Abstract
Objectives
To investigate cognitive impairment in older, ethnically diverse individuals with a broad range of kidney function, to evaluate a spectrum of cognitive domains and to determine whether the relationship between CKD and cognitive function is independent of demographic and clinical factors.
Design
Cross sectional.
Setting
Chronic Renal Insufficiency Cohort Study.
Participants
825 adults ≥55 years with CKD.
Measurements
We estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) using the four-variable Modification of Diet in Renal Disease equation. We compared cognitive scores on six cognitive tests across eGFR strata using linear regression; multivariable logistic regression was used to examine level of CKD and clinically significant cognitive impairment (score ≤1 sd from mean).
Results
Mean age of the participants was 64.9 years, 50% were male and 45% were Black. After multi-variable adjustment, participants with lower eGFR had lower cognitive scores on most cognitive domains (P<0.05). In addition, compared with persons who had mild or moderate CKD (eGFR 45-59), participants with advanced CKD (eGFR <30) were more likely to have clinically significant cognitive impairment on global cognition (adjusted odds ratio [OR] 2.0, 95% CI 1.1-3.9), naming (OR 1.9, 95% CI 1.0-3.3), attention (OR 2.4, 95%CI 1.3-4.5), executive function (OR 2.5, 95%CI 1.9- 4.4) and delayed memory, (OR=1.5, 95%CI 0.9-2.6) but not on category fluency (OR=1.1, 95% CI 0.6-2.0).
Conclusions
Among older adults with CKD, lower level of kidney function was associated with lower cognitive function on most domains. Our results suggest that older patients with advanced CKD should be screened for cognitive impairment.
Keywords: Chronic kidney disease, cognitive impairment, cognitive function
Introduction
The prevalence of chronic kidney disease (CKD) increases with advancing age. Similarly, cognitive impairment is more common in the elderly. Both conditions are associated with increased health-care utilization and decreased quality of life, and are of increasing public health significance.1, 2 Whether CKD is associated with accelerated cognitive aging has not been rigorously addressed. There are several potential mechanisms including cerebrovascular disease,3-5 metabolic dysregulation6 and direct effects of kidney disease7-9 that may link CKD with impaired cognitive function.
The few studies that have investigated the association between CKD prior to kidney failure and cognitive outcomes suggest that elders with very low levels of kidney function have worse cognitive function compared to those without CKD.10-14 However, little is known about the specific cognitive domains affected in CKD because most of the prior research studies have used global cognitive screening measures rather than tests of specific domains. In addition, they had limited representation of minority subjects and a limited range of kidney function.
The Chronic Renal Insufficiency Cohort (CRIC) Study, a prospective observational cohort study, was established to examine the risk factors and mechanisms of progression of CKD and cardiovascular disease (CVD). The CRIC Cognitive (CRIC COG) Study, an ancillary study to CRIC, provided the opportunity to investigate cognitive impairment in older, ethnically diverse individuals with a broad range of kidney function, to evaluate a spectrum of cognitive domains and to determine whether the relationship between CKD and cognitive function is independent of demographic and clinical factors.
Methods
Study Design
The CRIC Study is a prospective cohort study designed to evaluate the risk factors and mechanisms of progression of CKD and CVD among adults with mild-to-moderate CKD. A total of 3612 participants (47% with diabetes mellitus (DM)) were enrolled at seven clinical centers from May, 2003 to March, 2007, and are being followed annually until death or withdrawal of informed consent. The study design and methods have been published previously.15
The present study of cognitive function, called the CRIC COG Study, is ancillary to the parent CRIC Study. Similar to its parent study, the CRIC COG is a prospective observational cohort study which recruited study participants from 4 of the 7 clinical CRIC centers located in Philadelphia, PA; Cleveland, OH; Oakland, CA; and Chicago, IL. Only persons enrolled in the CRIC Study and who were 55 years of age or older were eligible for the cognitive function study. At their next scheduled in-clinic visit, eligible participants were enrolled in the CRIC COG Study and administered a cognitive battery. This served as the baseline visit for the study but may not have coincided with the baseline visit for the parent study. Thus “baseline” in this report is the participants' first visit as part of CRIC COG Study.
The CRIC COG Study was approved by institutional review boards at each of the participating sites as well as at UCSF (University of California, San Francisco), and all participants gave written informed consent.
Study Population
To be enrolled in the CRIC study, participants must be between 21 and 74 years old; and have an estimated glomerular filtration rate (eGFR) of 20-70 ml/min/1.73 m2 if aged 21-44 years, 20-60 ml/min/1.73 m2 if aged 45-64 years, and 20-50 ml/min/1.73 m2 if aged 65-74. They were not eligible if they were institutionalized, did not provide informed consent, had previously undergone dialysis for longer than one month, were diagnosed with polycystic kidney disease, had received a prior organ/bone marrow transplant, were taking immunosuppressive drugs for kidney in the past 6 months, or were currently participating in a clinical trial or an ongoing cohort study at some of the centers, the African American Study of Kidney Disease and Hypertension. Other exclusions included New York Heart Association Class III/IV heart failure, cirrhosis, HIV infection or AIDS, chemotherapy for cancer within 2 years, multiple myeloma, or renal cell carcinoma.15 The CRIC COG Study required that participants be enrolled in the CRIC study and at least 55 years of age. This minimum age requirement was established to increase the likelihood of observing clinically significant cognitive decline in the sample. The target sample size was 720, with equal recruitment from the four clinical centers.
Predictor Variable
The primary predictor of interest was level of kidney function. Estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD) estimating equation.16 Whenever possible we used the eGFR value determined at the CRIC visit coinciding with the CRIC COG baseline visit. In cases (N=8 or 1%) where visits did not coincide or when eGFR was missing, we used the most recent eGFR value (mean interval of 1.4 years).
Cognitive Function Measures
Six cognitive function tests (with 7 scores) were administered in the CRIC COG Study: the Modified Mini-Mental Status Examination (3MS), Trails A and B, Category (verbal) Fluency, Buschke Selective Reminding Test (immediate and delayed memory), and Boston Naming. The 3MS is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory with scores ranging from 0-100, higher scores indicating better function.17 Trails A and B measure visuospatial scanning, motor speed, executive function, and attention.18 Trails B is primarily a test of executive function while Trails A is predominantly a test of attention. The scores are based on the time to complete a particular task, and therefore, lower scores indicate higher function. Category (verbal) Fluency measures verbal production, semantic memory, and language with higher scores indicating better performance.19 Both Category Fluency and Trails B are typically impaired in the context of subcortical vascular disease.20 Buschke Selective Reminding Test is a well-established test of verbal memory with immediate and delayed components.21 Verbal memory is one of the earliest cognitive domains to be affected in Alzheimer's disease (AD) and is a hallmark feature of all dementias.22 Higher scores indicate higher function. The Modified Boston Naming test is a 15-item test of language function that requires the participant to name objects presented in pictures.19 Language deficits, such as those found with naming, are a hallmark feature of early AD. Higher scores indicate higher function.
Clinically significant impairment was defined as having a score one standard deviation (SD) below the mean or lower, except for the Trails A and B in which case impairment was a score at least one SD above the mean.
Covariates
Covariates included additional data collected as part of the CRIC study including sociodemographic characteristics, relevant medical history (diabetes mellitus, diagnosed hypertension; self-reported at each annual clinic visit if a doctor told them they had that condition), body mass index (BMI; measured as weight (kilograms) divided by the square of height (meters)) and resting blood pressure (systolic and diastolic; mean of three seated measurements, 30 seconds apart), each measured or updated annually. The Beck Depression Inventory (BDI) was collected biannually and moderate to severe depression was defined as a score of >14.23
Statistical Methods
We compared baseline characteristics across eGFR categories (≥60, 45-59, 30-44, and <30 ml/min/1.73 m2) using analysis of variance for normally distributed continuous data, Kruskal-Wallis test for non-normally distributed continuous data, and chi-square tests for categorical data. Tests for trend on categorical variables were done using the Cochran-Armitage Trend Test and for continuous variables, using orthogonal polynomial contrasts within the general linear models. The outcome variables of interest are the six cognitive function tests, resulting in seven scores. The primary analysis was a comparison of each score across category of eGFR level using a linear regression model, so we could test for an association between kidney function and cognitive function. Adjustment was made for the following possible confounding variables based on their association (p<0.1) with CKD and cognitive function: age, education, gender, BMI, diabetes, hypertension, and depression. Additionally, adjustment was made for race based on prior published studies showing separate associations between race and CKD9-11 as well as race and cognitive function.24, 25
We further tested for differences in likelihood of clinically significant cognitive impairment across eGFR strata using logistic regression models, with adjustment for age, race, education, gender, BMI, diabetes, hypertension, and depression score. For these analyses, the reference group was the subgroup of participants with eGFR of 45-59 ml/min/1.73 m2 (we did not chose the reference to be the 80 participants with eGFR ≥ 60 ml/min/1.73 m2 as they had demonstrated lower levels of kidney function at the time of screening several months before and did not seem to appropriately represent elders with eGFR ≥ 60). Results are summarized with odds ratios (OR) and 95% confidence intervals. Models were run with and without adjustment for possible confounders. All analyses were done using SAS version 9.1.
Results
A total of 825 participants were recruited into the CRIC COG Study (of those who were eligible, 983 were asked to participate). This cohort had a mean (SD) age of 64.9 (5.6) years and was 50% male and 45% Black. Site enrollment included 205 participants from the University of Pennsylvania center, 223 participants from the Case Western Reserve center, 196 from the University of Illinois at Chicago center, and 201 from the Kaiser Permanente of Northern California/University of California San Francisco center. At the time of cognitive testing, mean (SD) eGFR was 41.0 (14.3) ml/min/1.73 m2 with 80 participants having an eGFR ≥ 60 (mean 61) ml/min/1.73 m2, 289 with eGFR 45-59 (mean 49) ml/min/1.73 m2, 299 with eGFR 30-44 (mean 37) ml/min/1.73 m2 and 157 with eGFR <30 (mean 24) ml/min/1.73 m2. The characteristics of the CRIC COG participants were compared across eGFR category (Table 1). Participants with lower eGFR tended to be older, have less educational attainment and greater comorbidity burden including diabetes and hypertension.
Table 1.
Characteristics of the 825 Participants Enrolled in the CRIC Cognitive Function Study.
| Estimated Glomerular Filtration Rate (ml/min/1.73 m2) | |||||
|---|---|---|---|---|---|
| Variable | ≥60 | 45-59 | 30-44 | <30 | P-value† |
| N (%) or Mean (SD)* | (n=80) | (n=289) | (n=299) | (n=157) | |
| Age (years) | 61.5 (3.5) | 64.8 (5.6) | 65.5 (5.7) | 65.6 (5.7) | <0.001 |
| Men | 44 (55%) | 162 (56%) | 139 (47%) | 71 (45%) | 0.05 |
| Race | 0.70 | ||||
| White | 42 (53%) | 144 (50%) | 145 (49%) | 70 (45%) | |
| Black | 33 (41%) | 120 (42%) | 138 (46%) | 76 (48%) | |
| Other | 5 (6%) | 20 (7%) | 14 (5%) | 11 (7%) | |
| Hispanic ethnicity | 2 (3%) | 11(4%) | 6 (2%) | 3 (2%) | 0.51 |
| Education ≤ High School | 22 (28%) | 82 (28%) | 124 (42%) | 67 (43%) | 0.0004 |
| Married/Domestic Partner | 50 (63%) | 165 (57%) | 151 (51%) | 83 (53%) | 0.18 |
| Diabetes mellitus | 32 (40%) | 125 (43%) | 176 (59%) | 88 (56%) | <0.001 |
| Current Smoker | 10 (13%) | 27 (9%) | 40 (13%) | 14 (9%) | 0.33 |
| Hypertension | 62 (78%) | 251 (87%) | 289 (97%) | 150 (96%) | <0.001 |
| Stroke | 5 (6%) | 30 (10%) | 37 (12%) | 20 (13%) | 0.40 |
| Depression | 13 (16%) | 31 (11%) | 59 (20%) | 21 (13%) | 0.02 |
| Body mass index, kg/m2 | 31.0 (5.9) | 31.3 (7.8) | 32.9 (7.8) | 32.1 (8.5) | 0.06 |
| Systolic Blood Pressure | 119.3 (14.3) | 126.5 (21.2) | 129.9 (24.2) | 130.9 (22.3) | <0.001 |
| Diastolic Blood Pressure | 68.7 (11.8) | 67.6 (12.2) | 67.1 (12.5) | 65.7 (11.5) | 0.28 |
SD = standard deviation
P-value is for test of differences across levels of eGFR; a p-value in bold face indicates the test for trend was significant at p<0.05.
In unadjusted analyses, for each of the cognitive tests, a consistent pattern of worse cognitive scores in participants with greater CKD severity was observed. For example, for the 3MS test, participants with eGFR ≥ 60 ml/min/1.73 m2 had a mean (SD) score of 95.2 (5.0), those with eGFR 45-59 ml/min/1.73 m2 had a score 94.0 (7.6), those with eGFR 30-44 ml/min/1.73 m2 had a score 92.1 (7.1) and those with eGFR <30 ml/min/1.73 m2 had a score of 91.4 (7.8) (p<0.001 for trend). For the Trails B test, mean (SD) scores were 116 (70), 126 (70), 151 (83) and 164 (85), respectively (p<0.001 for trend). A similar pattern emerged for the other four cognitive tests (5 subscores). Multivariable adjustment for age, gender, race, education, BMI, diabetes, hypertension, and depression did not alter the findings with the exception of the Category Fluency and Buschke tests (Table 2).
Table 2.
Adjusted Mean Cognitive Function Score by Baseline Level of eGFR (Adjusted for Age, Race, Education, Gender, Diabetes, Hypertension, BMI and Depression).
| Cognitive Test | Estimated Glomerular Filtration Rate (ml/min/1.73 m2) | |||||
|---|---|---|---|---|---|---|
| Mean (SE and range*) | N | ≥60 | 45-59 | 30-44 | <30 | P-value† |
| 3MS‡ | 813 | 92.8 (0.82) | 92.2 (0.61) | 91.2 (0.62) | 91.0 (0.70) | 0.015 |
| Trails A‡ | 810 | 49.3 (4.44) | 53.7 (3.30) | 62.9 (3.37) | 68.0 (3.77) | <0.0001 |
| Trails B‡ | 809 | 143.3 (8.58) | 148.1 (6.38) | 161.2 (6.38) | 170.0 (7.28) | 0.0008 |
| Boston Naming‡ | 811 | 13.6 (0.18) | 13.5 (0.11) | 13.3 (0.11) | 13.2 (0.13) | 0.015 |
| Category Fluency‡ | 811 | 17.7 (0.61) | 16.4 (0.46) | 16.5 (0.46) | 16.6 (0.52) | 0.11 |
| Buschke Immediate Recall‡ | 813 | 6.4 (0.24) | 6.3 (0.18) | 6.1 (0.18) | 6.1 (0.20) | 0.14 |
| Buschke Delayed Recall‡ | 804 | 7.9 (0.37) | 7.4 (0.28) | 7.4 (0.28) | 7.3 (0.32) | 0.13 |
SE =Standard Error, 3MS scores range from 0-100; Trails A and B scores range from 0 to 300; Boston Naming scores range from 0 to 15; Category Fluency scores range from 0-45; Buschke Immediate and Delayed Recall scores range from 0-12; higher scores indicate higher function for all tests except for Trails A and B in which lower scores are better.
P-value for test of trend
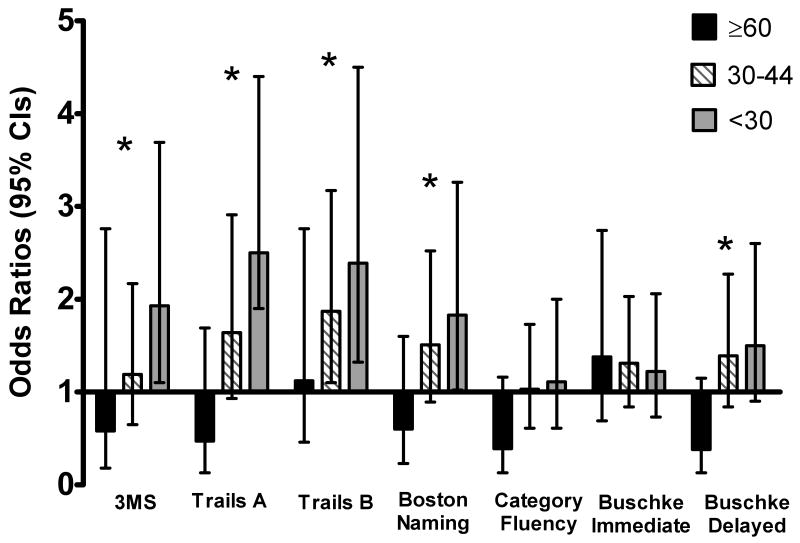
We next determined the association between severity of CKD and clinically significant cognitive impairment defined as a score 1 SD or more severe from the mean (Table 3). Participants with more severe CKD had higher prevalence of cognitive impairment on global cognition (22% for eGFR<30 ml/min/1.73 m2, 14% for eGFR 30-44 ml/min/1.73 m2, 10% for eGFR 45-59 ml/min/1.73 m2, 5% for eGFR ≥60 ml/min/1.73 m2, p<0.001 for trend), on attention (20%, 17%, 9%, 4%, respectively, p<0.001), on executive function (28%, 23 %, 12%, 11%, respectively, p<0.001), naming (24%, 20%, 13%, 8%, respectively, p<0.001), on category fluency (17%, 15%, 13%, 5%, respectively, p=0.02), and on delayed memory (19%, 17%, 13%, 5%, respectively, p=0.003). While this pattern was also evident for the test of immediate memory (22%, 22%, 17%, 19%, respectively, p=0.16), the differences were not statistically significant. After multivariable adjustment for age, gender, race, education, hypertension, depression, BMI, and diabetes, participants with more severe CKD continued to demonstrate greater likelihood of cognitive impairment on most tests (Figure). Compared with persons who had mild or moderate CKD (eGFR 45-59), participants with advanced CKD (eGFR <30) were more likely to have cognitive impairment on tests of global cognition (adjusted odds ratio [OR] 2.0, 95% CI 1.1 to 3.9), naming (OR 1.9, 95% CI 1.0 to 3.3), attention (OR 2.4, 95%CI 1.3 to 4.5), executive function (OR 2.5, 95% CI 1.9 to 4.4) and delayed memory (OR=1.5, 95%CI 0.9 to 2.6) but not on category fluency (OR=1.1, 95% CI 0.6 to 2.0) or immediate memory (OR= 1.2, 95% CI 0.7 to 2.1).
Table 3.
Prevalence and Unadjusted Odds Ratios of Clinically Significant Cognitive Impairment, by eGFR Group.
| Cognitive Test* | Estimated Glomerular Filtration Rate (ml/min/1.73 m2) | |||
|---|---|---|---|---|
| ≥60 | 45-59 | 30-44 | <30 | |
| 3MS | 5% 0.5 (0.2, 1.4) |
10% Reference |
14% 1.5 (0.9, 2.5) |
22% 2.6 (1.5, 4.4) |
| Trails A | 4% 0.4 (0.1, 1.4) |
9% Reference |
17% 2.1 (1.3, 3.5) |
20% 2.7 (1.5, 4.7) |
| Trails B | 11% 0.9 (0.4, 2.0) |
12% Reference |
23% 2.2 (1.4, 3.4) |
28% 2.8 (1.7, 4.6) |
| Boston Naming | 8% 0.6 (0.2, 1.4) |
13% Reference |
20% 1.8 (1.1, 2.8) |
24% 2.2 (1.3, 3.6) |
| Category Fluency | 5% 0.4 (0.1, 1.1) |
13% Reference |
15% 1.3 (0.8, 2.0) |
7% 1.4 (0.8, 2.4) |
| Buschke Immediate Recall | 19% 1.1 (0.6, 2.1) |
17% Reference |
22% 1.4 (0.9, 2.1) |
22% 1.4 (0.9, 2.3) |
| Buschke Delayed Recall | 5% 0.3 (0.1, 1.0) |
13% Reference |
17% 1.4 (0.9, 2.2) |
19% 1.5 (0.9, 2.6) |
Cognitive test with prevalence (%) of impairment, odds ratios and 95% confidence intervals.
Figure.
The likelihood of cognitive impairment (adjusted odds ratios and 95% confidence intervals) across eGFR category (reference group is 45-59 ml/min/1.73m2). Models were adjusted for age, gender, race, education, diabetes, hypertension, BMI, and depression. * P-value for trend test <0.05. 3MS scores range from 0-100; Trails A and B scores range from 0 to 300; Boston Naming scores range from 0 to 15; Category Fluency scores range from 0-45; Buschke Immediate and Delayed Recall scores range from 0-12; higher scores indicate higher function for all tests except for Trails A and B in which lower scores are better.
In subgroup analyses defined a priori by a diagnosis of diabetes or not, race (black compared to white) and gender, we did not observe consistent interactions for these strata (p>0.1 for all) and the association between level of eGFR and cognitive function. We also stratified the cohort by ages 55-64, 65-74 and ≥75 years and found that the prevalence of global cognitive impairment (defined by 3MS) was 9.1%, 18.4% and 13.3% respectively, but there was no interaction by age on the association between CKD severity and cognitive function.
Discussion
In the CRIC COG Study, we found that baseline cognitive function measured across multiple domains was worse among those participants with more severe kidney disease, even after adjustment for several potential confounders including demographic characteristics and relevant comorbidities. The proportion of participants with clinically meaningful cognitive impairment was much greater for those with more severe CKD as well. This pattern was observed for most of the cognitive domains including global cognition, verbal memory, attention, naming and executive function but not for verbal fluency. Similar findings were observed for participants with and without diabetes, men and women and blacks and whites.
Our findings support and extend beyond the findings observed by several published cross-sectional studies. Among 1015 postmenopausal women with cardiovascular disease, for every 10 mL/min/1.73 m2 reduction in GFR, there was a 27% increase in likelihood of global cognitive impairment. In over 20,000 US adults, lower levels of kidney function were found to be associated with increased prevalence of cognitive impairment.13 Level of eGFR was associated with cognitive impairment on executive function, memory, and language measures26. In other studies, subjects with CKD, had significantly poorer performance on tests of executive function, verbal memory, and global performance.27, 28 Similarly, patients with CKD performed significantly worse on both executive and memory measures compared with controls in a small clinical sample.14 The few longitudinal studies that have been conducted on CKD and cognition also support our observations. Among more than 3000 elders, those with CKD (defined as an eGFR <60 ml/min/1.73m2) had greater 3MS score decline over 4 years compared to those without CKD although there were few participants with severe CKD.10 In one of the only studies to investigate the association between CKD and dementia, Seliger et al. reported an elevated risk of incident dementia in elderly subjects with CKD participating in the Cardiovascular Health Cognition Study.11 However, another longitudinal study did not find an association between kidney function and global cognitive impairment or risk in cognitive decline.12 This may be due to differences in study populations, as that study cohort was comprised of older community-dwelling men primarily without CKD.12
There are several mechanisms that might link CKD with cognitive impairment. Individuals with CKD have an increased prevalence of established cardiovascular disease as well as cardiovascular risk factors, and these same risk factors increase risk of developing dementia including both Alzheimer's dementia and vascular dementia.3 CKD is also associated with a number of other factors that also may induce cognitive impairment, including anemia, increased levels of inflammatory cytokines, oxidative stress, and alterations in lipid and homocysteine metabolism.29-33
This study is an important step in advancing our understanding of the important role that kidney function may play in cognitive aging. Strengths of our study include: 1) its recruitment of patients from the ongoing CRIC study which is comprised of a large number of patients with a range of CKD severity, allowing us to explore the association of severity of CKD with cognitive function; 2) the administration of sensitive cognitive tests that evaluate a range of cognitive domains; 3) our ability to adjust for differences across levels of kidney function in important possible confounders to determine if the association between CKD and cognitive function was independent of demographics and comorbidities.
Some limitations of our study deserve mention. As a cross-sectional analysis, the ability to draw causal conclusions between level of eGFR and cognitive function is limited. The pattern of cognitive deficits is more suggestive of subcortical vascular disease involvement rather than underlying Alzheimer's disease but without structural brain imaging results we cannot determine the etiology of clinically significant cognitive impairment. Despite the relatively large, diverse sample of patients with CKD, our results may not be fully generalizable to the broader population with CKD in the U.S. and those CRIC participants with eGFR ≥ 60 ml/min/1.73 m2 most certainly are not reflective of people with better kidney function as they at one time had eGFR <60 ml/min/1.73 m2. Finally, while the effect sizes may seem small for some of the cognitive tests (eg. 3MS), similar effect sizes have been found in studies investigating more well-studied risk factors for cognitive impairment such as hypertension or dyslipidemia.34, 35
In summary, we found that lower level of eGFR was independently associated with impaired cognitive functioning across multiple different domains, especially related to tests of executive function, even after adjustment for sociodemographic and clinical factors. Our results suggest that screening older patients with advanced CKD for cognitive impairment might be important, but future research is needed to determine the appropriate screening tools and the sensitivity and specificity of various cut-points for screening. We hope that the ongoing longitudinal assessments of the CRIC COG study will facilitate a better understanding of the clinical importance, etiology and mechanisms explaining the association between CKD severity and cognition.
Acknowledgments
This study was funded by: NIH Grants U01-DK060990, U01 DK60980, U01 DK 60902, U01 DK61021, U01 DK60984 and R01 DK069406 (CRIC COG). Dr. Yaffe is also supported in part by K24 AG 031155 and an anonymous foundation.
Footnotes
An abstract from this study will be shared at the 2009 International Conference on Alzheimer’s disease and Associated Disorders (ICAD) in Vienna, Austria.
Conflict of Interest: The authors have no financial or personal conflicts of interest to report or disclosures to make.
Author contributions: Study concept and design (KY, MKT, JWK, ASG), acquisition of subjects and data analysis (KY, ASG), interpretation of data (KY, LA, MKT, JWK, ARS, ASG), preparation of manuscript (KY, PLB, JWK, ARS, DC, CA, LA, KD, AO, SS, NR, GM).
References
- 1.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics and Memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launer LJ. Demonstrating the case that AD is a vascular disease: Epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 4.Seliger SL, Gillen DL, Longstreth WT, Jr, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Seliger SL, Longstreth WT, Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 6.Burn D, Bates D. Neurology and the kidney. J Neurol Neurosurg Psychiatry. 1998;65:810–821. doi: 10.1136/jnnp.65.6.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng A, Irizarry MC, Nitsch RM, et al. Elevation of Cystatin C in susceptible neurons in Alzheimer's disease. Am J Pathol. 2001;159:1061–1068. doi: 10.1016/S0002-9440(10)61781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer's amyloid β inhibits in vitro amyloid fibril formation. Neurobiol Aging. 2004;25:1033–1043. doi: 10.1016/j.neurobiolaging.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin-C as a marker of cognitive function in elders: Findings from the Health ABC study. Ann Neurol. 2008;63:798–802. doi: 10.1002/ana.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 11.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 12.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56:2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura MK, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. American Journal of Kidney Diseases. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton WL, Shapiro RJ, Deria S, et al. Differential impact of age on verbal memory and executive functioning in chronic kidney disease. J Int Neuropsychol Soc. 2007;13:344–353. doi: 10.1017/S1355617707070361. [DOI] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 16.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 19.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 20.Saxton J, Ratcliff G, Newman A, et al. Cognitive test performance and presence of subclinical cardiovascular disease in the cardiovascular health study. Neuroepidemiology. 2000;19:312–319. doi: 10.1159/000026270. [DOI] [PubMed] [Google Scholar]
- 21.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 22.Masur DM, Fuld PA, Blau AD, et al. Predicting development of dementia in the elderly with the Selective Reminding Test. J Clin Exp Neuropsychol. 1990;12:529–538. doi: 10.1080/01688639008400999. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 24.Hou CE, Yaffe K, Perez-Stable EJ, et al. Frequency of dementia etiologies in four ethnic groups. Dement Geriatr Cogn Disord. 2006;22:42–47. doi: 10.1159/000093217. [DOI] [PubMed] [Google Scholar]
- 25.Mehta KM, Simonsick E, Rooks R, et al. Black and white differences in cognitive function test scores: What explains the difference? J Am Geriatr Soc. 2004;52:2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Elias MF, Elias PK, Seliger SL, et al. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009 Mar 18; doi: 10.1093/ndt/gfp107. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurella M, Chertow GM, Luan J, et al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 29.Abramson JL, Jurkovitz CT, Vaccarino V, et al. Chronic kidney disease, anemia and incident stroke in a middle-aged, community-based population: The ARIC Study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 30.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 32.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Lindquist K, Pennix BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 34.Johnson KC, Margolis KL, Espeland MA, et al. A prospective study of the effect of hypertension and baseline blood pressure on cognitive decline and dementia in postmenopausal women: The Women's Health Initiative Memory Study. J Am Geriatr Soc. 2008;56:1449–1458. doi: 10.1111/j.1532-5415.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 35.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]