min).
- 25.Fdhila F, Vazquez V, Sanchez JL, Riguera R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J Nat Prod. 2003;66:1299–1301. doi: 10.1021/np030233e. [DOI] [PubMed] [Google Scholar]
- 26.Schupp P, Proksch P, Wray V. Further new staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J Nat Prod. 2002;65:295–298. doi: 10.1021/np010259a. [DOI] [PubMed] [Google Scholar]
- 27.Utz I, Spitaler M, Rybczynska M, Ludescher C, Hilbe W, Regenass U, Grunicke H, Hofmann J. Reversal of multidrug resistance by the staurosporine derivatives CGP 41251 and CGP 42700. Int J Cancer. 1998;77:64–69. doi: 10.1002/(sici)1097-0215(19980703)77:1<64::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Cho KW, Lee HS, Rho JR, Kim TS, Mo SJ, Shin J. New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod. 2001;64:664–667. doi: 10.1021/np000599g. [DOI] [PubMed] [Google Scholar]
- 29.Mukku VJ, Speitling M, Laatsch H, Helmke E. New butenolides from two marine streptomycetes. J Nat Prod. 2000;63:1570–1572. doi: 10.1021/np0001676. [DOI] [PubMed] [Google Scholar]
- 30.J774.1 macrophages were cultured in complete medium (RPMI with NaHCO3, 10% FCS, 2 mM glutamine, 10 mM Hepes pH 7.2, 100 U/mL penicillin, 50 μg/mL gentamicin, 50 μM 2-mercaptoethanol) without phenol red in the absence or presence of increasing concentrations of the compounds at a cell density of 1 × 105 cells/mL (200 μL) for 24 h at 37 °C, 5% CO2 and 95% humidity. Following the addition of 20 μL of Alamar Blue, the plates were incubated and the ODs measured at 24 h, 48 h and 72 h. The same Alamar blue assay previously described for Leishmania was followed [21]. Kidney epithelial 293T cells were also tested in the same manner as the macrophages but using complete DMEM medium (4.5 g/L solution of DMEM high glucose solution with sodium pyruvate but without L-glutamine, FBS superior at final concentration of 20%, 200 mM L-glutamine 100x) and cell density (2 × 104 cells/mL).
- 31.Ponte-Sucre A, Vicik R, Schultheis M, Schirmeister T, Moll H. Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob Agents Chemother. 2006;50:2439–2447. doi: 10.1128/AAC.01430-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber W, Koella JC. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706x(93)90083-n. [DOI] [PubMed] [Google Scholar]