Abstract
Dietary protein is theorized to hold both anabolic effects on bone and demineralizing effects mediated by the diet acid load of sulfate derived from methionine and cysteine. The relative importance of these effects is unknown but relevant to osteoporosis prevention. Post-menopausal women (n=161, mean±SD 67.9±6.0 y) were assessed for areal bone mineral density (aBMD) of lumbar spine (LS) and total hip (TH) using dual X-ray absorptiometry, and dietary intakes of protein, sulfur-containing amino acids and minerals using a USDA multiple-pass 24 h recall. The acidifying influence of the diet was estimated using the ratio of protein / potassium intake, the potential renal acid load (PRAL) and intake of sulfate equivalents from protein. aBMD was regressed onto protein intake, then protein controlled for estimated dietary acid load. A step-down procedure assessed potential confounding influences (weight, age, physical activity and calcium and vitamin D intakes). Protein alone did not predict LS aBMD (P=0.81); however, after accounting for a negative effect of sulfate (β=− 0.28, P<0.01), the direct effect of protein intake was positive (β=0.22, P=0.04). At the TH protein intake predicted aBMD (β=0.18, P=0.03), but R2 did not improve with adjustment for sulfate (P=0.83). PRAL and the protein / potassium ratio were not significant predictors of aBMD. Results suggest that protein intake is positively associated with aBMD, but benefit at the LS is offset by a negative impact of the protein sulfur acid load. If validated experimentally, these findings harmonize conflicting theories on the role of dietary protein in bone health.
Keywords: bone mineral density, protein, sulfur, diet acid load
Introduction
Recent literature reflects discordant views on the role of dietary protein in bone health (1). Protein appears to hold an anabolic influence on bone, mediated by bone-active hormones, particularly insulin-like growth factor-1 (1), and may increase calcium absorption (2). Conversely sulfate equivalents derived from methionine and cysteine metabolism are exchanged in the kidney for acid equivalents (3); such a dietary acid load has been demonstrated to cause bone demineralization in animals (4,5) and is associated with reduced bone mineral mass in humans (6,7).
It has been proposed that bone demineralization is promoted by a mild but chronic dietary acid load characteristic of the Western diet (7). This acid load can be characterized by nutrient intake as the estimated net endogenous acid production (NEAP), calculated using a ratio of protein to potassium intake (8,9), or using a function of protein, calcium, potassium, magnesium and phosphorus intake known as the potential renal acid load (PRAL) (3,10). These methods assume the sulfur content of protein is a fixed ratio; however, it is acknowledged that the estimation of actual sulfur intakes improves estimations of dietary acid load (11,12), as actual methionine and cysteine contents vary according to protein source. Currently nutrient databases are available which account for variation in sulfur-containing amino acids (13).
The primary aim of this study was to elucidate the role of dietary protein in bone health status as estimated by dual X-ray absorptiometry (DXA) measures of areal bone mineral density (aBMD). Diet record analysis was used to estimate intakes of total protein and sulfate from amino acids for calculation of the dietary acid load. We anticipated that although dietary protein would be positively related to aBMD, this relationship would be suppressed by a negative association of aBMD with the dietary acid load related to protein intake.
Methods
Participants
Our sample consisted of 161 post-menopausal women (mean±SD 67.9±6.0 y) from Champaign County, IL participating in an ongoing study of the relation between physical activity, gait ability and self-efficacy. Subjects were recruited using local media advertising, churches, senior centers and health facilities. Women with neurological illness or severe orthopedic or cognitive limitations preventing physical testing for the broader study were excluded. Cross-sectional data were used for the present analysis. Study participants provided written, informed consent; all study procedures met ethical standards of and were approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
DXA
For bone measures, women changed into medical “scrubs” or wore light-weight clothing and removed all jewelry and other clothing except underwear. Lumbar spine (LS) and total hip (TH) aBMD were measured by DXA using a Hologic QDR 4500A bone densitometer (software version 11.2, Bedford, Massachusetts). LS scans included lumbar vertebrae L1–L4. Short and long-term accuracy of the densitometer were verified by scanning a manufacturer’s hydroxyapatite spine phantom of a known density. All DXA scans were performed by an Illinois state licensed x-ray technologist and analyzed by the same investigator trained in scan analysis by Hologic, Inc. (EM Evans). Precision for DXA BMD measures of interest are 1 – 1.5% in our laboratory with CV% calculated from duplicate scans of both young adults and postmenopausal women.
Dietary Intake and Estimation of Acid Load
Intake was assessed using the USDA multiple-pass 24 h dietary recall method (14,15). Participants completed an interview with researchers to screen for missed foods, portion size clarification, and recall completeness. Diet records were analyzed for total energy, protein, methionine, cystine and micronutrients of interest for calculation estimated NEAP: potassium, calcium, magnesium and phosphorus. Nutrient analysis was performed using Nutritionist Pro version 2.3.1 (First Data Bank, San Bruno, CA).
Protein sulfur load was calculated as mEq/d using intakes of methionine and cystine divided by their molecular weights, as described by Frassetto et al. (8).
| (Eq.1) |
The PRAL of the diet was estimated according to the method of Remer, Dimitriou and Manz (3, 10):
| (Eq.2) |
The protein / potassium ratio estimation of NEAP was calculated according to the method of Frasetto et al. (8):
| (Eq.3) |
Statistical Analyses
Distributions were assessed for normality and outliers using the Shapiro-Wilk statistic in conjunction with box plot outlier labeling (16). Correlations for energy intake, protein, protein sulfur, minerals of interest, vitamin D, aBMD and body composition (weight, fat mass and lean mass) were calculated for descriptive purposes.
Statistical tests followed the prescriptions of MacKinnon, Krull and Lockwood (17) for modeling suppression and mediation effects. Mediation effects, more commonly described as intermediate endpoints in epidemiology, describe a situation where all or part of the influence of X on Y is transmitted through a third, intermediate variable. Modeling these effects is similar statistically, but different conceptually from confounding variables, in which a spurious association between two variables is explained by a third, non-intermediate variable which presumably causes both X and Y. A suppressor effect is a special case of an intermediate endpoint in which X has a direct association with Y and an indirect association, transmitted through a third variable, which is opposite in sign to the direct effect (17). For example, our present hypothesis posits that protein intake will be positively associated with aBMD (the direct effect), but that a third, intermediate variable, the acid load associated with protein intake, will be negatively associated with aBMD, thereby suppressing the association of protein intake with aBMD unless the model is statistically controlled for the intermediate variable.
Sulfate, PRAL and the protein / potassium calculation were entered individually as the second block of a regression equation containing dietary protein to predict aBMD of the LS and TH. The change in R2 was observed between first and second blocks, indicating the improvement in the model conferred by the inclusion of respective estimates of diet acid load. A step-down regression procedure was then applied to examine the potentially confounding influences of body weight, age, physical activity and calcium as well as vitamin D intakes. Because the interaction of dietary protein and calcium intakes have been reported to impact bone health (1,18), a protein by calcium interaction term was also tested for statistical control. At each step, covariates yielding a P value >0.1 were removed from the model. Covariates were tested before and after the addition of sulfate to the model to ascertain any confounding influences. The variance inflation factor was observed as a test of multicollinearity.
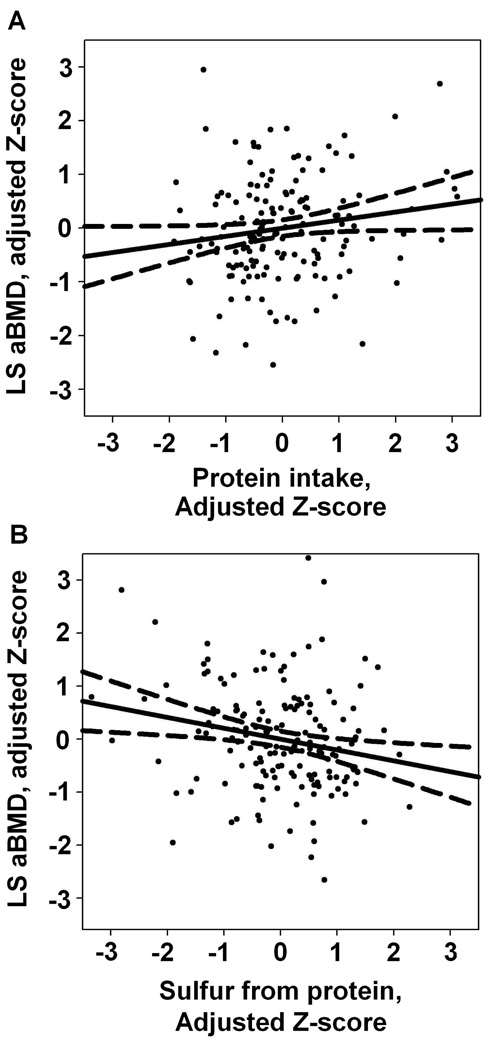
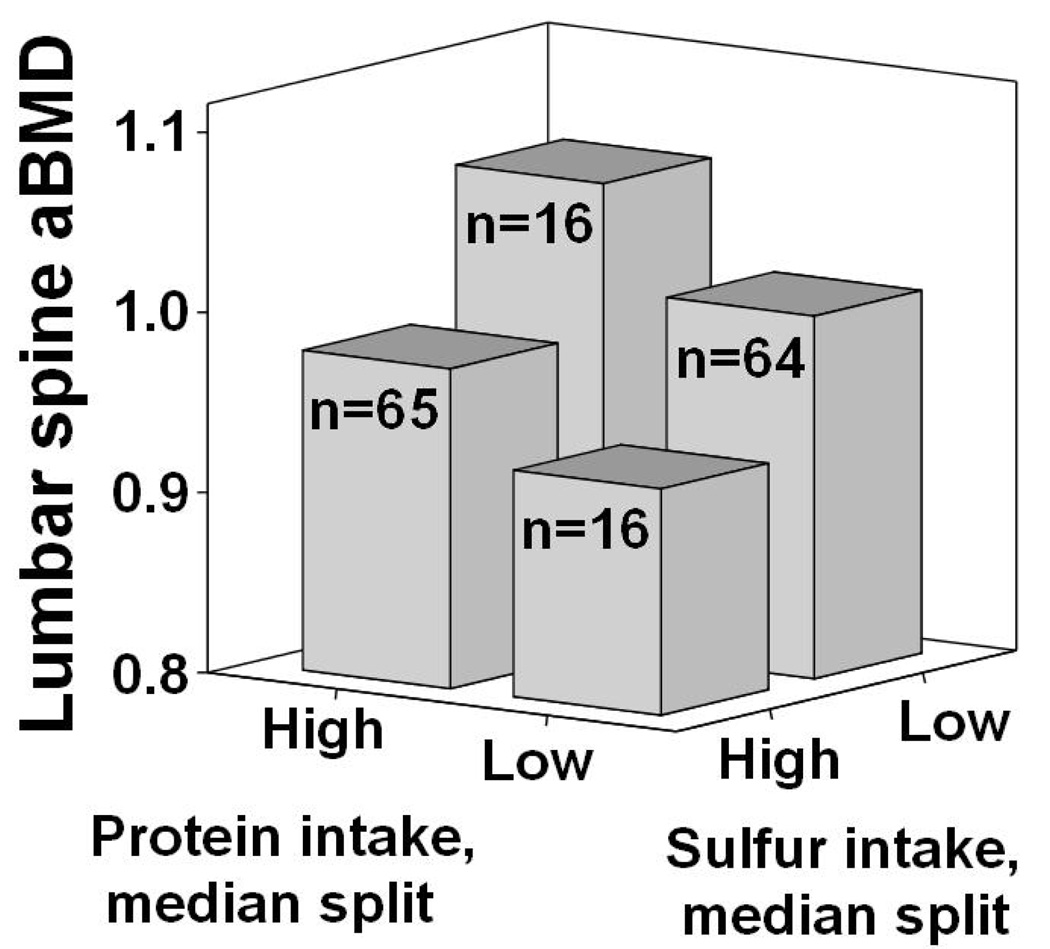
Where change in R2 was significant, unique protein effects and sulfur-mediated protein effects were estimated and tested for significance. The “Indirect” mediation macro for SPSS by Preacher and Hayes (19) provided bootstrapped variance estimates for the indirect or sulfur-mediated effect using 10,000 resamples with replacement. To describe the individual contributions of protein and related sulfur intakes, scatter plots and least squares regression lines were produced using sample Z-scores (difference between observation and sample mean divided by SD) of aBMD and protein adjusted for sulfur, then of aBMD and sulfur, adjusted for protein (Figure 1). Additionally mean LS aBMD was compared for subjects split across the median for both total protein and sulfur from protein (Figure 2). SPSS 14.0 was used in all analysis.
Figure 1.
Scatterplot with least squares regression line and mean 95% CI of lumbar spine areal bone mineral density (aBMD, g/cm2) with protein, adjusted for associated sulfur intake (A) and with sulfur from protein, adjusted for total protein intake (B); n=161 postmenopausal women. Values are sample Z-scores, adjusted by ordinary multiple regression. Regressions were also controlled for body weight. For aBMD on protein, β=0.21 (P=0.04). For aBMD on sulfur, β=−0.28 (P<0.01).
Figure 2.
Descriptive representation of median lumbar spine areal bone mineral density (aBMD) in 161 postmenopausal women with intakes above (high) and below (low) median intakes of protein and sulfur from protein.
Results
Among participants, 128 (80%) were taking supplemental calcium (defined as a daily supplement containing at least 100 mg calcium), 34 (20%) were taking a prescribed osteoporosis medication, 50 (31%) were taking hormone replacement therapy and an additional 64 (40%) had taken hormone replacement therapy in the past. One participant completed the LS scan, but not the TH scan, reducing the sample size for TH tests to 160. Parametric and robust descriptive statistics are presented in Table 1. Spearman’s correlations of aBMD with protein, energy and estimates of the diet acid load are presented in Table 2. Protein, sulfur, PRAL, and the protein / potassium index of NEAP were positively correlated with one another (all P<0.01), but of the dietary variables only sulfur exhibited a significant, negative correlation with LS aBMD.
Table 1.
Descriptive statistics, 161 postmenopausal women.
| Mean | SD | Median | IQR1 | |
|---|---|---|---|---|
| Age, y | 68 | 6.0 | 66 | 63 – 72 |
| Weight, kg | 73.1 | 15.1 | 70.7 | 62.7 – 80.7 |
| BMI, kg/m2 | 28.2 | 5.3 | 27.7 | 24.4 – 31.4 |
| kJ/d | 7063 | 2335 | 6749 | 5402 – 8548 |
| Protein, g/d | 74.7 | 25.0 | 76.9 | 54.7 – 88.9 |
| Calcium, mg/d | 840 | 34.5 | 781 | 513 – 1079 |
| Vitamin D, mg/d | 3.36 | 2.73 | 2.85 | 1.08 – 4.90 |
| PRAL2, mEq/d | 2.4 | 16.6 | 1.9 | −9.9 – 13.3 |
| Est. NEAP3 | 49.2 | 22.2 | 45.0 | 35.2 – 57.6 |
| Protein Sulfur4, mEq/d | 28.4 | 15.6 | 27.4 | 17.3 – 38.6 |
| LS aBMD5, g/cm2 | 0.99 | 0.15 | 0.98 | 0.88 – 1.08 |
| TH aBMD, g/cm2 | 0.88 | 0.13 | 0.87 | 0.78 – 0.97 |
Table 2.
Correlations of dietary intakes, diet acid load estimations and areal bone mineral density (aBMD) of the lumbar spine (LS) and total hip (TH) in postmenopausal women.
| Protein | Energy | PRAL1 | NEAP2 | Sulfur | K+ | LS BMD | |
|---|---|---|---|---|---|---|---|
| Energy, kJ | 0.59 | ||||||
| P | <0.01 | ||||||
| PRAL1 | 0.43 | 0.05 | |||||
| P | <0.01 | 0.56 | |||||
| Est. NEAP2 | 0.41 | 0.02 | 0.88 | ||||
| P | <0.01 | 0.82 | <0.01 | ||||
| Sulfur3 | 0.67 | 0.33 | 0.33 | 0.27 | |||
| P | <0.01 | <0.01 | <0.01 | 0.03 | |||
| K+ | 0.59 | 0.56 | −0.33 | −0.41 | 0.47 | ||
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| LS aBMD4 | −0.01 | 0.10 | −0.03 | −0.03 | −0.17 | −.04 | |
| P | 0.94 | 0.19 | 0.75 | 0.74 | 0.03 | 0.66 | |
| TH aBMD | 0.08 | 0.08 | 0.12 | 0.08 | 0.04 | −.04 | 0.59 |
| P | 0.30 | 0.32 | 0.12 | 0.30 | 0.64 | 0.63 | <0.01 |
Estimated Net Endogenous Acid Production (8), calculated using the ratio of protein to potassium intake according to formula 3.
Sulfur content of reported dietary protein sources, calculated according to formula 1.
Areal bone mineral density, g/cm2. Values are Spearman’s Rho and associated P values, n=161.
At the lumbar spine, the step-down procedure eliminated, in order, vitamin D (P=0.83), calcium×protein interaction (P=0.77), energy/wk expended in physical activity (P=0.55), age (P=0.63) and calcium (P=0.10), leaving only weight as a covariate in the final model (P<0.01). In the first block, protein held no association with LS aBMD (P=0.81). Neither the addition of PRAL (P=0.66) nor estimated NEAP using the protein / potassium ratio (P=0.97) significantly improved the model fit; however, adding sulfate demonstrated a negative association of sulfur from amino acids with LS aBMD, and a positive association of protein with LS aBMD (Table 3).
Table 3.
Regression of lumbar spine areal bone mineral density (aBMD, g/cm2) on protein and sulfur from protein, controlled for body weight1.
| B | SE | β | P | |
|---|---|---|---|---|
| Entered in separate regression models: | ||||
| Protein | 1.11e−4 | 4.65e−4 | 0.02 | 0.81 |
| Sulfur | −1.35e−3 | 7.39e−4 | −0.14 | 0.07 |
| Entered simultaneously, i.e., mutually adjusted: | ||||
| Protein | 1.35e−3 | 6.30e−4 | 0.22 | 0.04 |
| Sulfur | −2.82e−3 | 1.01e−3 | −0.28 | <0.01 |
Estimated using ordinary multiple regression. Final model controlling for weight was determined using a step-down procedure removing age, physical activity, calcium and vitamin D intakes and an interaction of dietary protein with calcium. n=161 postmenopausal women.
The standardized coefficient for sulfate regressed onto protein intake was 0.69 (P<0.01). The standardized indirect coefficient of protein, or that portion of protein’s total predictive influence that is mediated by its sulfur content, was −0.19 (95% CI, −0.35, −0.04), opposite in sign and similar in magnitude to the estimated β for sulfur-controlled protein, 0.21 (Table 3). Despite correlations between protein and acid-load estimators, the largest Variance Inflation Factor observed was 1.9 (for protein and sulfur), well below the recommended threshold of 10 for detecting problematic multicollinearity (20). The relationships of LS aBMD with protein and LS aBMD with sulfur were linear within the range of reported protein and sulfur intakes (Figure 1).
At the total hip, step-down regression removed energy expended in physical activity weekly (P=0.95) vitamin D (P=0.33) and the calcium · protein interaction (P=0.26) terms, but retained age (P<0.01), weight (P<0.01) and calcium intake (P=0.02) as covariates. Protein was a significant (P=0.03), positive (β=0.18) predictor of TH aBMD; however, no improvement in R2 was observed with the addition of PRAL (P=0.87), estimated NEAP using the protein / potassium ratio (P=0.29) or sulfate (P=0.83) to the model. The largest Variance Inflation Factor observed was 2.1.
Discussion
Our findings suggest that, within the range of intake reported in our sample, increasing dietary protein is beneficial to aBMD of the lumbar spine and total hip of postmenopausal women, but that this benefit is suppressed at the lumbar spine by the dietary acid load associated with sulfur containing amino acids. Neither the PRAL nor estimated NEAP using the protein / potassium ratio contributed to the prediction of aBMD at either site. The observed regression coefficients are small but clinically meaningful. A participant consuming mean levels of sulfate, but protein at +1 SD would be predicted to have 3.2% (95% CI: 0.12 to 6.4) additional LS aBMD above the sample mean. Conversely a subject consuming mean protein but +1 SD sulfate would be predicted to have 4.3% (−7.4 to −1.2) lower LS aBMD. Although further research is necessary to validate these cross-sectional data, the observed differences support hypotheses that improving intake of low-sulfate protein sources, or alternatively improving protein intake with a corresponding reduction in the dietary acid load may be beneficial in osteoporosis prevention.
Protein deficiency is detrimental to bone health (21). Observational studies of bone health tend to promote a positive view of protein intakes above 0.8 g.kg−1.d−1 (22–26), though not all studies are favorable (27–29). It has long been established that increasing protein intake elevates calcium losses in urine; in many studies fecal calcium measures indicated no apparent compensation in calcium absorption, promoting the view that urinary calcium must reflect mineral lost from bone (30). More recently, however, Kerstetter et al. (2) have demonstrated not only increased calcium absorption, but a reduction in urinary calcium of bone origin in subjects consuming 2.1 g.kg−1.d−1 protein compared to 1.0 g.kg−1.d−1 in a kinetic study using dual stable calcium isotopes. In the same study, no net difference in bone mineral mass were observed between levels of protein intake, however there was a trend toward reduced bone turnover.
In light of the influence of protein on urinary calcium excretion, Dawson-Hughes (1,31) suggests that increased protein intake is beneficial provided that calcium intake is sufficient to support bone growth and urinary losses. Skov et al. (32) and unpublished data in our lab (in review) demonstrate benefits of protein above current recommended levels to bone mineral mass, in the presence of adequate calcium, during weight loss. In the present study the interaction of calcium and protein intake was not significant.
Proposed mechanisms whereby dietary protein may enhance bone health include providing substrate for collagen deposition and increasing circulating levels of insulin-like growth factor-1, a known growth factor for bone (1). A recent prospective study by Alexy et al. (33) demonstrated improved bone health in youth with increasing protein intake. Conversely, bone status was negatively associated with the diet acid load, estimated by the PRAL, which includes estimation of the acidifying effect of dietary protein. The authors noted that this index of dietary acid load does not directly assess methionine and cysteine content of individual proteins, but assumes a fixed proportion of sulfur to protein. The young, growing population is distinct in several important ways from the older population of the present study, but the results illustrate a similar concept: protein is positively associated with bone mineral mass, but also contributes to an acid load with negative ramifications for bone.
Total protein and protein sulfur are highly and intuitively correlated, but the actual ratio depends on protein source. Methionine is an essential amino acid, and deficiency causes adverse health outcomes. However, our results suggest that the addition of lower sulfate protein to a diet that is already adequate in all essential amino acids may be beneficial in osteoporosis prevention; further research is necessary to test this hypothesis. Soy is implicated as a protein source with a low sulfur to protein ratio (13), estimated by Massey (34) at 39.8 mEq sulfur / 100 g protein, compared to a mid-range 54.8 mEq / 100 g protein for milk and 59.4 mEq / 100 g protein for beef, and a higher 73.0 mEq / 100 g protein for pork and 82.2 mEq / 100 g protein for oatmeal. Experiments have tested the impact of soy protein, with and without isoflavones, on calcium balance with mixed results (35–38). Alternatively increased protein from all sources in connection with enhanced intake of alkalizing nutrients may be beneficial. It has been shown that a more alkaline diet is associated with improved bone density (39–43), and that supplementation of potassium bicarbonate or potassium citrate attenuates bone turnover (44,45). Jajoo et al. (46) demonstrated that replacing some cereal, an acidifying diet component, with more base-producing fruits and vegetables resulted in reduced levels of parathyroid hormone, bone resorption and calcium excretion relative to controls.
It is conceptually important that the positive association in this sample between protein and LS aBMD, controlling for sulfur, was almost perfectly negated by the negative association of sulfate equivalents from methionine and cysteine and aBMD. This suppressor effect indicates that any study evaluating the association between protein intake and bone mineral status without controlling for actual sulfur content of protein may observe no significant correlation, despite real positive and negative protein effects. A neutral combined influence of protein and sulfur on aBMD prediction would mask true effects in both studies hypothesizing detrimental acid load effects to bone, and studies hypothesizing beneficial effects of dietary protein. This suppressor effect might explain the discordance of reports concerning the impact of dietary protein or acid load on bone health. Likewise it is possible that uncontrolled influences of the total dietary acid load might explain conflicting reports. If true, this would suggest that future studies of associations between protein intake and bone density must account for the dietary acid load in order to produce unbiased results.
Our results suggest that direct estimation of sulfur from amino acids may perform better than the protein / potassium ratio or PRAL in studies of acid-base balance and bone mineral mass. These constructs were developed and validated for the estimation of the acidifying influence of dietary intake (3,8–10). However because each estimates the acidifying effect of protein as a fixed ratio of total protein intake, they may not be well suited to the investigation of bone, where the negative acid effect seems to be opposed by an anabolic protein effect. Indeed our results suggest that the negative and positive effects of protein may neatly cancel one another out at the LS, leaving the estimated influence on bone of total protein, as used in the protein / potassium ratio and PRAL, neutral in spite of otherwise reliable estimations of dietary acid load. The PRAL is also a function of calcium and phosphorus intake, which have well established influences on the calcium economy (47). These factors may further confound the influence of the PRAL on bone. In light of these complex associations, it may be advisable to use direct intakes of protein, amino-acid sulfur (the acidifying agent of protein), and other relevant minerals such as potassium in lieu of estimated NEAP when calcium balance or bone mineral mass are investigated.
It is not clear why this suppressive association of sulfur intake would describe aBMD at the lumbar spine and not at the total hip, although differences in the response of these two sites are commonly reported (48–50). The change in R2 at the LS indicated a small likelihood of a type I error, or inappropriate rejection of the null hypothesis that sulfur intake does not predict aBMD. Conversely, a post-hoc power analysis of the regression at the hip was performed, and indicated a power of 0.74 to detect an improvement in R2 as large as that observed at the spine. Though it is not entirely improbable that our regression failed to detect a true difference at the hip, it is very possible that a real difference between protein associations at the hip and spine explain divergent findings. Differences in observations between these measurement sites may relate to differing levels of trabecular compared to cortical bone content; specifically, the ratio of trabecular to cortical bone is greater at the spine than at the hip. It has been theorized that trabecular bone, being more metabolically active, may be more sensitive to dietary intervention, at least in the short term (51,52). However a post-hoc analysis of the trochanter sub region, with a greater proportion of trabecular bone, did not mimic the findings at the LS in this sample (data not shown). Alternatively it is possible that the weight-bearing load at the hip during ambulation may blunt the effects of dietary factors.
This study is not without limitations; the constraints of cross-sectional data are recognized. This research is performed in the context of a broader body of literature suggesting causal connections between protein intake and bone mineral mass (31,32), sulfate intake and acid-base balance (53,54) and acid base balance and bone demineralization (4,5,6,44,55). The results are theoretically consistent and harmonize apparently conflicting research; however, this observational study offers no basis for new causal inference and is best interpreted as a rationale for additional investigation.
In conclusion, protein intake is positively associated with aBMD in postmenopausal women, but this association is suppressed by a negative association of sulfur from amino acids at the lumbar spine. This observation may reconcile reports of positive impacts of dietary protein on bone health with reports of a negative impact of the acid load from sulfur-containing amino acids. At the total hip dietary protein intake is positively associated with aBMD, and may not exhibit the same negative association with sulfur from protein as observed at the spine. These results highlight the need to evaluate actual sulfur contents of varying dietary protein sources rather than assuming a fixed ratio of sulfur to protein. Future research in this line of inquiry should evaluate the role of dietary protein in preserving bone health in populations at higher risk for fracture such as the elderly.
Abbreviations
- aBMD
areal bone mineral density
- DXA
dual x-ray absorptiometry
- LS
lumbar spine
- NEAP
net endogenous acid production
- PRAL
potential renal acid load
- TH
total hip
Footnotes
Support for this research was provided by National Institute on Aging Grant R01 AG20118 to Edward McAuley.
The authors have no conflicts of interest to disclose.
Previous abstract presentation:
Thorpe M. Mojtahedi MC. Chapman-Novakofski KM. McAuley E. Evans EM. 2007.
Associations of dietary protein, calcium, potential renal acid load and bone mineral density in elderly women. 2007 Experimental Biology meeting, Washington, D.C. The FASEB Journal, 21, Abstract #6432.
Literature Cited
- 1.Dawson-Hughes B. Interaction of dietary calcium and protein in bone health in humans. J Nutr. 2003;133(3):852S–854S. doi: 10.1093/jn/133.3.852S. [DOI] [PubMed] [Google Scholar]
- 2.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90(1):26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 3.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 4.Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr. 1998;128(6):1051–1053. doi: 10.1093/jn/128.6.1051. [DOI] [PubMed] [Google Scholar]
- 5.Macleay JM, Olson JD, Turner AS. Effect of dietary-induced metabolic acidosis and ovariectomy on bone mineral density and markers of bone turnover. J Bone Miner Metab. 2004;22(6):561–568. doi: 10.1007/s00774-004-0524-0. [DOI] [PubMed] [Google Scholar]
- 6.Lemann J, Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol. 2003;285(5):F811–F832. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 7.New SA. Intake of fruit and vegetables: Implications for bone health. Proc Nutr Soc. 2003;62(4):889–899. doi: 10.1079/PNS2003310. [DOI] [PubMed] [Google Scholar]
- 8.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3):576–583. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 9.Frassetto LA, Lanham-New SA, Macdonald HM, et al. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137(6):1491–1492. doi: 10.1093/jn/137.6.1491. [DOI] [PubMed] [Google Scholar]
- 10.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77(5):1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 11.Remer T, Manz F. Paleolithic diet, sweet potato eaters, and potential renal acid load. Am J Clin Nutr. 2003;78(4):802–803. doi: 10.1093/ajcn/78.4.802. author reply 803-4. [DOI] [PubMed] [Google Scholar]
- 12.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC., Jr Estimation of the net acid load of the diet of ancestral preagricultural homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76(6):1308–1316. doi: 10.1093/ajcn/76.6.1308. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Agriculture, Agricultural Research Service. USDA nutrient database for standard reference. 2006 release 19 < http://www.ars.usda.gov/ba/bhnrc/ndl>.
- 14.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J Am Diet Assoc. 2004;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 16.Thode HC. Testing for Normality. New York: Marcel Dekker, Inc.; 2002. [Google Scholar]
- 17.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP. Excess dietary protein may not adversely affect bone. J Nutr. 1998;128(6):1054–1057. doi: 10.1093/jn/128.6.1054. [DOI] [PubMed] [Google Scholar]
- 19.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 20.Myers R. Classical and Modern Regression with Applications. 2nd ed. Boston, MA: Duxbury; 1990. [Google Scholar]
- 21.Bonjour JP. Dietary protein: An essential nutrient for bone health. J Am Coll Nutr. 2005;24(6 Suppl):526S–536S. doi: 10.1080/07315724.2005.10719501. [DOI] [PubMed] [Google Scholar]
- 22.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128(10):801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69(1):147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 24.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: The framingham osteoporosis study. J Bone Miner Res. 2000;15(12):2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 25.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly : The rancho bernardo study. Am J Epidemiol. 2002;155(7):636–644. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 26.Wengreen HJ, Munger RG, West NA, et al. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of utah. J Bone Miner Res. 2004;19(4):537–545. doi: 10.1359/JBMR.040208. [DOI] [PubMed] [Google Scholar]
- 27.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143(5):472–479. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- 28.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Worldwide incidence of hip fracture in elderly women: Relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci. 2000;55(10):M585–M592. doi: 10.1093/gerona/55.10.m585. [DOI] [PubMed] [Google Scholar]
- 29.Weikert C, Walter D, Hoffmann K, Kroke A, Bergmann MM, Boeing H. The relation between dietary protein, calcium and bone health in women: Results from the EPIC-potsdam cohort. Ann Nutr Metab. 2005;49(5):312–318. doi: 10.1159/000087335. [DOI] [PubMed] [Google Scholar]
- 30.Kerstetter JE, O'Brien KO, Insogna KL. Low protein intake: The impact on calcium and bone homeostasis in humans. J Nutr. 2003;133(3):855S–861S. doi: 10.1093/jn/133.3.855S. [DOI] [PubMed] [Google Scholar]
- 31.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75(4):773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 32.Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A. Effect of protein intake on bone mineralization during weight loss: A 6-month trial. Obes Res. 2002;10(6):432–438. doi: 10.1038/oby.2002.60. [DOI] [PubMed] [Google Scholar]
- 33.Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82(5):1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 34.Massey LK. Dietary animal and plant protein and human bone health: A whole foods approach. J Nutr. 2003;133(3):862S–865S. doi: 10.1093/jn/133.3.862S. [DOI] [PubMed] [Google Scholar]
- 35.Spence LA, Lipscomb ER, Cadogan J, et al. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: A randomized crossover study. Am J Clin Nutr. 2005;81(4):916–922. doi: 10.1093/ajcn/81.4.916. [DOI] [PubMed] [Google Scholar]
- 36.Zemel MB. Calcium utilization: Effect of varying level and source of dietary protein. Am J Clin Nutr. 1988;48(3 Suppl):880–883. doi: 10.1093/ajcn/48.3.880. [DOI] [PubMed] [Google Scholar]
- 37.Kerstetter JE, Wall DE, O'Brien KO, Caseria DM, Insogna KL. Meat and soy protein affect calcium homeostasis in healthy women. J Nutr. 2006;136(7):1890–1895. doi: 10.1093/jn/136.7.1890. [DOI] [PubMed] [Google Scholar]
- 38.Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: Effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab. 2005;90(1):181–189. doi: 10.1210/jc.2004-0393. [DOI] [PubMed] [Google Scholar]
- 39.New SA, Bolton-Smith C, Grubb DA, Reid DM. Nutritional influences on bone mineral density: A cross-sectional study in premenopausal women. Am J Clin Nutr. 1997;65(6):1831–1839. doi: 10.1093/ajcn/65.6.1831. [DOI] [PubMed] [Google Scholar]
- 40.New SA, Robins SP, Campbell MK, et al. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr. 2000;71(1):142–151. doi: 10.1093/ajcn/71.1.142. [DOI] [PubMed] [Google Scholar]
- 41.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69(4):727–736. doi: 10.1093/ajcn/69.4.727. [DOI] [PubMed] [Google Scholar]
- 42.Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005;81(4):923–933. doi: 10.1093/ajcn/81.4.923. [DOI] [PubMed] [Google Scholar]
- 43.New SA, MacDonald HM, Campbell MK, et al. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79(1):131–138. doi: 10.1093/ajcn/79.1.131. [DOI] [PubMed] [Google Scholar]
- 44.Sellmeyer DE, Schloetter M, Sebastian A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J Clin Endocrinol Metab. 2002;87(5):2008–2012. doi: 10.1210/jcem.87.5.8470. [DOI] [PubMed] [Google Scholar]
- 45.Marangella M, Di Stefano M, Casalis S, Berutti S, D'Amelio P, Isaia GC. Effects of potassium citrate supplementation on bone metabolism. Calcif Tissue Int. 2004;74(4):330–335. doi: 10.1007/s00223-003-0091-8. [DOI] [PubMed] [Google Scholar]
- 46.Jajoo R, Song L, Rasmussen H, Harris SS, Dawson-Hughes B. Dietary acid-base balance, bone resorption, and calcium excretion. J Am Coll Nutr. 2006;25(3):224–230. doi: 10.1080/07315724.2006.10719536. [DOI] [PubMed] [Google Scholar]
- 47.Calvo MS. Dietary phosphorus, calcium metabolism and bone. J Nutr. 1993;123(9):1627–1633. doi: 10.1093/jn/123.9.1627. [DOI] [PubMed] [Google Scholar]
- 48.Sahota O, Pearson D, Cawte SW, San P, Hosking DJ. Site-specific variation in the classification of osteoporosis, and the diagnostic reclassification using the lowest individual lumbar vertebra T-score compared with the L1-L4 mean, in early postmenopausal women. Osteoporos Int. 2000;11(10):852–857. doi: 10.1007/s001980070044. [DOI] [PubMed] [Google Scholar]
- 49.Aoki TT, Grecu EO, Srinivas PR, Prescott P, Benbarka M, Arcangeli MM. Prevalence of osteoporosis in women: Variation with skeletal site of measurement of bone mineral density. Endocr Pract. 2000;6(2):127–131. doi: 10.4158/EP.6.2.127. [DOI] [PubMed] [Google Scholar]
- 50.Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: The postmenopausal health study. Br J Nutr. 2006;96(6):1140–1148. doi: 10.1017/bjn20061977. [DOI] [PubMed] [Google Scholar]
- 51.Erdman JW, Jr, Stillman RJ, Boileau RA. Provocative relation between soy and bone maintenance. Am J Clin Nutr. 2000;72(3):679–680. doi: 10.1093/ajcn/72.3.679. [DOI] [PubMed] [Google Scholar]
- 52.Baeksgaard L, Andersen KP, Hyldstrup L. Calcium and vitamin D supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporos Int. 1998;8(3):255–260. doi: 10.1007/s001980050062. [DOI] [PubMed] [Google Scholar]
- 53.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59(6):1356–1361. doi: 10.1093/ajcn/59.6.1356. [DOI] [PubMed] [Google Scholar]
- 54.Remer T. Influence of diet on acid-base balance. Semin Dial. 2000;13(4):221–226. doi: 10.1046/j.1525-139x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 55.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC., Jr Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;330(25):1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]