Abstract
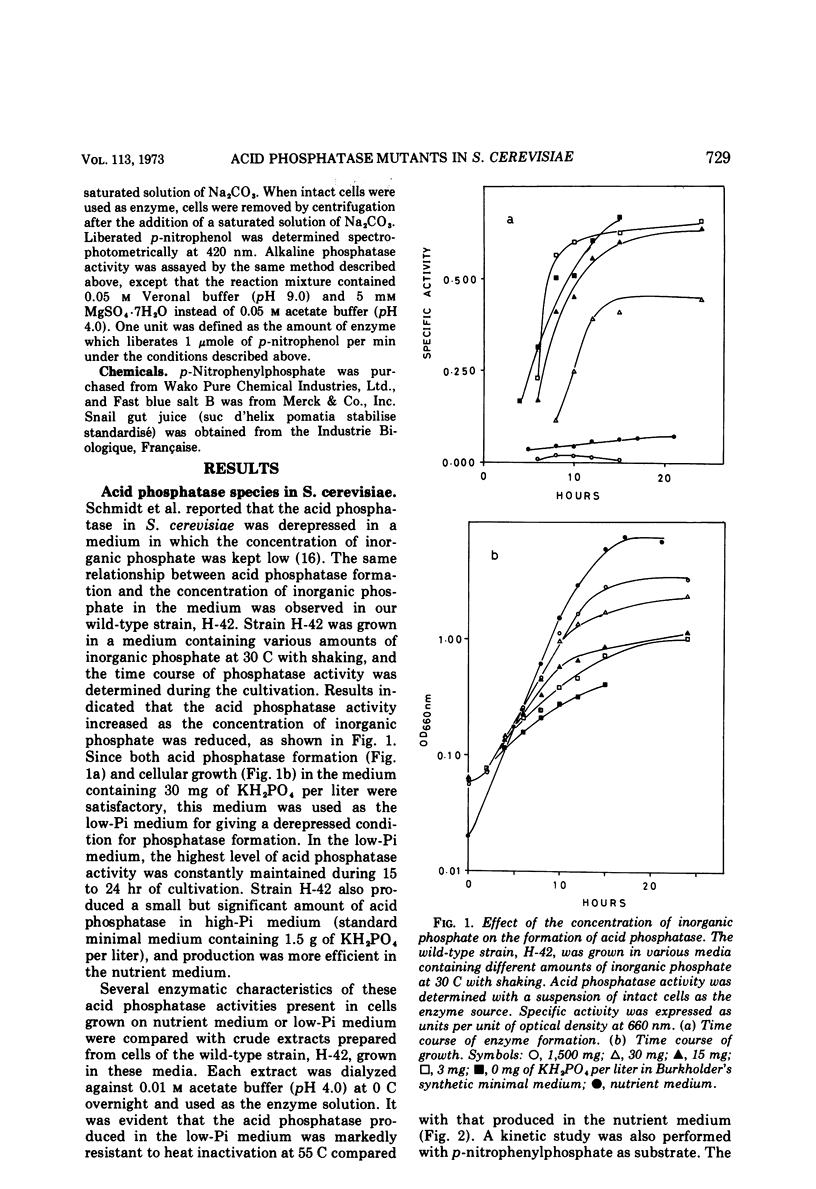
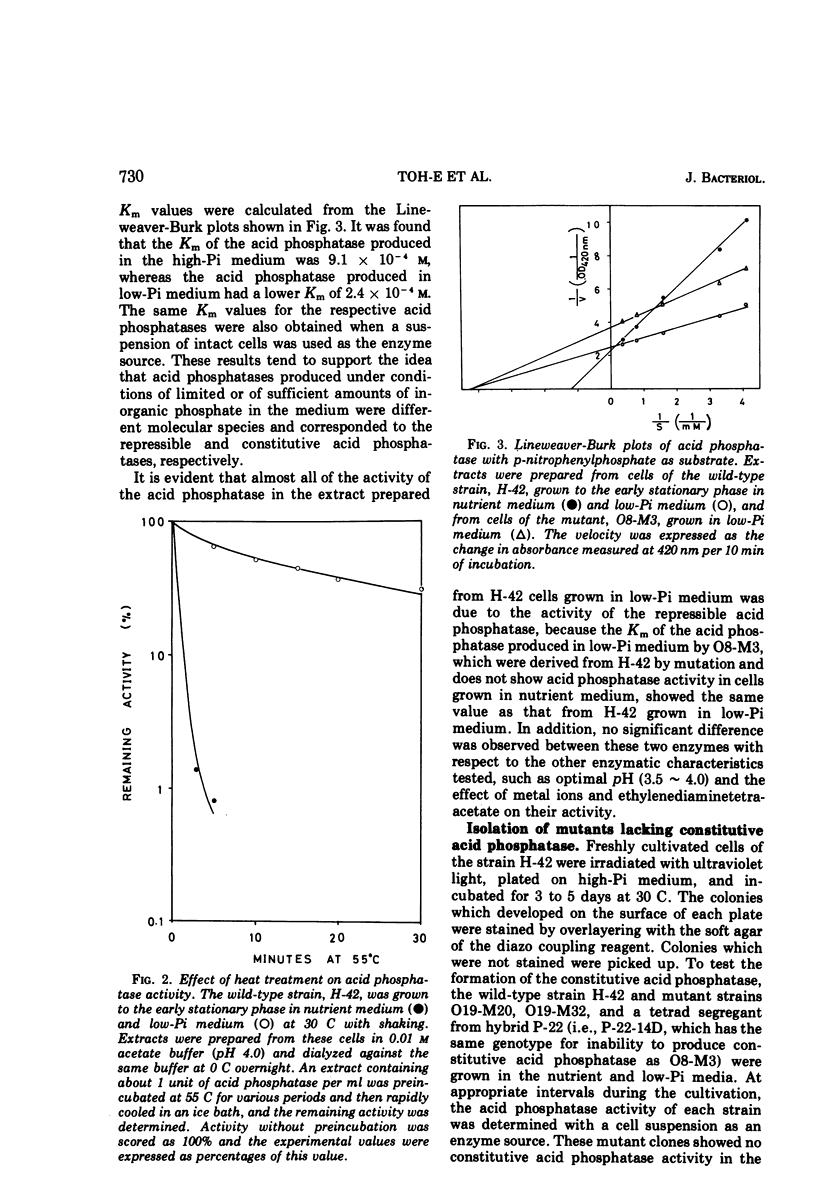
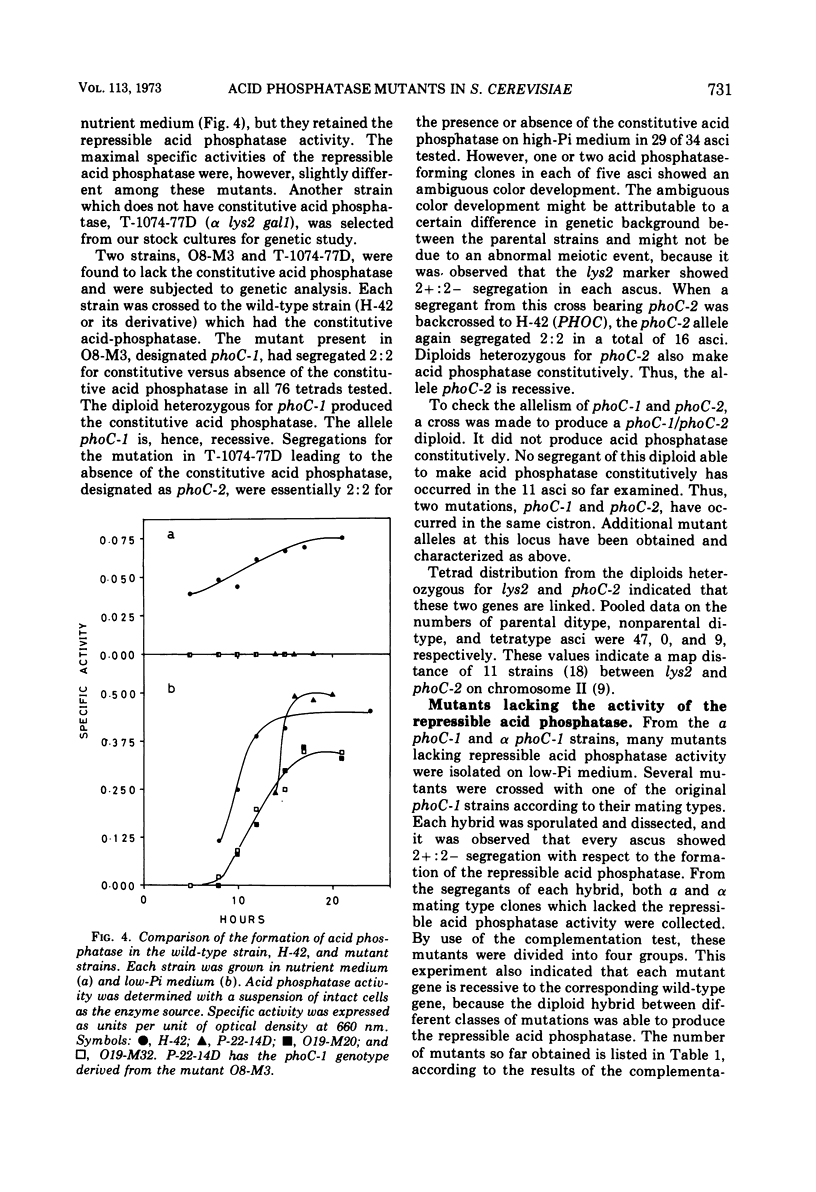
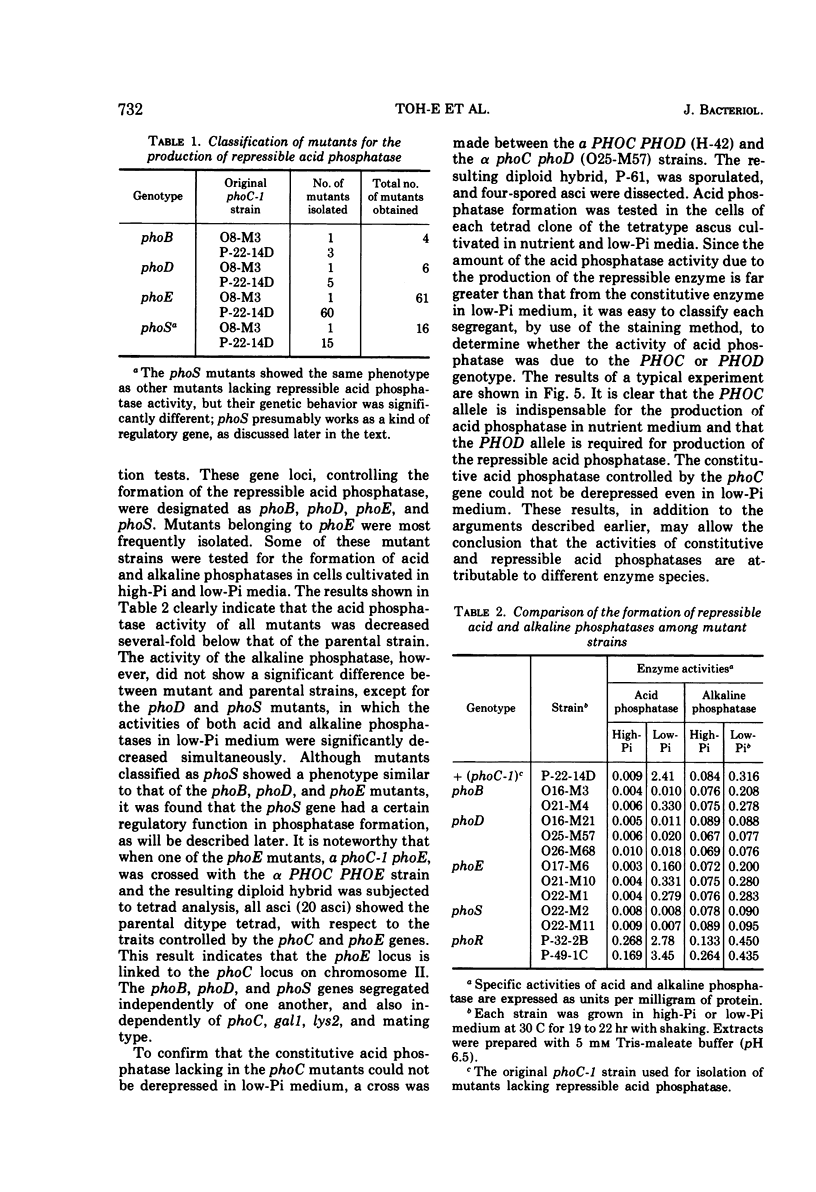
Saccharomyces cerevisiae strain H-42 seems to have two kinds of acid phosphatase: one which is constitutive and one which is repressible by inorganic phosphate. The constitutive enzyme was significantly unstable to heat inactivation, and its Km of 9.1 × 10−4m for p-nitrophenylphosphate was higher than that of the repressible enzyme (2.4 × 10−4m). The constitutive and the repressible acid phosphatases are specified by the phoC gene and by the phoB, phoD, or phoE gene, respectively. Results of tetrad analysis suggested that the phoC and phoE genes are linked to the lys2 locus on chromosome II. Since both repressible acid and alkaline phosphatases were affected simultaneously in the phoR, phoD, and phoS mutants, it was concluded that these enzymes were under the same regulatory mechanism or that they shared a common polypeptide. The phoR mutant produced acid phosphatase constitutively, and the phoR mutant allele was recessive to its wild-type counterpart. The phoS mutant showed a phenotype similar to that of a mutant defective in one of the phoB, phoD, or phoE genes. However, the results of genetic analysis of the phoS mutant clearly indicated that the phoS gene is not a structural gene for either of the repressible acid and alkaline phosphatases, but is a kind of regulatory gene. According to the proposed model, the phoS gene controls the expression of the phoR gene, and inorganic phosphate would act primarily as an inducer for the formation of the phoR product which represses phosphatase synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUM J. J. OBSERVATIONS ON THE ACID PHOSPHATASES OF EUGLENA GRACILIS. J Cell Biol. 1965 Feb;24:223–234. doi: 10.1083/jcb.24.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORN G. GENETIC ANALYSIS OF THE PHOSPHATASES IN ASPERGILLUS NIDULANS. Genet Res. 1965 Feb;6:13–26. doi: 10.1017/s0016672300003943. [DOI] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Mortimer R. K. Genetic mapping of nonsense suppressors in yeast. Genetics. 1968 Dec;60(4):735–742. doi: 10.1093/genetics/60.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEGREN C. C., SHULT E. E. Mapping methods in tetrad analysis. I. Provisional arrangement and ordering of loci preliminary to map construction by analysis of tetrad distribution. Genetica. 1956;28(1-2):165–176. doi: 10.1007/BF01694317. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindegren C. C., Lindegren G. A New Method for Hybridizing Yeast. Proc Natl Acad Sci U S A. 1943 Oct 15;29(10):306–308. doi: 10.1073/pnas.29.10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyc J. F. A repressible acid phosphatase in Neurospora crassa. Biochem Biophys Res Commun. 1967 Apr 20;27(2):183–188. doi: 10.1016/s0006-291x(67)80059-7. [DOI] [PubMed] [Google Scholar]
- Nyc J. F., Kadner R. J., Crocken B. J. A repressible alkaline phosphatase in Neurospora crassa. J Biol Chem. 1966 Apr 10;241(7):1468–1472. [PubMed] [Google Scholar]
- SCHMIDT G., BARTSCH G., LAUMONT M. C., HERMAN T., LISS M. Acid phosphatase of bakers' yeast: an enzyme of the external cell surface. Biochemistry. 1963 Jan-Feb;2:126–131. doi: 10.1021/bi00901a022. [DOI] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Ishikawa T. Genetic control of the synthesis of repressible phosphatases in Neurospora crassa. Genetics. 1971 Nov;69(3):339–351. doi: 10.1093/genetics/69.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]


