Abstract
Background
The present study evaluates the reversal of diabetes mediated impairment of angiogenesis in myocardial infarction (MI) model of Type I diabetic rats by intramyocardial administration of adenoviral vector encoding Thioredoxin-1 (Ad.Trx1). Various studies have linked diabetes mediated impairment of angiogenesis to dysfunctional antioxidant systems in which Trx1 plays a central role.
Methods and Results
Ad.Trx1 was intramyocardially administered immediately after MI to non-diabetic and diabetic rats. Ad.LacZ was similarly administered to the respective control groups. The hearts were excised for molecular and immunohistochemical analysis at predetermined time points. The myocardial function was measured by echocardiography 30 days after the intervention. The Ad.Trx1 administered group exhibited reduced fibrosis, oxidative stress and cardiomyocyte and endothelial cell apoptosis as compared to diabetic MI group along with increased capillary and arteriolar density. Western blot and immunohistochemical analysis demonstrated myocardial overexpression of Trx1, HO-1, VEGF, p38MAPKβ and decreased p-JNK and p38MAPKα in the Ad.Trx1 treated diabetic group. Alternatively, we have observed significant reduction in the expression of VEGF in SnPP (HO-1 enzyme inhibitor) treated non-diabetic and diabetic animals even after Ad.Trx1 therapy. Echocardiographic analysis after 4 weeks of MI revealed significant improvement in the myocardial functional parameters such as the ejection fraction, fractional shortening and E/A ratio in the Ad.Trx1 administered group as compared to the diabetic MI group.
Conclusion
We demonstrate that the infarcted myocardium can be rescued from diabetes related impairment of angiogenesis and reduce myocardial functional disorder by Trx1 gene therapy in streptozotocin induced diabetic rats.
Keywords: Thioredoxin (Trx1), Gene Therapy, Myocardial Infarction, Diabetes, Angiogenesis
Introduction
Diabetic individuals often suffer from complications such as coronary artery disease, peripheral artery disease and stroke which contribute to the morbidity or mortality among these subjects 1. This higher incidence of cardiovascular complications as well as the unfavorable prognosis among diabetic individuals who develop such complications has been correlated to diabetes mediated impairment of angiogenesis 2. Inhibited angiogenesis is known to contribute to impaired coronary collateral vessel formation and wound healing 2. Various studies have linked diabetes mediated impaired angiogenesis to improper degradation of the basement membrane, alterations to the delicate balance of angiogenic growth factors and cytokines regulating vascular stability, problems in signal transduction and to a large extent to diabetes mediated oxidative stress 2. Evidences suggest, Reactive Oxygen Species (ROS) mediated oxidative stress to be the primary contributor of vascular and myocardial dysfunction thereby compromising the recovery from such conditions 3, 4.
Therefore, maintaining a balanced redox status in the microenvironment might aid in alleviating many of the diabetic mediated complications specially those related to impaired angiogenesis. Balanced and responsive antioxidant systems are vital for proper regulation of the redox status of the cell. The cells are normally able to defend themselves against the oxidative stress induced damage through the use of several antioxidant systems 5. The thioredoxin (Trx) system is one of the main ubiquitously expressed thiol-reducing antioxidant systems. The classical 12kDa cytosolic thioredoxin-1(Trx1) is the most studied of the different forms of Trx 5, 6. Trx1 has been described as growth regulator, transcriptional factor regulator, co-factor apart from its very important anti-oxidative role 7.
Key biological activities of Trx1 include antioxidative as well as anti-apoptotic and growth stimulatory properties through its interactions with nuclear factor-kappa B (NFκB), apoptosis signal regulation kinase-1 (ASK-1) and hypoxia inducible factor-1 alpha (HIF-1α) 8–10. Induction of ASK-1 is known to activate the pro-apoptotic JNK and p38MAPK signaling pathways11. In addition, it was reported that transgenic mouse hearts over expressing Trx1 displayed significantly improved post-ischemic ventricular recovery, reduced myocardial infarct size and resistance to ischemic reperfusion injury as compared to the hearts from wild-type mice 7, 12. Inhibition of Trx1 expression in the heart has been associated with increased oxidative stress and apoptosis13. Recent reports suggest the contribution of Trx system in the upregulation of Hemeoxygenase-1 (HO-1) protein levels as well as HO-1 promoter activity under conditions associated with inflammation and increased oxidative stress 14, 15. HO-1 is known to be a stress response protein that has important redox regulatory functions 15, 16. Some of our recent studies have demonstrated that the mechanism responsible for the enhanced angiogenesis as well as the cardioprotective effect of resveratrol and sildenafil operates through Trx1, HO-1 and Vascular Endothelial Growth Factor (VEGF) 16–18. Recently, we have also observed that resveratrol alleviates cardiac dysfunction in streptozotocin induced diabetic rats through the induction of Trx1, HO-1 and VEGF 17. We have recently reported the possible role of decreased VEGF in the pathogenesis of diabetes mediated impairment of angiogenesis in the myocardium 19. The process of angiogenesis was shown to be highly impaired in diabetic mice models of hind limb ischemia and wound healing due to decreased expression of VEGF 20, 21. Moreover, Trx1 has been shown to promote the expression and activity of HIF-1α leading to the elevated expression of VEGF and thus enhance vascular growth.10
There have been several attempts at the preclinical and clinical levels to induce myocardial angiogenesis by overexpressing the angiogenic factors such as VEGF in the peri-infarct zone after MI. Recently, we have shown that intramyocardial co-administration of a combination of adenoviral vectors encoding VEGF and Angiopoietin-1 (Ang-1) induced and stabilized the process of angiogenesis in the ischemic diabetic myocardium and reduced ventricular remodeling leading to cardioprotection 19.
In view of, the increased functional severity of cardiac failure in diabetic post-myocardial infarction (MI) subjects, the given role of oxidative stress mediated reduction in myocardial angiogenesis and the anti-oxidative, growth regulatory and angiogenic potential of Trx1 through induction of VEGF, we hypothesized that overexpression of Trx1 alone in the myocardium can prove to be a useful prophylaxis of subsequent heart failure after diabetes associated MI. Therefore, to address the importance of Trx1 in in vivo angiogenesis during myocardial ischemia in a diabetic scenario and identify a potential therapeutic tool, we investigated the effect of Trx1 gene therapy with an adenoviral vector carrying the Trx1 gene in the MI model of streptozotocin (STZ) induced diabetic rats. In the present study we investigated the effect of Trx1 gene delivery on myocardial fibrosis, oxidative stress, cardiomyocyte and endothelial cell apoptosis, capillary and arteriolar density and left ventricular remodeling in diabetic rats. We have demonstrated for the first time that the myocardium can be rescued from diabetes related impairment of angiogenesis, severity of functional disorder and subsequent heart failure by Trx1 gene therapy in streptozotocin induced diabetic rats.
Materials and Methods
Experimental Animals
This study was performed in accordance with the principles of laboratory animal care formulated by the National Society for Medical Research and with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication No. 85-23, revised 1985). The experimental protocol was approved by the Institutional Animal Care Committee of the University of Connecticut Health Center (Farmington, CT). Male SD rats (300–325gm) were randomly separated into normal and diabetic rats as they received an (i.p.) injection of vehicle (0.1mol/l citrate buffer, pH 4.5) alone or streptozotocin (STZ) at a dosage of 65mg/kg body weight dissolved in 0.1mol/l citrate buffer. Five days after STZ injection, hyperglycemia was documented by measuring the glucose content of tail vein blood with Freestyle Flash blood glucose monitoring system. Rats with blood glucose concentrations ≥ 300mg/dl were used for the study.
Experimental Design
Myocardial Infarction (MI) was induced in the diabetic animals 30 days after the induction of diabetes as previously described 16. Age matched non-diabetic animals were used as comparable controls. Sham-operated rats underwent thoracotomy and pericardiectomy without MI. The rats were randomized into eight groups. 1) Control Sham (CS), 2) Control + MI (CMI), 3) CMI + Ad.LacZ (CMI-AdLacZ), 4) CMI + Ad.Trx1 (CMI-AdTrx1), 5) Diabetic Sham (DS), 6) Diabetic + MI (DMI) 7) DMI + Ad.LacZ (DMI-AdLacZ) and 8) DMI + Ad.Trx1 (DMI-AdTrx1).
The adenoviral vector encoding Trx1 (Ad.Trx1, 1 × 109 pfu) 22 was intramyocardially administered (in 100μl of PBS, using a 30g needle) at 4 different sites into the left anterior free wall adjacent to the point of ligation (border zone surrounding the infarct) immediately after inducing an MI. Adeno-LacZ (Ad.LacZ, 1 × 109 pfu) 22 was used as the control. Ad.Trx1 and Ad.LacZ were generous gifts from Dr. Sadoshima J, New Jersey Medical School, Newark 13, 23.
In our study, we have used STZ induced diabetic rats and MI was induced 30 days after the induction of hyperglycemia. These rats even before the first surgical intervention tend to lose weight, become weak, shows lesser physical activity and vital sign indices deteriorate. There was significant reduction in the heart rate in these animals in the sham group as well as after MI 19. Overall there was a higher rate of mortality in the diabetic animals after MI (45–52%) than compared to the non-diabetic MI controls (8–12%). Though we considered a second thoracotomy for gene delivery at a later time after the induction of MI, owing to the higher rate of mortality and the stress and pain that the diabetic animals have to undergo, we decided to administer the adenoviral vectors immediately after MI.
Another group of non-diabetic and diabetic animals were treated with tin-protoporphyrin IX (SnPP, an inhibitor of HO-1 activity, 50μmol/kg, i.p.) to determine whether HO-1 plays an important role in Trx1 mediated VEGF expression 16. These SnPP treated animals were then subjected to MI and treated with Ad.Trx1 as described above.
For detailed descriptions of the Materials and Methods and details regarding the time point of harvesting the tissue for various experiments please refer to the supplementary material provided.
Statistical Analysis
All data were analyzed by statistical software ‘GraphPad Prism 5.0’ (SanDiego, CA, USA). Statistical analysis was performed by using one way analysis of variance (ANOVA). Post-hoc comparisons between the groups were performed by Newman-Keuls Multiple Comparison Test. Results are presented as mean ± SEM with p ≤ 0.05 used to indicate statistical significance.
Results
Transfection efficiency of Ad.LacZ and Ad.Trx1 in HUVECs
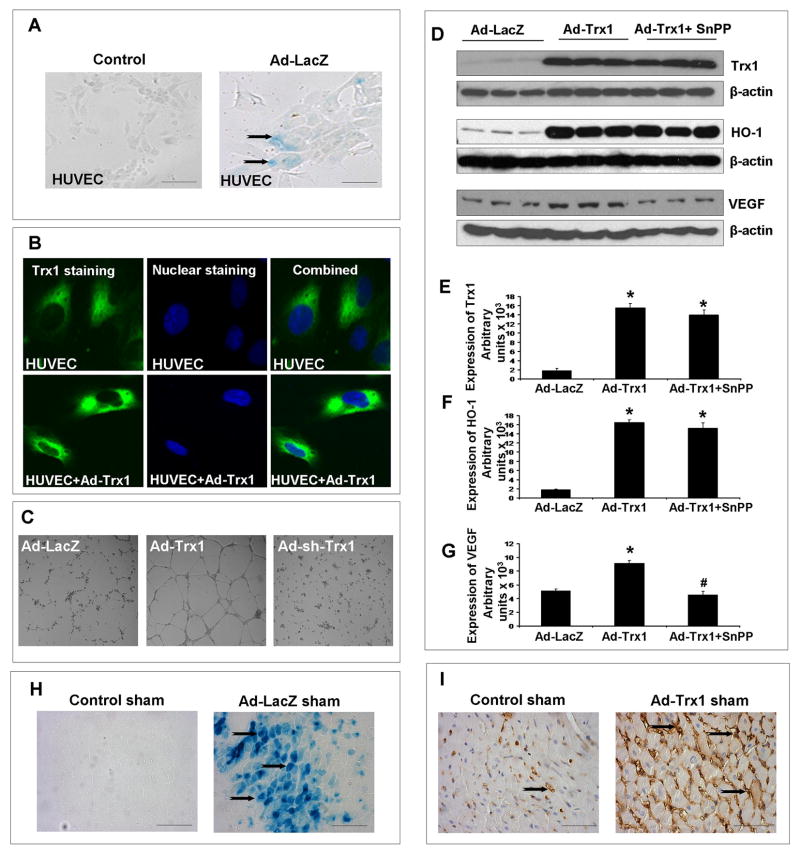
To examine the transfection efficiency of Ad.LacZ and Ad.Trx1, cytochemistry was performed after 48hrs to examine the extent of β-galactosidase (β-gal) and immunocytochemistry for Trx1 expression in the cells. Figure 1A and 1B represents the expression of β-gal and Trx1, respectively in the Ad.LacZ and Ad.Trx1 treated and untreated HUVECs. Significant β-gal staining was also observed in Ad.LacZ treated HUVECs (Figure 1A). Significant increase in the expression of Trx1 was observed in the Ad.Trx1 treated cells as compared to the untreated HUVECs (Figure 1B).
Figure 1.
(A) Representative micrographs showing the in vitro transfection efficiency of Ad.LacZ in HUVECs. (B) Representative micrographs showing the in vitro transfection efficiency of Ad.Trx1 in HUVECs. (C) Representative micrographs showing the angiogenic potential of overexpressing Trx1 in HUVECs by in vitro Matrigel Assay. The tuburogenesis was significantly abolished by using Ad-sh-Trx1. (D) Representative Western Blots showing the effect of Ad.Trx1 transfection in HUVECs on Trx1, HO-1 and VEGF. The use of SnPP significantly reduced the expression of VEGF. Bar graphs (E), (F) and (G) represent the quantitative difference in expression of the Trx1, HO-1 and VEGF, respectively between the groups. Values are represented as mean ± SEM (n=3/group), *p≤0.05 when compared to the Ad.LacZ treated HUVEC controls and #p≤0.05 when compared to Ad.Trx1 treated HUVECs. Micrographs (H) and (I) represents the in vivo transfection efficiency of Ad.LacZ and Ad.Trx1 in the non-diabetic Sham operated groups. Scale bar = 50μm.
Effect of Ad.Trx1 gene transfection on tuborogenesis in HUVECs
To determine whether Ad.Trx1 gene transfection has angiogenic potential, we performed in vitro matrigel assay with HUVECs to analyze the extent of tube formation as shown in Figure 1C. We have observed significant increase tuborogenesis in the Ad.Trx1 treated HUVECs when compared to the Ad.LacZ treated control. This Ad.Trx1 mediated tuborogenesis was found to be significantly abolished when HUVECs were transfected with Ad-sh-Trx1 along with Ad.Trx1 (Figure 1C), thereby documenting the angiogenic potential of Trx1.
Ad.Trx1 transfection increased the expression of Trx1, HO-1 & VEGF in HUVECs
Our in vitro matrigel assay experiments have documented the angiogenic potential of Trx1. In order to examine the molecular basis of the angiogenic potential of Ad.Trx1 treatment in HUVECs we examined the effect of Ad.Trx1 transfection on the expression levels of HO-1 and VEGF (n=3/group). We have observed significant increase in the expression of Trx1 (8.7 fold, Figure 1D and 1E), HO-1 (9.2 fold, Figure 1D and 1F) and VEGF (1.8 fold, Figure 1D and 1G) in HUVECs treated with Ad.Trx1 when compared to the Ad.LacZ treated cells. However, when the cells were transfected with Ad.Trx1 and treated with SnPP, we have observed significant reduction in VEGF expression (2 fold) eventhough there was no significant change in Trx-1 and HO-1 expression, when compared to the Ad.Trx1 treated group (Figure 1D–1G).
Histochemistry for the transfection efficiency of Ad.LacZ and Ad.Trx1 (in vivo)
Once we verified the angiogenic potential of Trx1 and the molecular basis of this angiogenic capacity we intended to study the effect of Ad.Trx1 gene therapy in the infarcted diabetic myocardium. In order to examine the efficiency of our gene transfer technique, in vivo, we have evaluated the adenoviral gene expression 4 days after intramyocardial adenoviral gene administration in the Sham operated non-diabetic control groups. We injected the adenoviral vector encoding β-galacatosidase (β-gal, Ad.LacZ) and Trx1 (Ad.Trx1) at 4 different sites (in 100μl of PBS, using a 30g needle) into the left anterior wall of the myocardium in non-diabetic sham animals (n=3/group). We have observed robust infection and expression of the respective proteins in the myocardium as assessed by X-gal staining (Figure 1H) and Trx1 (Figure 1I) immunohistochemical staining in the myocardium surrounding the sites of gene transfer. The β-gal expression was not evident in the remote areas such as the septum and the right ventricular muscles.
Biological Effects of Trx1 gene therapy in the Diabetic Ischemic Myocardium
Trx1 gene therapy reduced myocardial fibrosis
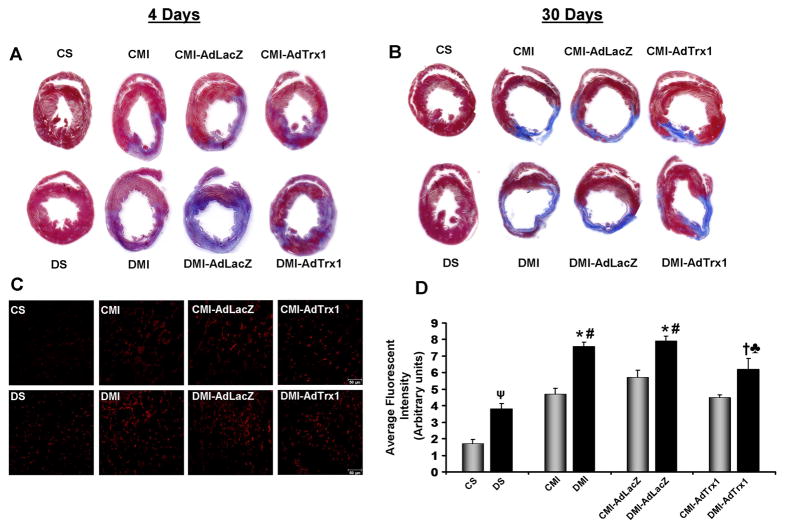
4 days after MI and gene therapy the hearts (n=3/group) were harvested and Masson’s trichrome staining was performed on paraffin embedded tissue sections to visualize the infarct scar (Figure 2A). There was no evident difference in the infarct scar extension and thickness in the Ad.LacZ treated CMI-AdLacZ and DMI-AdLacZ when compared to their respective non-treated controls, CMI and DMI respectively. Upon Trx1 gene therapy the non-diabetic CMI-AdTrx1 group showed a thicker/muscular infarct, lesser scar region and more islands of viable cardiac tissue when compared to the CMI group. Similarly, the diabetic MI group that received the Trx1 gene therapy (DMI-AdTrx1) demonstrated evidently lesser scar region and more islands of viable cardiac tissue when compared to the DMI group.
Figure 2.
Effect of Trx1 gene therapy on: - (A and B) Myocardial fibrosis (by Masson’s trichrome staining). Representative images show myocardial fibrosis (A) 4 days after and (B) 30 days after MI and intervention. A thinner infarct and significant fibrosis is evident in the DMI and DMI-AdLacZ groups compared to the CMI and CMI-AdLacZ groups, respectively. Trx1 gene therapy in the CMI-AdTrx1 and DMI-AdTrx1 groups resulted in a thicker infarct containing islands of viable cardiac tissue with evidently lesser scar extension. (C) Oxidative stress (by DHE staining for O2·− production), representative images show DHE stained myocardial sections from the different groups 4 days after MI and intervention, Scale bar = 50μm. (D) Graph represents the quantitative analysis (arbitrary units) of the average fluorescent intensity of DHE fluorescence. Trx1 gene therapy in the DMI-AdTrx1 groups resulted in significant reduction in oxidative stress in the diabetic ischemic myocardium. The average fluorescent intensity of DHE fluorescence was calculated from 5–8 images per heart and 3–4 hearts per group. CS represents control Sham, CMI represent control group with MI, CMI-AdLacZ represents CMI rats injected with Ad.LacZ, CMI-AdTrx1 represents CMI rats injected with Ad.Trx1, DS represents diabetic Sham, DMI represent diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ and DMI-AdTrx1 represent DMI rats injected with Ad.Trx1. ψp≤0.05 when DS compared to CS, *p≤0.05 when compared with CMI, #p≤0.05 when compared with CMI-AdLacZ, †p≤0.05 when compared with DMI and ♣p≤0.05 when compared with DMI-AdLacZ.
30 days after MI and gene therapy the hearts were harvested and Masson’s trichrome staining was performed on paraffin embedded tissue sections to visualize the extent of collagen deposition and myocardial fibrosis (Figure 2B). The infarct scar extension and the thinning of the infarct were evident 30 days after MI in the DMI groups compared to the CMI group. There was no marked difference in the extent of collagen deposition and myocardial fibrosis in the Ad.LacZ treated CMI-AdLacZ and DMI-AdLacZ when compared to their respective non-treated controls, CMI and DMI respectively. Evidently, there was a less prominent scar extension, collagen deposition and fibrosis in the Ad.Trx1 treated non-diabetic (CMI-AdTrx1) and diabetic rats (DMI-AdTrx1) compare to the CMI and DMI rats, respectively.
Trx1 gene therapy reduced oxidative stress (ROS) in the myocardium
The amount of ROS mediated oxidative stress was determined 4 days after MI and gene therapy by the evaluation of superoxide anion (O2·−) formation by means of dihydroethidium (DHE) staining 24 (n=3–4/group, Figure 2C-2D). MI alone increased the levels of ROS in the non diabetic MI (CMI) and non-diabetic Ad.LacZ treated MI (CMI-AdLacZ) groups compared to the non-diabetic sham (CS) group. However, this increase in myocardial ROS was exacerbated in all the diabetic MI groups compared to its respective non-diabetic controls. Myocardial ROS levels significantly increased in the diabetic sham (DS) group compared to the non-diabetic sham (CS) group. The induction of MI significantly intensified the formation of ROS in the DMI and DMI-AdLacZ groups compared to the DS group. Though there was a reduction in MI induced ROS levels in the CMI-AdTrx1 group compared to the CMI and CMI-AdLacZ groups, the change was not significant. However, Ad.Trx1 gene therapy significantly reduced the ROS levels in the treated DMI-AdTrx1 myocardium compared to the DMI and DMI-AdLacZ myocardium thereby reducing ROS mediated oxidative stress (Figure 2C–2D).
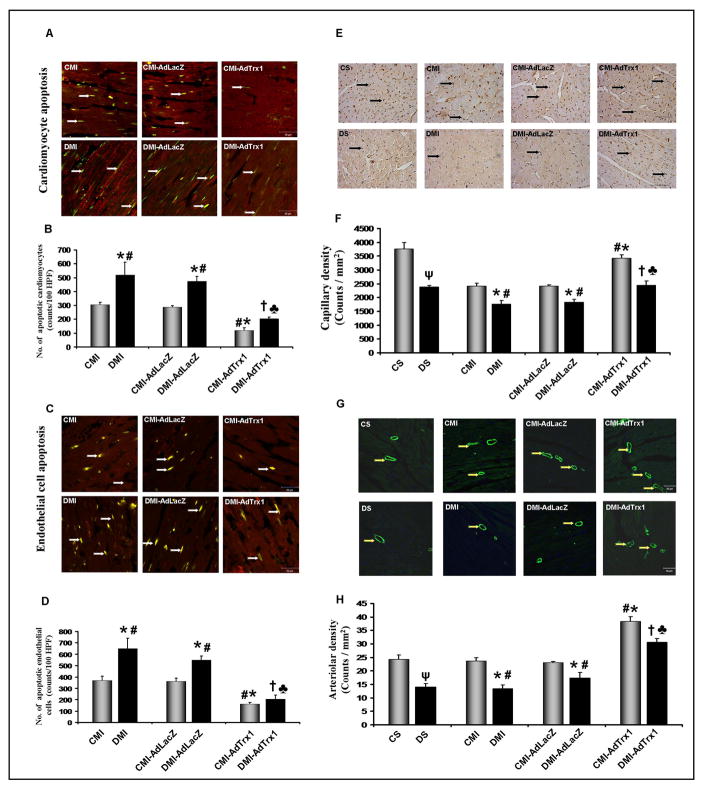
Trx1 gene therapy significantly reduced cardiomyocyte and endothelial cell apoptosis
In order to understand whether the Trx1 gene therapy was paralleled by reduction in cardiomyocyte and endothelial cell apoptosis we performed the TUNEL assay followed by antibody staining with either anti-α-sarcomeric actin or anti-von-Willebrand factor (Figure 3A–3D) was used to measure (counts/100HPF) the cardiomyocyte and endothelial cell apoptosis, respectively (n=4/group). We have observed significant increase in the apoptotic cardiomyocytes (520±92.6 vs 306±16.3) and endothelial cells (650±90.5 vs 369±38.7) in the DMI group when compared to the CMI group. There was no significant difference in the number of apoptotic cardiomyocytes or endothelial cells between the Ad.LacZ treated CMI-AdLacZ or DMI-AdLacZ group when compared to their respective non-treated controls (CMI or DMI). However, Trx1 gene therapy significantly reduced the cardiomyocyte (119±21.5 vs 306±16.3) and endothelial cell apoptosis (163±15.4 vs 369±38.7) in the CMI-AdTrx1 group when compared to the CMI group. Similarly, Trx1 gene therapy in the DMI-AdTrx1 group significantly reduced cardiomyocyte (204±13.1 vs 520±92.6) and endothelial cell apoptosis (208±32.8 vs 650±90.5) when compared to the DMI group (Figure 3A–3D).
Figure 3.
Effect of Trx1 gene therapy on: - (A and B) Cardiomyocyte Apoptosis, A) Representative digital micrographs showing cardiomyocyte apoptosis in the different experimental groups, Scale bar = 20μm. B) Graph represents the quantitative analysis of cardiomyocyte apoptosis 4 days after MI in counts/100HPF. (C and D) Endothelial Cell Apoptosis C) Representative digital micrographs showing endothelial cell apoptosis in the different experimental groups, Scale bar = 20μm. D) Graph represents the quantitative analysis of endothelial cell apoptosis 4 days after MI in counts/100HPF. (E and F) Capillary Density, E) Representative digital micrographs showing capillary density/CD31 immunostaining in the different experimental groups, Scale bar = 50μm. F) Graph represents the quantitative analysis of capillary density in counts/mm2. (G and H) Arteriolar Density, G) Representative digital micrographs showing arteriolar density/α-Smooth Muscle Actin immunostaining in the different experimental groups, Scale bar = 50μm. H) Graph represents the quantitative analysis of arteriolar density in counts/mm2. Values are mean ± SEM. (n = 4/group). CS represents control Sham, CMI represent control group with MI, CMI-AdLacZ represents CMI rats treated with Ad.LacZ, CMI-AdTrx1 represents CMI rats treated with Ad.Trx1, DS represents diabetic Sham, DMI represent diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ and DMI-AdTrx1 represent DMI rats injected with Ad.Trx1. ψp ≤ 0.05 when DS compared to CS, *p ≤ 0.05 when compared with CMI, #p ≤ 0.05 when compared with CMI-AdLacZ, †p ≤ 0.05 when compared with DMI and ♣p ≤ 0.05 when compared with DMI-AdLacZ.
Trx1 gene therapy increased capillary and arteriolar density
We performed immunohistochemical analysis for CD31/PECAM-1 (an endothelial cell marker) for capillary density (counts/mm2) and α-Smooth Muscle Actin for arteriolar density (counts/mm2) (n=4/group, Figure 3E–3H). There was significant reduction in the capillary density (1773±126 vs 2415±105, Figure 3E–3F) and arteriolar density (13.4±1.4 vs 23.7±1.3, Figure 3G–3H) in the DMI group as compared to the CMI group. There was no significant difference in the capillary or arteriolar density between the Ad.LacZ treated CMI-AdLacZ or DMI-AdLacZ group when compared to their respective non-treated controls (CMI or DMI). However, Trx1 gene therapy significantly increased the capillary (3433±121 vs 2415±49, Figure 3E–3F) and arteriolar (38.4±1.7 vs 23.7±1.3, Figure 3G–3H) density in the CMI-AdTrx1 group when compared to the CMI group. Similarly, significant increase in the capillary density (2450±153 vs 1773±126, Figure 3E–3F) and arteriolar density (30.6±1.5 vs 13.4±1.4, Figure 3G–3H) was observed upon Trx1 gene therapy in DMI-AdTrx1 group as compared to the DMI group.
Molecular Basis for the Angiogenic and Cardioprotective Effect of Trx1 Gene Therapy in the Diabetic Ischemic Myocardium
Trx1 gene therapy significantly increased the expression of Trx1, HO-1 & VEGF expression
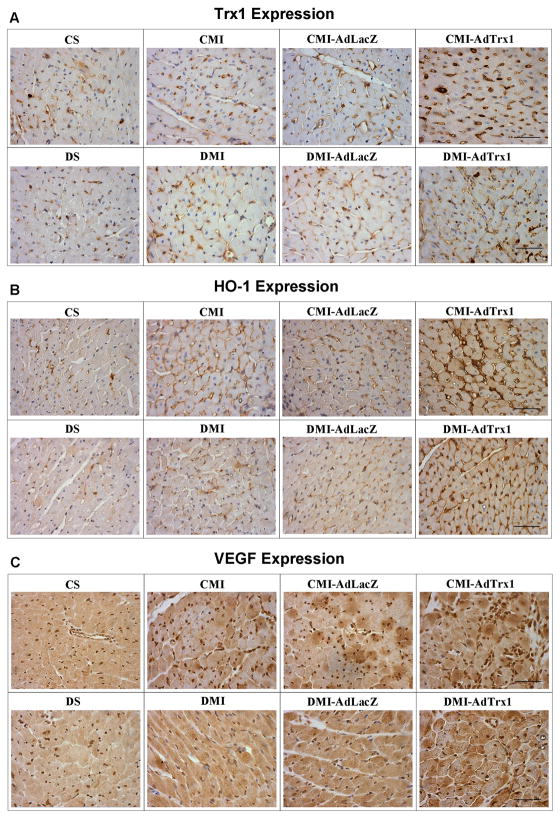
Immunohistochemical analysis was carried out for protein expression of Trx1, HO-1 and VEGF, 4 days after MI and gene therapy (n=3–4/group). We have observed evident reduction in the expression of Trx1 (Figure 4A), HO-1(Figure 4B) and VEGF (Figure 4C) in the diabetic sham operated (DS) and MI induced (DMI and DMI-AdLacZ) groups when compared to their respective non-diabetic sham operated (CS) and MI induced (CMI and CMI-AdLacZ) controls. However there was an evident increased in the expression of Trx1, HO-1 and VEGF in the Ad.Trx1 administered CMI-AdTrx1 group when compared to the CMI or CMI-AdLacZ groups. Similarly, Trx1 gene therapy has shown increased expression of these proteins in DMI-AdTrx1 group as compared to DMI and DMI-AdLacZ groups (Figure 4A–4C).
Figure 4.
Effect of Trx1 gene therapy on A) Trx1, B) HO-1 and C) VEGF expression (immunohistochemical analysis by DAB staining) 4 days after MI and gene therapy. (n = 3–4/group). CS represents control Sham, CMI represent control group with MI, CMI-AdLacZ represents CMI rats treated with Ad.LacZ, CMI-AdTrx1 represents CMI rats treated with Ad.Trx1, DS represents diabetic Sham, DMI represent diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ and DMI-AdTrx1 represent DMI rats injected with Ad.Trx1. Scale bar = 50μm.
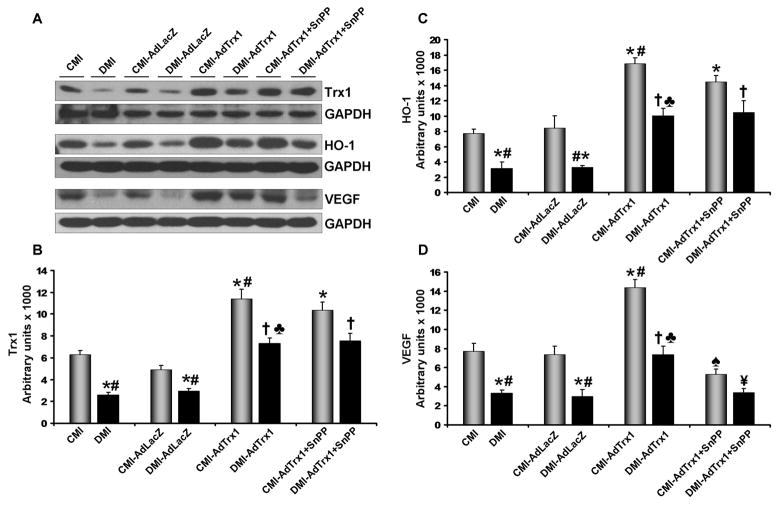
Our Western blot analysis, 4 days after gene therapy (n=3–4/group) also revealed that Trx1 gene therapy in the CMI-AdTrx1 group significantly increased the expression of Trx1 (1.8 fold, Figure 5A and 5B), HO-1 (2.2 fold, Figure 5A and 5C) and VEGF (1.9 fold, Figure 5A and 5D) when compared to the CMI group. Ad.LacZ treatment in the CMI-AdLacZ or DMI-AdLacZ group did not show any significant difference in the expression of these proteins when compared to their respective non-treated controls (CMI and DMI). The expression of Trx1 (2.4 fold), HO-1 (2.5 fold) and VEGF (2.3 fold) was found to be significantly decreased in DMI group as compared to the CMI group. However, Trx1 gene therapy significantly increased the expression of Trx1 (2.8 fold), HO-1 (3.2 fold) and VEGF (2.2 fold) in DMI-AdTrx1 when compared to the DMI group (Figure 5A and 5D).
Figure 5.
Effect of Trx1 gene therapy on Trx1, HO-1 and VEGF expression (Western blot analysis): - (A) Representative Western blots show the expression of Trx1, HO-1 and VEGF. Bar graphs (B), (C) and (D) represent the quantitative difference in expression of Trx1, HO-1 and VEGF respectively in arbitrary units. There was significant decrease in the expression of Trx1, HO-1 and VEGF in the DMI and DMI-AdlacZ compared to the CMI and CMI-AdLacZ groups, respectively. There was no significant difference in the expression of these proteins in the Ad.LacZ treated group compared to the respective non-treated MI groups. Trx1 gene therapy significantly increased the expression of Trx1, HO-1 and VEGF when compared to non-treated and Ad.LacZ treated MI groups, in both non-diabetic and diabetic myocardium. SnPP treatment significantly reduced the Trx1 gene therapy mediated increase in VEGF in both non-diabetic and diabetic myocardium, while there was no significant difference in the expression of Trx1 and HO-1 in these groups. Values are expressed as mean ± SEM. (n = 3–4/group). CMI represent control group with MI, CMI-AdLacZ represents CMI rats injected with Ad.LacZ, CMI-AdTrx1 represents CMI rats injected with Ad.Trx1, DMI represents diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ, DMI-AdTrx1 represent DMI rats injected with Ad.Trx1, CMI-AdTrx1 + SnPP represents the non-diabetic animals that were treated with SnPP and then subjected to MI and Ad.Trx1 treatment and DMI-AdTrx1 + SnPP represents the diabetic animals that were treated with SnPP and then subjected to MI and Ad.Trx1 treatment. *p ≤ 0.05 when compared with CMI, #p ≤ 0.05 when compared with CMI-AdLacZ, †p ≤ 0.05 when compared with DMI, ♣p ≤ 0.05 when compared with DMI-AdLacZ, ♠p ≤ 0.05 when compared to CMI-AdTrx1 and ¥p ≤ 0.05 when compared to DMI-AdTrx1.
SnPP treatment significantly reduced Trx1 mediated VEGF expression (in vivo)
In order to examine whether HO-1 mediated the Trx1 induced VEGF expression in the Ad.Trx1 treated animals we intramyocardially administered Ad.Trx1 after MI to SnPP treated non-diabetic and diabetic animals (n=3/group). There was no significant difference in the expression of Trx1 and HO-1 between the SnPP treated and non-treated (in both non-diabetic and diabetic group) animals that received the Ad.Trx1 gene therapy. However, we have observed a significant downregulation of VEGF (2.7 and 2.2 fold, respectively) inspite of the increase in the expression of Trx1 and HO-1 in the SnPP treated non-diabetic (CMI-AdTrx1 + SnPP) and diabetic (DMI-AdTrx1 + SnPP) animals even after Ad.Trx1 injections when compared to the non-diabetic (CMI-AdTrx1) and diabetic (DMI.AdTrx1) animals which were treated with Ad.Trx1 but not SnPP. (Figure 5A and 5D).
Trx1 gene therapy increased the expression of anti-apoptotic p38MAPKβ while reducing the expression of pro-apoptotic p-JNK and p38MAPKα
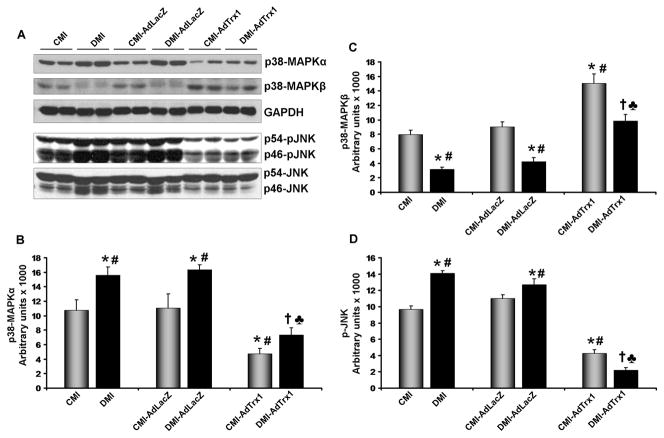
In the present study we have evaluated the expression of the phosphorylated JNK and levels p38MAPK (α and β) proteins (n=3–4/group, Figure 6). Trx1 gene therapy in the CMI-AdTrx1 group significantly increased the expression of p38MAPKβ (1.9 fold, Figure 6A and 6C), and decreased the expression of p38MAPKα (2.3 fold, Figure 6A and 6B) and p-JNK (2.3 fold, Figure 6A and 6D) when compared to the CMI group. Ad.LacZ treatment in the CMI-AdLacZ or DMI-AdLacZ group did not show any significant difference in the expression of these proteins when compared to their respective non-treated controls (CMI and DMI). Significant increase in the activation of pro-apoptotic p-JNK (1.5 fold) and p38MAPKα (1.5 fold) expression and downregulation of anti-apoptotic p38MAPKβ (2.5 fold) was observed in the DMI as compared to the CMI. However, Trx1 gene therapy increased the expression of p38MAPKβ (3.1 fold), and decreased the expression of p-JNK (6.4 fold) and p38MAPKα (2.1 fold) in the DMI-AdTrx1 when compared to DMI group (Figure 6A–6D).
Figure 6.
Effect of Trx1 gene therapy on p38MAPKα, p38MAPKβ and p-JNK expression (Western blot analysis): - (A) Representative Western blots show the expression of p38MAPKα, p38MAPKβ and p-JNK Bar graphs (B), (C) and (D) represent the quantitative difference in expression of p38MAPKα, p38MAPKβ and p-JNK, respectively. There was significant increase in the expression of pro-apoptotic p38MAPKα and p-JNK and significant decrease in the expression of anti-apoptotic p38MAPKβ in the DMI and DMI-AdLacZ groups compared to the CMI and CMI-AdLacZ groups, respectively. There was no significant difference in the expression of these proteins in the Ad.LacZ treated group compared to the respective non-treated MI groups. Trx1 gene therapy significantly increased the expression of p38MAPKβ while decreasing the expression of p38MAPKα and p-JNK when compared to non-treated and Ad.LacZ treated MI groups, in both non-diabetic and diabetic myocardium. Values are mean ± SEM. (n = 3–4/group) CMI represent control group with MI, CMI-AdLacZ represents CMI rats injected with Ad.LacZ, CMI-AdTrx1 represents CMI rats injected with Ad.Trx1, DMI represents diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ and DMI-AdTrx1 represent DMI rats injected with Ad.Trx1. *p ≤0.05 when compared with CMI, #p ≤ 0.05 when compared with CMI-AdLacZ, †p ≤0.05 when compared with DMI and ♣p ≤ 0.05 when compared with DMI-AdLacZ.
Preservation of myocardial functions after acute MI in the diabetic myocardium through Trx1 gene therapy
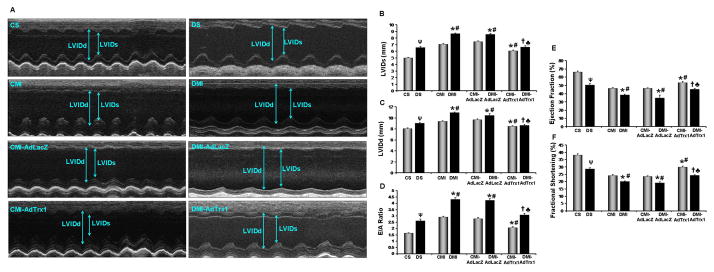
Echocardiographic assessment of myocardial function was performed 30 days after the surgical intervention, MI and gene therapy in the experimental animals (n=4–5/group). The representative picture (Figure 7A–7F) shows the parasternal short axis view in M-mode. The quantitative representations of echocardiographic measurements were given in Figure 7B–7F. There was significant functional disorder in all the diabetic groups compared to their respective non-diabetic controls. Trx1 gene therapy significantly improved the myocardial functions both in the non-diabetic as well as diabetic groups compared to their respective non-treated or Ad.LacZ treated controls.
Figure 7.
Effect of Trx1 gene therapy on left ventricular myocardial functions (echocardiography). (A) Representative echocardiograph pictures of parasternal short axis images, 30 days after MI and gene therapy. Bar graphs represent: - (B) Left Ventricular Inner Diameter in systole (LVIDs, in mm); (C) Left Ventricular Inner Diameter in diastole (LVIDd, in mm); (D) E/A ratio; (E) Ejection Fraction (EF in %) and (F) Fractional Shortening (FS in %). There was significant diastolic (as evidenced by increased E/A ratio) and systolic (as evidenced by reduced EF and FS) functional disorder in the DMI and DMI-AdLacZ groups compared to the CMI and CMI-AdLacZ groups, respectively. There was no significant difference in the myocardial functions in the Ad.LacZ treated group compared to the respective non-treated MI groups. Trx1 gene therapy significantly improved both diastsolic and systolic functional parameters when compared to non-treated and Ad.LacZ treated MI groups, in both non-diabetic and diabetic myocardium. Values are mean ± SEM. (n = 4–5/group). CS represents control Sham, CMI represent control group with MI, CMI-AdLacZ represents CMI rats injected with Ad.LacZ, CMI-AdTrx1 represents CMI rats injected with Ad.Trx1, DS represents diabetic Sham, DMI represent diabetic group with MI, DMI-AdLacZ represent DMI rats injected with Ad.LacZ and DMI-AdTrx1 represent DMI rats injected with Ad.Trx1. ψp ≤ 0.05 when DS compared to CS, *p ≤ 0.05 when compared with CMI, #p ≤ 0.05 when compared with CMI-AdLacZ, †p ≤ 0.05 when compared with DMI and ♣p ≤ 0.05 when compared with DMI-AdLacZ.
Improved contraction along with improved end-systolic and diastolic dimensions were observed with Trx1 treatment which preserves the contractile function of the heart and suggests attenuated remodeling in the treated animals. Increased Left Ventricular Internal Diameter in systole (LVIDs, 8.7±0.1mm vs 7.1±0.1mm) and Left Ventricular Internal Diameter in diastole (LVIDd, 10.9±0.1mm vs 9.3±0.1mm) was observed in DMI rats as compared to CMI (Figure 7B and 7C). Trx1 gene therapy reduced the LVIDs (6.6±0.2mm vs 8.6±0.1mm) and LVIDd (8.6±0.2mm vs 10.9±0.1mm) in DMI-AdTrx1 group as compared to DMI though significant difference was not observed in DMI-AdLacZ group which shows the functional recovery of the ischemic myocardium following Trx1 gene delivery (Figure 7B and 7C). Improved systolic parameters were observed in Trx1 treated rats compared to DMI controls, as assessed by Ejection Fraction (EF) and Fractional Shortening (FS). Ejection Fraction as well as Fractional Shortening was improved in DMI-AdTrx1 rats (EF=45.3±0.9% vs 38.5±0.8%, FS=24.3±0.4% vs 20±1.4%), compared to DMI controls (Figure 7E and 7F). There was no significant difference was observed in the EF or FS in DMI-AdLacZ group as compared to DMI group.
There was also significant diastolic dysfunction as evidenced by higher E/A ratio, in the diabetic groups compared to the respective non-diabetic controls. After 30 days of MI the DMI-AdTrx1 group showed a significant improvement in diastolic function as evidenced by the E/A ratio (3.1±0.1 vs 4.3±0.1) as compared to the DMI group (Figure 7D).
Discussion
Our present study is the first to document that inducing the expression of Trx1 can rescue the diabetic myocardium from diabetes/oxidative stress related impairment of myocardial angiogenesis by reducing oxidative stress and through the enhanced expression of HO-1 and VEGF. In addition, we have observed decreased expression of pro-apoptotic proteins such as p-JNK and p38MAPKα and increased expression of anti-apoptotic protein p38MAPKβ in the DMI-AdTrx1 as compared to DMI. Associated with these molecular changes we have also observed reduced myocyte and endothelial cell apoptosis, reduced fibrosis and increased capillary and arteriolar density with significant improvement of the myocardial functions as assessed by echocardiography. Our preclinical results indicate that the suppression of oxidative stress by Trx1 induction has beneficial effects in the treatment of diabetes related cardiac failure.
Preclinical and clinical observations have linked diabetes mediated oxidative stress to increased myocyte and endothelial cell apoptosis and reduction in the anti-oxidative and angiogenic factors 4, 25. In the current study we have observed that Trx1 gene therapy in the ischemic diabetic myocardium was able to significantly reduce oxidative stress as evidenced from the reduction in ROS generation. This reduction in ROS generation can be correlated to the reduction in myocyte and endothelial cell apoptosis and increase in the expression of angiogenic VEGF.
The pro-angiogenic effect of resveratrol through the activation of Trx1, HO-1 and VEGF was blocked by SnPP, a specific inhibitor of HO-1 activity 16. HO-1 is a stress inducible enzyme system that catalyzes the breakdown of pro-oxidant heme into biliverdin, carbon monoxide and iron and is well known for its anti-apoptotic and anti-inflammatory activities 26, 27. The expression and activity of HO-1 has been shown to be impaired in a diabetic milieu 25. It was reported that HO-1 can inhibit post-myocardial infarction remodeling and restore ventricular function 28. Evidences suggest that HO-1 promotes neovascularization in the ischemic heart by the induction of VEGF 29. Rivard A and colleagues have reported that the impairment in new blood vessel formation in hind limb ischemia model of diabetic mice was a result of reduced expression of VEGF and that VEGF gene therapy could induce neovascularization in this diabetic mice model 20. It was also shown that adenovirus mediated gene transfer of VEGF could promote wound healing by the induction of angiogenesis in CD1 diabetic mice 21.
Upon Trx1 gene therapy we have observed a significant increase in the expression of Trx1, HO-1 and VEGF in the DMI-AdTrx1 group as compared to the DMI group thereby suggesting that Trx1 gene therapy has proven to be effective in inducing the expression of these proteins that were downregulated in a diabetic condition. However, in the SnPP treated, Ad.Trx1 injected non-diabetic and diabetic MI animals there was significant downregulation of VEGF even though there was no significant difference in the expression of Trx1 and HO-1 when compared to the CMI-AdTrx1 and DMI-AdTrx1 groups, respectively, suggesting that the Trx1 induced expression of VEGF is mediated by HO-1.
The fact that Trx1 gene therapy significantly reduced the number of apoptotic cardiomyocytes and endothelial cells in the DMI-AdTrx1 group as compared to the DMI group suggests that Trx1 gene therapy significantly aids in the rescue of the diabetic myocardium from MI induced degeneration and also might support the neovascularization process. The decrease in the number of apoptotic cardiomyocytes and endothelial cells in the treatment group can be correlated to the increase in the expression of Trx1 and HO-1 both of which have been shown to be antioxidative, anti-apoptotic and growth stimulatory 9, 10, 28.
We have also observed decreased expression of p-JNK and p38MAPKα (pro-apoptotic signal) and increased p38MAPKβ (anti-apoptotic signal) in the DMI-AdTrx1 which also might have resulted in decreased cardiomyocyte and endothelial cell apoptosis. Several reports have shown that ischemia and doxorubicin induced cardiomyocyte apoptosis were attenuated by pharmacological p38 inhibition 30, 31. Four splice variants of p38 family such as p38MAPKα, β, γ and δ were identified 32. Differential activation of the p38MAPKα and p38MAPKβ has been reported 33. While p38MAPKα is shown to contribute to pro-apoptotic pathway, p38MAPKβ activation through ischemic preconditioning is shown to decrease the infarct size which demonstrated the anti-apoptotic pathway activation 33. Moreover, the p38MAPKα dominant negative mice were reported to be less susceptible to ischemia reperfusion injury and subsequent apoptosis.34 In the present study we have observed increased activation of p38MAPKβ upon Ad.Trx1 treatment which might have resulted in decreased apoptosis and subsequent reduction in myocardial fibrosis.
We have also documented that the capillary and arteriolar density after 30 days of MI is significantly increased upon Trx1 gene therapy in the DMI-AdTrx1 group as compared to the DMI group. This can be correlated to the increase in the expression Trx1, HO-1 and VEGF in the treatment group. The anti-apoptotic and growth stimulatory Trx1 and HO-1 might have reduced endothelial cell apoptosis while the increase in VEGF should have stimulated the neovascularization process in the treatment group.
Therefore, the observed reduction in ROS, cardiomyocyte and endothelial cell apoptosis and the improvement in the capillary and arteriolar density in the treatment group might have led to reduction in the infarct scar extension and fibrosis in the Ad.Trx1 treatment groups. These ultimately can explain the fact that there was significant improvement in the left ventricular myocardial functions (assessed by echocardiogram) such as LVIDs, LVIDd, E/A ratio, EF and FS in the DMI-AdTrx1 group as compared to the DMI group. Reports have linked Type I diabetes to early diastolic dysfunction, progressive systolic dysfunction and subsequent heart failure 35. E/A ratio, an index of diastolic dysfunction has been shown to be significantly reduced in Type I diabetic patients 36. However, in cases of diabetes mediated advanced heart failure the E/A ratio tends to increase 36. In our current study the severity of heart failure can be assessed by the significantly higher E/A ratio in all the diabetic groups compared to the respective non-diabetic controls. However, our Trx1 gene therapy significantly reduced the magnitude of diastolic dysfunction in the diabetic rats. The magnitude and duration of hyperglycemia in a hypoinsulinemic setting is known to determine the extent of progressive systolic dysfunction in Type I diabetic subjects 35. There was significant systolic functional disorder in the diabetic myocardium after MI. However, Trx1 gene therapy significantly improved the systolic functional parameters in the treated diabetic MI rats compared to the non-treated diabetic MI rats.
In conclusion, our preclinical findings indicated that a precise balanced antioxidant system is essential for inducing functional neovasculature in diabetic ischemic myocardium. Our data is supportive of the concept that in a diabetic milieu the impaired angiogenesis is not only caused by the downregulation of angiogenic growth factors but also is dependent on the excessive oxidative stress and the lack of responsive antioxidant systems. Therefore, for the first time we propose to treat diabetes mediated impairment of angiogenesis by normalizing and stabilizing the redox microenvironment in the myocardium by means of Ad.Trx1 gene therapy thereby reducing oxidative stress, cell death and inducing neovessel formation and maturation, subsequently reducing ventricular remodeling after an MI. Our novel and unique approach of Trx1 gene delivery can prove to be a strategic therapeutic modality in the treatment of diabetes related cardiac failure and may ultimately improve the quality of life and may mitigate the disease progression in the affected subjects.
Clinical Perspective
The higher incidence of post-MI angina, larger infarcts, severity of myocardial functional disorder as well as the unfavorable prognosis among diabetic individuals who develop such complications has been correlated to oxidative stress mediated impairment of angiogenesis. Moreover, diabetes reflects a challenging condition where the usual revascularization techniques such CABG and PTCA tend to fail thereby leaving many of the diabetic-IHD subjects with no-option for treatment. Therefore, maintaining a balanced redox status in the microenvironment might aid in alleviating many of the diabetic mediated complications, especially those related to impaired angiogenesis. Our preclinical findings are supportive of the concept that in a diabetic milieu, impaired angiogenesis is not only caused by the downregulation of angiogenic growth factors but also is dependent on the excessive oxidative stress and the lack of responsive antioxidant systems. For the first time, our unique results have identified the in vivo angiogenic potential of Trx1 and substantiated its anti-oxidative and anti-apoptotic properties in the setting of MI in a diabetic milieu. We therefore propose to treat diabetes mediated impairment of angiogenesis by normalizing and stabilizing the redox microenvironment in the myocardium by means of Trx1 gene therapy thereby reducing oxidative stress, cell death and inducing neovessel formation and maturation, subsequently reducing ventricular remodeling after an MI. Our novel approach of Trx1 gene delivery can prove to be a strategic therapeutic modality in the treatment of diabetes related cardiac failure and may ultimately improve the quality of life and mitigate the disease progression in the affected subjects.
Supplementary Material
Acknowledgments
Funding Sources
This study was supported by the NIH grants HL-56803 and HL-69910 to Maulik N.
Footnotes
Disclosures
Samson Mathews Samuel: None
Mahesh Thirunavukkarasu: None
Suresh Varma Penumathsa: None
Srikanth Koneru: None
Lijun Zhan: None
Gautam Maulik: None
Perumana R Sudhakaran: None
Nilanjana Maulik: Research Grant: HL-56803 and HL-69910 to Maulik N., Amount: >= $ 10,000
References
- 1.Barzilay JI, Kronmal RA, Bittner V, Eaker E, Evans C, Foster ED. Coronary artery disease and coronary artery bypass grafting in diabetic patients aged > or = 65 years (report from the Coronary Artery Surgery Study [CASS] Registry) Am J Cardiol. 1994;74:334–339. doi: 10.1016/0002-9149(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 3.Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2003;29:579–585. doi: 10.1016/s1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- 4.Di Filippo C, Cuzzocrea S, Rossi F, Marfella R, D’Amico M. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24:77–87. doi: 10.1111/j.1527-3466.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Taniguchi-Ueda Y, Mori K, Yodoi J. A novel promoter sequence is involved in the oxidative stress-induced expression of the adult T-cell leukemia-derived factor (ADF)/human thioredoxin (Trx) gene. Nucleic Acids Res. 1996;24:2746–2752. doi: 10.1093/nar/24.14.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino Y, Shioji K, Nakamura H, Masutani H, Yodoi J. From oxygen sensing to heart failure: role of thioredoxin. Antioxid Redox Signal. 2007;9:689–699. doi: 10.1089/ars.2007.1575. [DOI] [PubMed] [Google Scholar]
- 8.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 11.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Shioji K, Nakamura H, Masutani H, Yodoi J. Redox regulation by thioredoxin in cardiovascular diseases. Antioxid Redox Signal. 2003;5:795–802. doi: 10.1089/152308603770380106. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ejima K, Layne MD, Carvajal IM, Nanri H, Ith B, Yet SF, Perrella MA. Modulation of the thioredoxin system during inflammatory responses and its effect on heme oxygenase-1 expression. Antioxid Redox Signal. 2002;4:569–575. doi: 10.1089/15230860260220067. [DOI] [PubMed] [Google Scholar]
- 15.Wiesel P, Foster LC, Pellacani A, Layne MD, Hsieh CM, Huggins GS, Strauss P, Yet SF, Perrella MA. Thioredoxin facilitates the induction of heme oxygenase-1 in response to inflammatory mediators. J Biol Chem. 2000;275:24840–24846. doi: 10.1074/jbc.M000835200. [DOI] [PubMed] [Google Scholar]
- 16.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin inudced diabetes: Role of nitric oxide, thioredoxin and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koneru S, Varma Penumathsa S, Thirunavukkarasu M, Vidavalur R, Zhan L, Singal PK, Engelman RM, Das DK, Maulik N. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med. 2008;12:2651–2664. doi: 10.1111/j.1582-4934.2008.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel SM, Akita Y, Paul D, Thirunavukkarasu M, Zhan L, Sudhakaran PR, Li C, Maulik N. Co-administration of Adenoviral VEGF and Ang-1 Enhances Vascularization and Reduces Ventricular Remodeling in the Infarcted Myocardium of Type I Diabetic Rats. Diabetes. 2010;59:51–60. doi: 10.2337/db09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–1277. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- 22.Koneru S, Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik N. Thioredoxin-1 gene delivery induces heme oxygenase-1 mediated myocardial preservation after chronic infarction in hypertensive rats. Am J Hypertens. 2009;22:183–190. doi: 10.1038/ajh.2008.318. [DOI] [PubMed] [Google Scholar]
- 23.Ago T, Yeh I, Yamamoto M, Schinke-Braun M, Brown JA, Tian B, Sadoshima J. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid Redox Signal. 2006;8:1635–1650. doi: 10.1089/ars.2006.8.1635. [DOI] [PubMed] [Google Scholar]
- 24.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D’Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–810. doi: 10.2337/diabetes.54.3.803. [DOI] [PubMed] [Google Scholar]
- 26.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 27.Wagener FA, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102:521–528. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. Faseb J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 29.Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Kang YJ, Zhou Z-X, Wang G-W, Buridi A, Klein JB. Suppression by Metallothionein of Doxorubicin-induced Cardiomyocyte Apoptosis through Inhibition of p38 Mitogen-activated Protein Kinases. J Biol Chem. 2000;275:13690–13698. doi: 10.1074/jbc.275.18.13690. [DOI] [PubMed] [Google Scholar]
- 31.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue T-L. Inhibition of p38 Mitogen-Activated Protein Kinase Decreases Cardiomyocyte Apoptosis and Improves Cardiac Function After Myocardial Ischemia and Reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 32.Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 33.Sumbayev VV, Yasinska IM. Regulation of MAP kinase-dependent apoptotic pathway: implication of reactive oxygen and nitrogen species. Arch Biochem Biophys. 2005;436:406–412. doi: 10.1016/j.abb.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Patten RD, Karas RH. Cardiomyocyte apoptosis in the ischemic and failing heart. Drug Discovery Today: Disease Mechanisms. 2005;2:47–53. [Google Scholar]
- 35.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 36.Young LH. Diastolic function and type 1 diabetes. Diabetes Care. 2004;27:2081–2083. doi: 10.2337/diacare.27.8.2081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.