Abstract
Retinal ischemia can be effectively modeled by permanent bilateral common carotid artery occlusion, which leads to chronic hypoperfusion-induced degeneration in the entire rat retina. The complex pathways leading to retinal cell death offer a complex approach of neuroprotective strategies. In the present review we summarize recent findings with different neuroprotective candidate molecules. We describe the protective effects of intravitreal treatment with: (i) urocortin 2; (ii) a mitochondrial ATP-sensitive K+ channel opener, diazoxide; (iii) a neurotrophic factor, pituitary adenylate cyclase activating polypeptide; and (iv) a novel poly(ADP-ribose) polymerase inhibitor (HO3089). The retinoprotective effects are demonstrated with morphological description and effects on apoptotic pathways using molecular biological techniques.
Keywords: BCCAO, ischemia, retinoprotection, urocortin 2, diazoxide, PACAP, PARP-inhibitor, rat retina
1. Retinal Ischemia
The retina is supplied by two arterial systems: the chorocapillary layer supplies the outer retina, while the central retinal artery supplies the inner retina. The rich capillary networks provide an excellent blood supply suiting the high energy demand of the retinal light processing events. When the retinal circulation does not meet the requirements of the retina, the retina suffers an ischemic damage, present in numerous human conditions and leading to various degrees of visual impairment [1–6]. The pathways leading to ischemic retinal damage and the potential retinoprotective strategies have been reviewed in several excellent papers [1,7–10]. In the present review we focus on four recently proven retinoprotective agents in one type of animal model of retinal ischemia: in bilateral common carotid artery occlusion (BCCAO).
The main factors involved in ischemia-induced retinal degeneration are thought to be the excitatory neurotransmitter release, glial dysfunction, Ca2+ overload, formation of free radicals, elevation of nitric oxide and release of potentially toxic mediators by activated inflammatory cells such as tumor necrosis factor and interleukin-1 [1]. This complex cascade of events finally leads to degeneration of certain cell populations or the entire retina depending on the strength and duration of the ischemic event.
There are numerous animal models of retinal ischemia, including high intraocular pressure, ligation of the ophthalmic vessels and BCCAO with or without the occlusion of the vertebral arteries [1,11]. BCCAO leads to moderate reduction in the cerebral blood flow in rats leading to subtle changes in biochemical and behavioral measures. It has been shown that BCCAO causes long-lasting white matter lesion, neuronal degeneration, microglial activation, astrocytosis, behavioral deficits and changes in several biochemical parameters [12]. In the retina, the effects of chronic BCCAO depend on the rat strain and technique used. It produces varying degrees of retinal degeneration from subtle changes to severe degeneration, paralleling the retinopathy of carotid artery occlusive disease in humans [13,14]. Numerous electroretinographical, immunohistochemical, histological and functional studies show that BCCAO leads to varying degrees of ischemic damage of the retina [7,15,17–20].
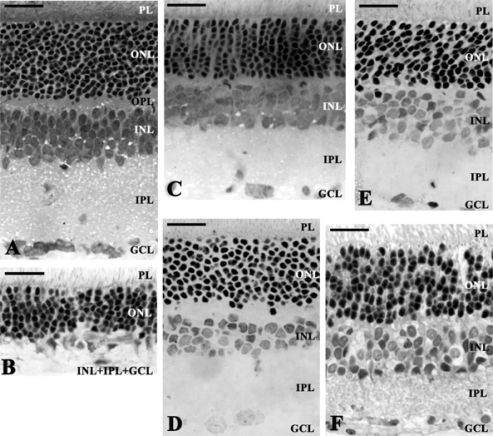
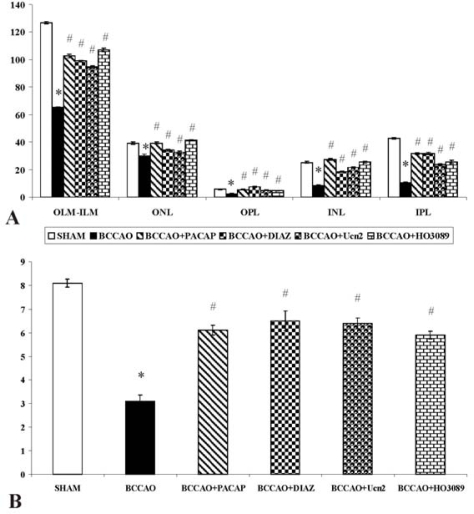
Previously, we have found that permanent BCCAO leads to a severe retinal damage, with all retinal layers bearing the marks of deterioration [20,21]. The most marked reduction in thickness can be observed in the plexiform layers, and as a consequence, the distance between outer limiting membrane and inner limiting membrane is significantly less than in control preparations (Figures 1A, 1B and 2). The photoreceptor layer also suffers degeneration: the outer segments become shorter and the geometric arrangement is disturbed. Numerous photoreceptors and possibly the second- and third-order neurons belonging to the same retinal circuitry have been found to be damaged. This assumption is further justified by our observation of degenerating bipolar cell terminals in the inner plexiform layer.
Figure 1.
Microphotographs of different retinal sections. (A) Histological sections of control animals; (B) effects of bilateral common carotid artery occlusion (BCCAO). Extreme swelling of neuronal cell bodies and the fusion of the INL, IPL and GCL layers were observed. BCCAO caused severe overall retinal degeneration ameliorated by intravitreal injection of (C) urocortin 2, (D) diazoxide, (E) pituitary adenylate cyclase activating polypeptide and (F) the poly(ADP-ribose) polymerase inhibitor HO3089. Scale bar: 20 μm. Abbreviations: PL: photoreceptor layer; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer.
Figure 2.
Quantification of the whole retina thickness (OLM-ILM), distinct retinal layers (A) and the cell number of GCL/100 μm in different conditions (B) by morphometrical analysis. Significant decreases were observed in BCCAO-induced retinal degeneration. The neuroprotective effects of urocortin 2; diazoxide; pituitary adenylate cyclase activating polypeptide and HO3089 treatments were quantified by the thickness of different retinal layers and also the cell number of GCL/100 μm. *p < 0.05 between untreated and BCCAO; #p < 0.05 BCCAO vs. BCCAO+different treatments. Results are presented as mean ± S.E.M. Statistical comparisons were made using the ANOVA test followed by Tukey-B`s post hoc analysis. Abbreviations: OLM: outer limiting membrane; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer; ILM: inner limiting membrane.
2. Potential Protective Strategies
Given the complexity of events leading to retinal cell death, a variety of pharmacological approaches has been shown to be protective in retinal ischemia. As glutamate-mediated excitotoxicity is one of the main factors in retinal ischemia, decreasing excitotoxicity is an important therapeutic approach [1]. Other strategies involve reducing the detrimental effects of free radicals and increased Ca2+ levels, counteracting mitochondrial failure, anti-inflammatory strategies and potentiating endogenous protective mechanisms [1,22]. Table 1 gives a brief overview of the protective strategies proven in animal models of retinal ischemia. In the following sections, we describe four novel neuroprotective strategies recently found to reduce ischemic retinal damage in the rat.
Table 1.
Brief overview of potential retinoprotective strategies proven in animal models of retinal ischemia.
| Substance | Main effects | References |
|---|---|---|
| Antioxidants (e.g., Vitamin E, lutein, flavonoids) | ↓ oxidative damage ↓ caspase3 ↑ glutathione ↓ nitrotyrosine ↓ nuclear PAR ↓ loss of ATP |
[23] [24] [25] |
| Ischemic preconditioning | protein kinase C activation ATP-sensitive K+ channel opening ↑ ferritin level adenosine A1 receptor stimulation STAT-3 activation ↑ HSP27 |
[26] [27] [28] [29] [30] |
| Ischemic postconditioning | ↓ glutamate | [31] |
| Adenosine | vasodilation ↓ neuronal activity ↑ glycogenolysis |
[32] |
| Growth factors (IGFII, NGF, BDNF, VEGF) | ↑ phosphate activated glutaminase (PAG) ↓ ammonia ↑ blood flow to the retina ↑ Bcl-2 ↓ Bax |
[33] [34] [35] |
| Erythropoietin | ↓ apoptosis ↑ ischemic preconditioning |
[36] [37] [38] |
| Statins | ↓ HSP27 | [39] |
| Estradiol | ↓ glutamate | [40] |
| Cannabinoids | ↓ peroxynitrite | [41] |
| Morphine | ↑ ischemic preconditioning | [42,43] |
| L-carnitine | ↓ oxidative stress | [44] |
| Glutamate receptor antagonists | ↓ glutamate excitotoxicity | [45] |
| Adrenergic receptor blockers | ↓ influx of sodium and calcium | [46] |
| Alpha2 adrenergic agonist (brimonidine) | ↓ glutamate and aspartate | [47] |
| Ca2+, K+, Na+ channel blockers | ↓ influx of sodium and calcium ↑ ischemic preconditioning ↓ c-jun, p-JNK |
[48,49] |
| Hypothermia | ↓ energy demand | [50] |
| Hyperglycaemia | ↑ HSP-27 | [51] |
3. Urocortin 2
Urocortins (Ucn 1, 2 and 3) are paralogs of corticotropin-releasing factor (CRF) [52]. In terms of primary structure, Ucn 2 most resembles Ucn 3, with both peptides acting as a preferential or selective CRF2 agonist, leading to their designation as the selective CRF2 agonists, Ucn 2 and Ucn 3 [53]. Ucns have been proposed to participate in many physiological functions, including anxiety, learning and memory, osmoregulation, thermoregulation, feeding, reproductive and cardiovascular functions [52,54–58]. Ucns confer protection when added to post-ischemic/hypoxic cardiomyocytes or to isolated intact heart during reperfusion after regional ischemia, preventing necrotic and apoptotic cell death and reducing infarct size, respectively [52,59–62]. Ucn 1 and 2 are known to exert cardioprotective effects against ischemic/hypoxic insults via a CRF2 dependent mechanism [52]. The neuroprotective activity of Ucn 1 is mediated by CRF1 receptors via cAMP-dependent pathways [63]. Less is known about the neuroprotective functions of Ucns. Ucn 1 has been shown to protect hippocampal neurons against excitotoxic and oxidative injuries [64]. mRNA transcripts for both CRF receptor subtypes, class B G-protein coupled receptors [65,66], and the CRF peptides also have been reported in retina [67–72], so further studies are needed to determine the mediating receptor subtype. The downstream mechanisms underlying Ucn 2 retinoprotection also remain uncertain. CRF-like immunoreactivity is present in amacrine, horizontal, and ganglion cells, as well as inner and outer nuclear and plexiform layers. The presence of CRF, Ucns, POMC and mRNAs of prohormone convertases 1 and 2 also has been shown in the retinal pigment epithelium [66]. Based on the retinal distribution of the CRF peptide family, it has been suggested that ocular tissues express CRF/Ucn-driven signaling systems that may play multiple roles in retinal function [66]. We have provided evidence that acute intravitreal Ucn 2 administration attenuates the marked degeneration of retinal layers that otherwise is seen two weeks following permanent BCCAO in rats. The in vivo findings demonstrate that protective actions of Ucn 2 extend to sparing retina from ischemic injury (Figures 1C and 2) [73].
4. Diazoxide
Mitochondrial dysfunction is involved in many key events of neuronal cell death in the retina [1]. ATP-sensitive K+ channels are located in different parts of the cell, including the inner mitochondrial membrane. 7-chloro-3-methyl-4H-1,2,4-benzothiadiazine-1,1-dioxide, diazoxide (DIAZ), a selective mitochondrial, ATP-dependent K+ channel opener that has been implicated in cytoprotection in cardiac and cerebral ischemia [12]. The cardio- and neuroprotective effects of various agents are attributed to the activation of these channels [74,75]. This type of neuroprotection represents a new mechanism of protection which is not dependent on blocking glutamatergic receptors or scavanging free radicals [74]. DIAZ is usually applied as pretreatment before CNS insults because the drug is known to mimic the effects of ischemic preconditioning [76–79]. When DIAZ is used prior to the insult in vitro, it protects against neuronal cell death induced by oxidative stress or glutamate [80,81]. DIAZ can target both mitochondrial and surface cation channels on the astrocytes, thereby modulating the astrocytic function [82]. DIAZ has mostly been applied in neuronal cell cultures exposed to oxygen–glucose deprivation [74,81,83–86]. In vivo, it has neuroprotective effects in various cerebral ischemic experimental conditions [74,83,87,88–90]. The protective effects of DIAZ have also been described both using pre- and postischemic administration in BCCAO [88,89,91,92], but relatively little is known about its putative protective effects in the retina. The mitochondrial ATP-sensitive K+ channels are also present in the retina, where the stimulatory effects of DIAZ have been reported [93]. It has been shown that DIAZ enhances survival of retinal ganglion cells, protects retinal neurons against excitotoxicity and inhibits the glutamate-induced mitochondrial depolarization in vitro [75,94]. DIAZ has also been reported to block the hypoxia-induced horizontal cell depolarization and the reduction of the light-evoked hyperpolarization in vitro [95]. In vivo, ischemic preconditioning can effectively be mimicked by DIAZ [96]. In an in vitro system, opening the mitochondrial K+ channels has been shown to inhibit the oxygen/glucose deprivation-induced glutamate release and to be protective in a model of retinal ischemia [97]. In a superfused retinal system, DIAZ has blocked the hypoxia-induced horizontal cell depolarization and the reduction of the light-evoked hyperpolarization [95]. We have recently reported that local administration of DIAZ is protective in retinal degeneration induced by neonatal monosodium-glutamate treatment or by BCCAO-induced ischemic damage of the retina (Figures 1D and 2) [98]. The mechanism could be multiple, including acute cytoprotective effects of the drug as well as early and late preconditioning. If DIAZ is available for the cells at the time of the ischemia/hypoxia or other kind of depolarization, its protective mechanism can be mediated by reduction of the mitochondrial calcium load [83].
5. Pituitary Adenylate Cyclase Activating Polypeptide
An important approach in neuroprotection is to potentiate or mimic endogenous protective mechanisms [1,96]. Several trophic factors have protective effects against retinal ischemia. Intravitreal injections of brain-derived neurotrophic factor, ciliary neurotrophic factor, basic fibroblast growth factor, hepatocyte growth factor and pigment epithelium derived factor result in significantly less damage in the inner retinal layers [99–101]. Pituitary adenylate cyclase activating polypeptide (PACAP) is a neurotrophic and neuroprotective peptide that has been shown to exert protective effects in different neuronal injuries, such as traumatic brain and spinal cord injury, models of neurodegenerative diseases and cerebral ischemia [102–104]. PACAP and its receptors are present in the retina [105,106]. PACAP is also a trophic factor in the nervous system and retina [107–109]. Increasing body of evidence shows that PACAP is an effective protective agent in retinal injuries. In vitro, the peptide has been shown to counteract the excitotoxic lesion induced by glutamate [110], cell death induced by anisomycin [111] and electrophysiological changes induced by anoxia [112]. In vivo, PACAP protects the retina against glutamate and kainate toxicity and optic nerve transection [113–118].
The neuroprotective effects of PACAP seem to be mediated predominantly by PAC1 receptors, involving protein kinase A and C (PKA and PKC) pathways. A major contribution to this effect has been shown to come from the PKA/MAPK (mitogen activated protein kinase) pathway and downstream, the inhibition of the apoptosis executor, caspase-3. In the retina, a part of this complex neuroprotective mechanism has been confirmed in an in vivo model, the monosodium-glutamate induced degeneration. PACAP has been shown to upregulate the antiapoptotic pathways, such as PKA, cAMP response element binding (CREB) and extracellular signal-regulated kinase (ERK) phosphorylation, and the PKA/Bad/14-3-3 protein cascade resulting in increased expression of the protective Bcl-xL and Bcl-2 [119–121]. At the same time, PACAP treatment downregulates the proapoptotic signaling, such c-Jun N-terminal kinase (JNK), apoptosis inducing factor (AIF), caspase-3, and the release of mitochondrial cytochrome c into the cytosol [119–121].
Recently, we have provided evidence that PACAP also reduces ischemic damage of the retina, protecting all inner retinal layers (Figures 1E and 2) [117,122]. This correlates with previous results showing the distribution of PAC1 receptor in the retina [105]. Strongest expression of the receptor is found in the GCL and INL, while weaker expressions are found in the ONL and OPL. This pattern of receptor expression provides basis for the sites of action by intraocular PACAP administration in our studies. Also, the PACAP antagonist PACAP6-38 could counteract the protective effects of PACAP mostly in the INL and GCL, where the strongest receptor expression has been described.
6. PARP Inhibition
The multifunctional nuclear enzyme, poly(ADP-ribose) polymerase (PARP) is implicated as a major regulator of the cell death process induced by a variety of environmental stimuli [123]. It is well established that overproduction of reactive oxygen species in response to the environmental stimuli cause DNA damage that leads to PARP activation [124]. Excessive PARP activation results in depletion of its substrate, NAD+ leading to ATP depletion and necrotic cell death as a consequence of energy loss. PARP activation facilitates other components of the cell death machinery too, namely; destabilization of the mitochondrial membrane systems [125,126], nuclear translocation of AIF [127] and activation of cell death promoting kinases such as JNK [128]. In addition, PARP activation suppresses the cytoprotective phosphatidyl-inositol-3 kinase-Akt pathway [129]. In the retina, increased activation of PARP contributes to retinal ganglion cell death in response to optic nerve transection [130], is involved in photoreceptor degeneration in the retinal degeneration-1 transgenic mouse model [131] and oxidative stress-induced apoptosis of ganglion cells [132]. PARP inhibition, on the other hand, has been demonstrated to decrease retinal damage in NMDA-induced cell death in the retina [133] and N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis [134].
Although involvement of PARP activation in various ischemia models has been thoroughly studied [135–137], only circumstantial evidences are available for the role of PARP activation in chronic hypoperfusion induced neurodegenerative processes [138]. We have recently provided evidence that a novel PARP inhibitor HO3089 suppresses the morphological and biochemical changes induced by chronic hypoperfusion [20]. Upon PARP inhibitor treatment, the normal morphological structure of the retina was preserved and the thickness of the retinal layers was increased compared to the control ischemic eyes (Figures 1F and 2). Western blot analysis revealed activation of poly-ADP-ribose (PAR) synthesis, which was inhibited by the PARP inhibitor indicating that PARP activation was a causative factor behind the hypoperfusion-induced retinal degeneration. Inhibition of PARP also led to increased activation of one of the most important cytoprotective pathways, the phosphatidyl-inositol-3 kinase-Akt system and its downstream target, GSK-3beta. The signal transduction pathways involving MAP kinases play key roles in cellular survival and adaptation in the retina. BCCAO induced phosphorylation of JNK and p38 MAPK. HO3089 decreased the phosphorylation of these proapoptotic MAPKs. In addition, HO3089 treatment induced phosphorylation that is activation, of the protective ERK signaling pathways [20].
7. Conclusions
In the present review we have summarized recent findings describing novel neuroprotective strategies in ischemic retinal lesions induced by chronic BCCAO. The described strategies encompass four substances acting on different protective pathways, such as endogenous neurotrophism (PACAP and Ucn 2), mitochondrial integrity (DIAZ) and PARP inhibition. The vast amount of retinoprotective agents proven to be effective in animal models provides a divergent array of possible therapeutic strategies in ischemic retinal injury. Further studies are necessary to determine the most effective combination of putative therapeutic treatments in human retinal diseases. The perspective we illustrate by histological studies may also yield novel perspectives on other hypoxic-ischemic retinal disorders, including diabetic retinopathy and age-related macular degeneration, diseases, which are characterized by both vascular and neural abnormalities of the retina.
Acknowledgments
This work was supported by Hungarian National Scientific Grants OTKA T061766, K72592, F67830, CNK 78480, ETT278-04/2009, Richter Gedeon Centenary Foundation, Bolyai Scholarship, University of Pecs Medical School Research Grant 2009.
References
- 1.Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Harris A, Jonescu-Cuypers CP, Kagemann L, Krieglstein GK. Atlas of Ocular Blood Flow–Vascular Anatomy, Pathophysiology, and Metabolism. Imprint of Butterworth Heinemann; Philadelphia, PA, USA: 2003. [Google Scholar]
- 3.Feigl B. Age-related maculopathy-linking aetiology and pathophysiologycal changes to the ischaemia hypothesis. Prog. Retin. Eye Res. 2009;28:63–86. doi: 10.1016/j.preteyeres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Chen CS, Miller NR. Ocular ischemic syndrome: review of clinical presentations, etiology, investigation, and management. Compr. Ophthalmol. 2007;8:17–28. [PubMed] [Google Scholar]
- 7.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv. Ophthalmol. 1999;1:102–128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 8.Bek T. Inner retinal ischemia: current understanding and needs for further investigations. Acta Ophthalmol. 2009;87:362–367. doi: 10.1111/j.1755-3768.2008.01429.x. [DOI] [PubMed] [Google Scholar]
- 9.Roth S. Endogenous neuroprotection in the retina. Brain Res. Bull. 2004;62:461–466. doi: 10.1016/j.brainresbull.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Fulton AB, Akula JD, Mocko JA, Hansen RM, Benador IY, Beck SC, Fahl E, Seeliger MW, Moskowitz A, Harris ME. Retinal degenerative and hypoxic ischemic disease. Doc. Ophthalmol. 2009;118:55–61. doi: 10.1007/s10633-008-9127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J. Neurogenereration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience. 2008;55:937–947. doi: 10.1016/j.neuroscience.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Slakter JS, Spertus AD, Weissman SS, Henkind P. An experimental model of carotid artery occlusive disease. Am. J. Ophtalmol. 1984;97:168–172. doi: 10.1016/s0002-9394(14)76086-6. [DOI] [PubMed] [Google Scholar]
- 14.Spertus AD, Slakter JS, Weissman SS, Henkind P. Experimental carotid occlusion: funduscopic and fluorescein angiographic findings. Br. J. Ophtalmol. 1984;68:47–57. doi: 10.1136/bjo.68.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block F, Schwarz M, Sontag KH. Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci. Lett. 1992;144:124–126. doi: 10.1016/0304-3940(92)90731-l. [DOI] [PubMed] [Google Scholar]
- 16.Osborne NN, Safa R, Nash MS. Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vision Res. 1999;39:3995–4002. doi: 10.1016/s0042-6989(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 17.Stevens WD, Fortin T, Pappas BA. Retinal and optic nerve degeneration after chronic carotid ligation. Stroke. 2002;33:1107–1112. doi: 10.1161/01.str.0000014204.05597.0c. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H, Schmidt-Kasner R, Hamasaki DI, Yamamoto H, Parel JM. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006;82:767–779. doi: 10.1016/j.exer.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Lavinsky D, Arterni NS, Achaval M, Netto CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006;244:199–204. doi: 10.1007/s00417-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 20.Mester L, Szabo A, Atlasz T, Szabadfi K, Reglodi D, Kiss P, Racz B, Tamas A, Gallyas F, Sumegi B, Hocsak E, Gabriel R, Kovacs K. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox. Res. 2009;18:68–76. doi: 10.1007/s12640-009-9049-6. [DOI] [PubMed] [Google Scholar]
- 21.Atlasz T, Babai N, Kiss P, Reglodi D, Tamas A, Szabadfi K, Toth G, Hegyi O, Lubics A, Gabriel R. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen. Comp. Endocrinol. 2007;153:108–114. doi: 10.1016/j.ygcen.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Vidal-Sanz M, Lafuente M, Sobrado-Calvo P, Selles-Navarro I, Rodriguez E, Mayor-Torroglosa S, Villegas-Perez MP. Death and neuroprotection of retinal ganglion cells after different types of injury. Neurotox. Res. 2000;2:215–227. doi: 10.1007/BF03033795. [DOI] [PubMed] [Google Scholar]
- 23.Dilsiz N, Sahaboglu A, Yildiz MZ, Reichenbach A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006;244:627–633. doi: 10.1007/s00417-005-0084-6. [DOI] [PubMed] [Google Scholar]
- 24.Li SY, Fu ZJ, Ma H, Jang WC, So KF, Wong D, Lo AC. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest. Ophthalmol. Vis. Sci. 2009;50:836–843. doi: 10.1167/iovs.08-2310. [DOI] [PubMed] [Google Scholar]
- 25.Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from ischemia in vitro. Exp. Eye Res. 2008;86:366–374. doi: 10.1016/j.exer.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest. Ophthalmol. Vis. Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- 27.Obolensky A, Berenshtein E, Konijn AM, Banin E, Chevion M. Ischemic preconditioning of the rat retina: protective role of ferritin. Free Radic. Biol. Med. 2008;44:1286–1294. doi: 10.1016/j.freeradbiomed.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto K, Yonoki Y, Kuwagata M, Saito M, Nakahara T, Ishii K. Histological protection against ischemia-reperfusion injury by early ischemic preconditioning in rat retina. Brain Res. 2004;1015:154–160. doi: 10.1016/j.brainres.2004.04.074. [DOI] [PubMed] [Google Scholar]
- 29.Chollangi S, Wang J, Martin A, Quinn J, Ash JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis. 2009;34:535–544. doi: 10.1016/j.nbd.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest. Ophthalmol. Vis. Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez DC, Chianelli MS, Rosenstein RE. Involvement of glutamate in retinal protection against ischemia/reperfusion damage induced by post-conditioning. J. Neurochem. 2009;111:488–498. doi: 10.1111/j.1471-4159.2009.06334.x. [DOI] [PubMed] [Google Scholar]
- 32.Macaluso C, Frishman LJ, Frueh B, Kaelin-Lang A, Onoe S, Niemeyer G. Multiple effects of adenosine in the arterially perfused mammalian eye. Possible mechanisms for the neuroprotective function of adenosine in the retina. Doc. Ophthalmol. 2003;106:51–59. doi: 10.1023/a:1022456615715. [DOI] [PubMed] [Google Scholar]
- 33.Tomita H, Ishiguro S, Abe T, Tamai M. Administration of nerve growth factor, brain-derived neurotrophic factor and insulin-like growth factor-II protects phosphate-activated glutaminase in the ischemic and reperfused rat retinas. Tohoku J. Exp. Med. 1999;187:227–236. doi: 10.1620/tjem.187.227. [DOI] [PubMed] [Google Scholar]
- 34.Nishijima K, Ng Y-S, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivilia S, Giuliani A, Fernández M, Turba ME, Forni M, Massella A, de Sordi N, Giardino L, Calzà L. Intravitreal NGF administration counteracts retina degeneration after permanent carotid artery occlusion in rat. BMC Neurosci. 2009;10:52. doi: 10.1186/1471-2202-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreixler JC, Hagevik S, Hemmert JW, Shaikh AR, Rosenbaum DM, Roth S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology. 2009;110:774–780. doi: 10.1097/ALN.0b013e31819c4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jehle T, Meschede W, Dersch R, Feltgen N, Bach M, Lagreze WA. Erythropoietin protects retinal ganglion cells and visual function after ocular ischemia and optic nerve compression. Ophthalmologe. 2009 doi: 10.1007/s00347-009-2030-1. in press. [DOI] [PubMed] [Google Scholar]
- 39.Schmeer C, Gamez A, Tausch S, Witte OW, Isenmann S. Statins modulate heat shock protein expression and enhance retinal ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Invest. Ophthalmol. Vis. Sci. 2008;49:4971–4981. doi: 10.1167/iovs.07-1597. [DOI] [PubMed] [Google Scholar]
- 40.Russo R, Cavaliere F, Watanabe C, Nucci C, Bagetta G, Corasaniti MT, Sakurada S, Morrone LA. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog. Brain Res. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 41.El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI. Neuroprotective effect of (−)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am. J. Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riazi-Esfahani M, Kiumehr S, Asadi-Amoli F, Lashay AR, Dehpour AR. Morphine pretreatment provides histologic protection against ischemia-reperfusion injury in rabbit retina. Retina. 2008;28:511–517. doi: 10.1097/IAE.0b013e31815960c3. [DOI] [PubMed] [Google Scholar]
- 43.Riazi-Esfahani M, Kiumehr S, Asadi-Amoli F, Dehpour AR. Effects of intravitreal morphine administered at different time points after reperfusion in a rabbit model of ischemic retinopathy. Retina. 2009;29:262–268. doi: 10.1097/IAE.0b013e31818a211d. [DOI] [PubMed] [Google Scholar]
- 44.Kocer I, Kulacoglu D, Altuntas I, Gundogdu C, Gullulu G. Protection of the retina from ischemia-reperfusion injury by L-carnitine in guinea pigs. Eur. J. Ophthalmol. 2003;13:80–85. doi: 10.1177/112067210301300112. [DOI] [PubMed] [Google Scholar]
- 45.Lombardi G, Moroni F. Glutamate receptor antagonists protect against ischemia-induced retinal damage. Eur. J. Pharmacol. 1994;271:489–495. doi: 10.1016/0014-2999(94)90810-9. [DOI] [PubMed] [Google Scholar]
- 46.Wood JP, Schmidt KG, Melena J, Chidlow G, Allmeier H, Osborne NN. The beta-adrenoceptor antagonists metipranolol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp. Eye Res. 2003;76:505–516. doi: 10.1016/s0014-4835(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 47.Donello JE, Padillo EU, Webster ML, Wheeler LA, Gil DW. Alpha(2)-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J. Pharmacol. Exp. Ther. 2001;296:216–223. [PubMed] [Google Scholar]
- 48.Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels [K(ATP)] in retina: a key role for delayed ischemic tolerance. Brain Res. 2001;890:118–129. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, Kawakami T, Shimada M, Yamaguchi A, Kuwagata M, Saito M, Nakahara T, Ishii K. Histological protection by cilnidipine, a dual L/N-type Ca(2+) channel blocker, against neurotoxicity induced by ischemia-reperfusion in rat retina. Exp. Eye Res. 2009;88:974–982. doi: 10.1016/j.exer.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Traustason S, Eysteinsson T, Agnarsson BA, Stefánsson E. GABA agonists fail to protect the retina from ischemia-reperfusion injury. Exp. Eye Res. 2009;88:361–366. doi: 10.1016/j.exer.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Holman MC, Chidlow G, Wood JP, Casson RJ. Hyperglycemia rescues retinal neurons from hypoperfusion-induced injury. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4191. in press. [DOI] [PubMed] [Google Scholar]
- 52.Fekete ÉM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol. Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- 54.Latchman DS. Molecules in focus urocortin. Biochem. Cell Biol. 2002;34:907–910. doi: 10.1016/s1357-2725(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 55.Pan W, Kastin AJ. Urocortin and the brain. Prog. Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul. Pept. 2000;93:85–92. doi: 10.1016/s0167-0115(00)00180-4. [DOI] [PubMed] [Google Scholar]
- 57.Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 2005;579:4259–4264. doi: 10.1016/j.febslet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 58.Uchida M, Suzuki M, Shimizu K. Effects of urocortin, corticotropin-releasing factor (CRF) receptor agonist, and astressin, CRF receptor antagonist, on the sleep-wake pattern: analysis by radiotelemetry in conscious rats. Biol. Pharm. Bull. 2007;10:1895–1897. doi: 10.1248/bpb.30.1895. [DOI] [PubMed] [Google Scholar]
- 59.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjøs OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 60.Liu CN, Yang C, Liu XY, Li S. In vivo protective effects of urocortin on ischemiareperfusion injury in rat heart via free radical mechanisms. Can. J. Physiol. Pharmacol. 2005;83:459–465. doi: 10.1139/y05-033. [DOI] [PubMed] [Google Scholar]
- 61.Rademaker MT. Urocortin: cardiovascular actions and therapeutic implications. Lett. Drug Des. Discov. 2004;1:168–172. [Google Scholar]
- 62.Tao J, Li S. Effects of UCN via ion mechanisms or CRF receptors? Biochem. Biophys. Res. Commun. 2005;336:731–736. doi: 10.1016/j.bbrc.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 63.Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, Strijbos PJLM. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacol. 2003;45:623–636. doi: 10.1016/s0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dautzenberg FM, Huber G, Higelin J, Py-Lang G, Kilpatrick GJ. Evidence for the abundant expression of arginine 185 containing human CRF2 receptors and the role of position 185 for receptor-ligand selectivity. Neuropharmacol. 2000;39:1368–1376. doi: 10.1016/s0028-3908(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 66.Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J. Endocrinol. 2007;193:157–169. doi: 10.1677/joe.1.06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skofitsch G, Jacobowitz DM. Corticotropin releasing factor-like immunoreactive neurons in the rat retina. Brain Res. Bull. 1984;12:539–542. doi: 10.1016/0361-9230(84)90169-2. [DOI] [PubMed] [Google Scholar]
- 68.Williamson DE, Eldred WD. Synaptic organization of two types of amacrine cells with CRF-like immunoreactivity in the turtle retina. Vis. Neurosci. 1991;6:257–269. doi: 10.1017/s095252380000626x. [DOI] [PubMed] [Google Scholar]
- 69.Williamson DE, Eldred WD. Amacrine and ganglion cells with corticotropinreleasing-factor-like immunoreactivity in the turtle retina. J. Comp. Neurol. 1989;280:424–435. doi: 10.1002/cne.902800308. [DOI] [PubMed] [Google Scholar]
- 70.Zhang DR, Gallagher M, Sladek CD, Yeh HH. Postnatal development of corticotrophin releasing factor-like immunoreactive amacrine cells in the rat retina. Brain Res. Dev. Brain Res. 1990;51:185–194. doi: 10.1016/0165-3806(90)90275-4. [DOI] [PubMed] [Google Scholar]
- 71.Zhang DR, Yeh HH. Corticotropin releasing factor-like immunoreactivity (CRFLI) in horizontal cells of the developing rat retina. Vis. Neurosci. 1991;6:383–391. doi: 10.1017/s0952523800006611. [DOI] [PubMed] [Google Scholar]
- 72.Zhang DR, Yeh HH. Histogenesis of corticotropin releasing factor-like immunoreactive amacrine cells in the rat retina. Brain Res. Dev. Brain Res. 1990;53:194–199. doi: 10.1016/0165-3806(90)90006-k. [DOI] [PubMed] [Google Scholar]
- 73.Szabadfi K, Atlasz T, Reglodi D, Kiss P, Danyáadi B, Fekete ÉM, Zorrilla EP, Tamas A, Szabo K, Gabriel R. Urocortin 2 protects against retinal degeneration following bilateral common carotid artery occlusion in the rat. Neurosci. Lett. 2009;455:42–45. doi: 10.1016/j.neulet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels - a novel approach to neuroprotection. Brain Res. Rev. 2004;46:282–294. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Yamauchi T, Kashii S, Yasuyoshi H, Zhang S, Honda Y, Akaike A. Mitochondrial ATP-sensitive potassium channel: a novel site for neuroprotection. Invest. Ophthalmol. Vis. Sci. 2003;44:2750–2756. doi: 10.1167/iovs.02-0815. [DOI] [PubMed] [Google Scholar]
- 76.Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke. 1999;30:2713–2718. doi: 10.1161/01.str.30.12.2713. [DOI] [PubMed] [Google Scholar]
- 77.Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J. Cereb. Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Minners J, McLeod CJ, Sack MN. Mitochondrial plasticity in classical ischemic preconditioning-moving beyond the mitochondrial KATP channel. Cardiovasc. Res. 2003;59:1–6. doi: 10.1016/s0008-6363(03)00337-7. [DOI] [PubMed] [Google Scholar]
- 79.Shake JG, Peck EA, Marban E, Gott VL, Johnston MV, Troncoso JC, Redmond JM, Baumgartner WA. Pharmacologically induced preconditioning with diazoxide: a novel approach to brain protection. Ann. Thorac. Surg. 2001;72:1849–1854. doi: 10.1016/s0003-4975(01)03192-7. [DOI] [PubMed] [Google Scholar]
- 80.Nagy K, Kis B, Rajapakse NC, Bari F, Busija DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J. Neurosci. Res. 2004;76:697–704. doi: 10.1002/jnr.20120. [DOI] [PubMed] [Google Scholar]
- 81.Teshima Y, Akao M, Li RA, Chong TH, Baumgartner WA, Johnston MV, Marban E. Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke. 2003;34:1796–1802. doi: 10.1161/01.STR.0000077017.60947.AE. [DOI] [PubMed] [Google Scholar]
- 82.Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija DW. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J. Neurosci. Res. 2003;73:206–214. doi: 10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- 83.Domoki F, Bari F, Nagy K, Busija DW, Siklós L. Diazoxide prevents mitochondrial swelling and Ca2+ accumulation in CA1 pyramidal cells after cerebral ischemia in newborn pigs. Brain Res. 2004;1019:97–104. doi: 10.1016/j.brainres.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 84.Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J. Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Sato T, Seharaseyon J, Szewczyk A, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels. Viable candidate effectors of ischemic preconditioning. Ann. NY Acad. Sci. 1999;874:27–37. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- 86.Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoK(ATP) opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am. J. Physiol. Heart Circ. Physiol. 2002;283:1005–1011. doi: 10.1152/ajpheart.00054.2002. [DOI] [PubMed] [Google Scholar]
- 87.Busija DW, Katakam P, Rajapakse NC, Kis B, Grover G, Domoki F, Bari F. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res. Bull. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 88.Farkas E, Annahazi A, Institoris A, Mihaly A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide alleviate experimental cerebral hypoperfusion-induced white matter injury in the rat brain. Neurosci. Lett. 2005;373:195–199. doi: 10.1016/j.neulet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Farkas E, Timmer NM, Domoki F, Mihaly A, Luiten PG, Bari F. Post-ischemic administration of diazoxide attenuates long-term microglial activation in the rat brain after permanent carotid artery occlusion. Neurosci. Lett. 2005;387:168–172. doi: 10.1016/j.neulet.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 90.Lenzser G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 91.Farkas E, Institoris A, Domoki F, Mihaly A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusion-related learning dysfunction and brain damage after carotid artery occlusion. Brain Res. 2004;1008:252–260. doi: 10.1016/j.brainres.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 92.Farkas E, Institoris A, Domoki F, Mihaly A, Bari F. The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res. 2006;1087:168–174. doi: 10.1016/j.brainres.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 93.Sheu SJ, Wu SN. Mechanism of inhibitory actions of oxidizing agents on calcium-activated potassium current in cultured pigment epithelial cells of the human retina. Invest. Ophthalmol. Vis. Sci. 2003;44:1237–1244. doi: 10.1167/iovs.02-0330. [DOI] [PubMed] [Google Scholar]
- 94.Pielen A, Kirsch M, Hofmann HD, Feuerstein TJ, Lagreze WA. Retinal ganglion cell survival is enhanced by gabapentin-lactam in vitro: evidence for involvement of mitochondrial KATP channels. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004;242:240–244. doi: 10.1007/s00417-004-0872-4. [DOI] [PubMed] [Google Scholar]
- 95.Hankins MW, Ikeda H. Consequences of transient retinal hypoxia on rod input to horizontal cells in the rat retina. Vision Res. 1993;33:429–436. doi: 10.1016/0042-6989(93)90250-z. [DOI] [PubMed] [Google Scholar]
- 96.Roth S, Dreixler JC, Shaikh AR, Lee KH, Bindokas V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest. Ophthalmol. Vis. Sci. 2006;47:2114–2124. doi: 10.1167/iovs.05-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jehle T, Lagreze WA, Blauth E, Knorle R, Schnierle P, Lucking CH, Feuerstein TJ. Gabapentin-lactam (8-aza-spiro[5,4]decan-9-on; GBP-L) inhibits oxygen glucose deprivation-induced [3H]glutamate release and is a neuroprotective agent in a model of acute retinal ischemia. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:74–81. doi: 10.1007/s002100000265. [DOI] [PubMed] [Google Scholar]
- 98.Atlasz T, Babai N, Reglodi D, Kiss P, Tamas A, Bari F, Domoki F, Gabriel R. Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox. Res. 2007;12:105–111. doi: 10.1007/BF03033919. [DOI] [PubMed] [Google Scholar]
- 99.Unoki K, La Vail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest. Ophtalmol. Vis. Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- 100.Ogata N, Wang L, Jo N, Tombran-Tink J, Takahashi K, Mrazek D, Matsumura M. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr. Eye Res. 2001;22:245–252. doi: 10.1076/ceyr.22.4.245.5506. [DOI] [PubMed] [Google Scholar]
- 101.Shibuki H, Katai N, Kuroiwa S, Kurokawa T, Arai J, Matsumoto K, Nakamura T, Yoshimura N. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2002;43:528–536. [PubMed] [Google Scholar]
- 102.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 103.Ohtaki H, Nakamachi T, Dohi K, Shioda S. Role of PACAP in ischemic neural death. J. Mol. Neurosci. 2008;36:16–25. doi: 10.1007/s12031-008-9077-3. [DOI] [PubMed] [Google Scholar]
- 104.Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide-review. Curr. Pharm. Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- 105.Seki T, Izumi S, Shioda S, Zhou CJ, Arimura A, Koide R. Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann. N. Y. Acad. Sci. 2000;921:366–369. doi: 10.1111/j.1749-6632.2000.tb06995.x. [DOI] [PubMed] [Google Scholar]
- 106.Seki T, Shioda S, Ogino D, Nakai Y, Arimura A, Koide R. Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci. Lett. 1997;238:127–130. doi: 10.1016/s0304-3940(97)00869-0. [DOI] [PubMed] [Google Scholar]
- 107.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 108.Bagnoli P, Dal Monte M, Casini G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol. 2003;18:1219–1242. doi: 10.14670/HH-18.1219. [DOI] [PubMed] [Google Scholar]
- 109.Borba JC, Henze IP, Silveira MS, Kubrusly RC, Gardino PF, de Mello MC, Hokoc JN, de Mello FG. Pituitary adenylate cyclase activating polypeptide (PACAP) can act as determinant of the tyrosine hydoxylase phenotype of dopaminergic cells during retina development. Dev. Brain Res. 2005;156:193–201. doi: 10.1016/j.devbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 110.Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, Sasa M. Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res. 1999;839:66–73. doi: 10.1016/s0006-8993(99)01690-x. [DOI] [PubMed] [Google Scholar]
- 111.Silveira MS, Costa MR, Bozza M, Linden R. Pituitary adenylate cyclase activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J. Biol. Chem. 2002;277:16075–16080. doi: 10.1074/jbc.M110106200. [DOI] [PubMed] [Google Scholar]
- 112.Rabl K, Reglodi D, Banvolgyi T, Somogyvari-Vigh A, Lengvari I, Gabriel R, Arimura A. PACAP inhibits anoxia-induced changes in physiological responses in horizontal cells in the turtle retina. Regul. Pept. 2002;109:71–74. doi: 10.1016/s0167-0115(02)00189-1. [DOI] [PubMed] [Google Scholar]
- 113.Seki T, Itoh H, Nakamachi T, Shioda S. Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J. Mol. Neurosci. 2008;36:57–60. doi: 10.1007/s12031-008-9091-5. [DOI] [PubMed] [Google Scholar]
- 114.Babai N, Atlasz T, Tamas A, Reglodi D, Toth G, Kiss P, Gabriel R. Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Ann. N.Y. Acad. Sci. 2006;1070:149–155. doi: 10.1196/annals.1317.003. [DOI] [PubMed] [Google Scholar]
- 115.Babai N, Atlasz T, Tamas A, Reglodi D, Toth G, Kiss P, Gabriel R. Degree of damage compensation by various PACAP treatments in monosodium glutamate-induced retinal degeneration. Neurotox. Res. 2005;8:227–233. doi: 10.1007/BF03033976. [DOI] [PubMed] [Google Scholar]
- 116.Tamas A, Gabriel R, Racz B, Denes V, Kiss P, Lubics A, Lengvari I, Reglodi D. Effects of pituitary adenylate cyclase activating polypeptide in retinal degeneration induced by monosodium-glutamate. Neurosci. Lett. 2004;372:110–113. doi: 10.1016/j.neulet.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 117.Atlasz T, Szabadfi K, Reglodi D, Kiss P, Tamas A, Toth G, Molnar A, Szabo K, Gabriel R. Effects of pituitary adenylate cyclase activating polypeptide (PACAP1-38) and its fragments on retinal degeneration induced by neonatal MSG treatment. Ann. NY Acad. Sci. 2009;1163:348–352. doi: 10.1111/j.1749-6632.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 118.Seki T, Nakatani M, Taki C, Shinonara Y, Ozawa M, Nishimura S, Shioda S. Neuroprotective effect of PACAP against kainic acid (KA)-induced neurotoxicity in rat retina. Ann. NY Acad. Sci. 2006;1070:531–534. doi: 10.1196/annals.1317.074. [DOI] [PubMed] [Google Scholar]
- 119.Racz B, Tamas A, Kiss P, Toth G, Gasz B, Borsiczky B, Ferencz A, Gallyas F, Jr, Roth E, Reglodi D. Involvement of ERK and CREB signalling pathways in the protective effect of PACAP on monosodium glutamate-induced retinal lesion. Ann. NY Acad. Sci. 2006;1070:507–511. doi: 10.1196/annals.1317.070. [DOI] [PubMed] [Google Scholar]
- 120.Racz B, Gallyas F, Jr, Kiss P, Toth G, Hegyi O, Gasz B, Borsiczky B, Ferencz A, Roth E, Tamas A, Lengvari I, Lubics A, Reglodi D. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways. Regul. Pept. 2006;137:20–26. doi: 10.1016/j.regpep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 121.Racz B, Gallyas F, Jr, Kiss P, Tamas A, Lubics A, Lengvari I, Roth E, Toth G, Hegyi O, Verzar Zs, Fabricsek Cs, Reglodi D. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox. Res. 2007;12:95–104. doi: 10.1007/BF03033918. [DOI] [PubMed] [Google Scholar]
- 122.Atlasz T, Szabadfi K, Kiss P, Tamas A, Toth G, Reglodi D, Gabriel R. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull. 2009 doi: 10.1016/j.brainresbull.2009.09.004. in press. [DOI] [PubMed] [Google Scholar]
- 123.Virag L, Szabo C. The therapeutic potential of poly(ADPribose) polymerase inhibitors. Pharmacol. Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 124.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Halmosi R, Berente Z, Osz E, Toth K, Literati-Nagy P, Sumegi B. Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system. Mol. Pharmacol. 2001;59:1497–1505. doi: 10.1124/mol.59.6.1497. [DOI] [PubMed] [Google Scholar]
- 126.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 127.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 128.Xu Y, Huang S, Liu ZG, Han J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 2006;281:8788–8795. doi: 10.1074/jbc.M508135200. [DOI] [PubMed] [Google Scholar]
- 129.Veres B, Gallyas F, Jr, Varbiro G, Berente Z, Osz E, Szekeres G, Szabo C, Sumegi B. Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem. Pharmacol. 2003;65:1373–1382. doi: 10.1016/s0006-2952(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 130.Weise J, Isenmann S, Bahr M. Increased expression and activation of poly(ADP-ribose) polymerase (PARP) contribute to retinal ganglion cell death following rat optic nerve transection. Cell Death Differ. 2001;8:801–807. doi: 10.1038/sj.cdd.4400872. [DOI] [PubMed] [Google Scholar]
- 131.Paquet-Durand F, Silva J, Talukdar T, Johnson LE, Azadi S, van Veen T, Ueffing M, Hauck SM, Ekstrom PA. Excessive activation of poly-(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. Neurobiol. Dis. 2007;27:10311–10319. doi: 10.1523/JNEUROSCI.1514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li GY, Osborne NN. Oxidative-induced apoptosis to an immortalized ganglion cell line is caspase independent but involves the activation of poly (ADP-ribose) polymerase and apoptosis-inducing factor. Brain Res. 2008;1188:35–43. doi: 10.1016/j.brainres.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 133.Goebel DJ, Winkler BS. Blockade of PARP activity attenuates poly(ADP-ribosyl)ation but offers only partial neuroprotection against NMDA-induced cell death in the rat retina. J. Neurochem. 2006;98:1732–1745. doi: 10.1111/j.1471-4159.2006.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uehara N, Miki K, Tsukamoto R, Matsuoka Y, Tsubura A. Nicotinamide blocks N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in rats through poly (ADP-ribose) polymerase activity and Jun N-terminal kinase/activator protein-1 pathway inhibition. Exp. Eye Res. 2006;82:488–495. doi: 10.1016/j.exer.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 135.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J. Neuropathol. Exp. Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 136.Meli E, Pangallo M, Baronti R, Chiarugi A, Cozzi A, Pellegrini-Giampietro DE, Moroni F. Poly(ADP-ribose) polymerase as a key player in excitotoxicity and post-ischemic brain damage. Toxicol. Lett. 2003;139:153–162. doi: 10.1016/s0378-4274(02)00429-0. [DOI] [PubMed] [Google Scholar]
- 137.Ikeda Y, Hokamura K, Kawai T, Ishiyama J, Ishikawa K, Anraku T, Uno T, Umemura K. Neuroprotective effects of KCL-440, a new poly(ADP-ribose) polymerase inhibitor, in the rat middle cerebral artery occlusion model. Brain Res. 2005;1060:73–80. doi: 10.1016/j.brainres.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 138.Cozzi A, Cipriani G, Fossati S, Faraco G, Formentini L, Min W, Cortes U, Wang ZQ, Moroni F, Chiarugi A. Poly(ADPribose) accumulation and enhancement of postischemic brain damage in 110-kDa poly(ADP-ribose) glycohydrolase null mice. J. Cereb. Blood Flow Metab. 2006;26:684–695. doi: 10.1038/sj.jcbfm.9600222. [DOI] [PubMed] [Google Scholar]