Abstract
Affective variables have been shown to impact working memory and cognitive control. Theoretical arguments suggest that the functional impact of emotion on cognition might be mediated through shifting action dispositions related to changes in motivational orientation. The current study examined the effects of positive and negative affect on performance via direct manipulation of motivational state in tasks with high demands on cognitive control. Experiment 1 examined the effects of monetary reward on task-switching performance, while Experiment 2 examined the effects of both rewards and punishments on working memory, using primary (liquid) reinforcers. In both experiments, dissociable trial-by-trial and contextual (block-related) enhancements of cognitive control during task performance were observed in relationship to motivational incentive value. Performance enhancements were equivalent in the reward and punishment conditions, but were differentially impacted by individual difference measures of trait reward and punishment sensitivity. Together, the results suggest both common and specific mechanisms by which approach and avoidance motivational states influence cognitive control, via activation of reward and punishment processing systems.
Introduction
Much of complex human behavior depends upon our ability to invoke cognitive control, such as inhibiting inappropriate but habitual action tendencies, selectively attending to goal-relevant aspects of the environment, and actively maintaining goal-relevant information over extended periods in service of demanding cognitive activities (e.g. problem-solving, reasoning). In the last two decades there has been an explosion of research that has begun to isolate and elucidate the psychological, computational, and neural mechanisms of cognitive control (Botvinick et al., 2001; Boucher et al., 2007; Braver & Ruge, 2006; Miller & Cohen, 2001; Monsell & Driver, 2000; Smith & Jonides, 1999). There is now general consensus in the field that a core component of cognitive control is the active representation and maintenance of behavioral goal information, which serves as a top-down bias over processing in task-specific pathways (Desimone & Duncan, 1995; Engle, 2002; Miller & Cohen, 2001; O'Reilly, 2006). Recently, it has become appreciated that these cognitive control processes might be influenced by affective states and traits (Ochsner & Gross, 2005; Bishop, 2007; Fales et al., 2008, Pessoa, 2008, Eysenck et al., 2007). In the current study, we extend this work through the use of motivational variables to examine how positive and negative affective information can influence cognitive control function.
We posit that the experimental approach of using motivational manipulations to induce affective states, and subsequently explore how affect-related factors influence cognitive control, has distinct advantages. First, there is general consensus among emotion theorists that a major function of affect is motivational – to create dispositions for action. Positive emotions typically prime approach-related behavioral drives, with negative emotions activating avoidance-related behaviors (Bradley, 2000; Carver & Scheier, 1990; Davidson et al., 1990; Frijda, 1986). Second, activation of approach and avoidance motivational systems is likely to involve specialized neural mechanisms, and consequently may have differential influences over cognitive control processes. For example, Davidson and other theorists have argued for a hemispheric asymmetry, with the left and right frontal cortex specialized for approach and avoidance drives, respectively (Davidson, 1995; Heller & Nitschke, 1997; Tomarken & Keener, 1998). Thus, approach vs. avoidance motivational states may differentially influence cognitive control functions that show frontal lateralization as well (Gray & Braver, 2002).
The importance of motivation might also be critical when considering individual difference effects during cognitive task performance. Personality research has suggested that there are two fundamental dimensions, either called extraversion/neuroticism, BAS/BIS (behavioral approach system, behavioral inhibition system), promotion/prevention, or positive/negative affect susceptibility, along which individuals vary (Eysenck, 1967; Gray, 1994; Higgins, 1997; Humphreys & Revelle, 1984; Larsen & Ketelaar, 1991), For many theorists, the dimensions are also primarily motivational in orientation, and relate to individual differences in the strength or sensitivity of approach and avoidance systems (Carver & White, 1994; Gray, 1981). Thus, in Carver & White's (1994) BIS/BAS scales, BIS refers to sensitivity to cues of punishment while BAS refers to sensitivity to cues of reward. Punishment cues will result in greater anxiety among high BIS individuals, while reward cues will result in greater happiness among high BAS individuals. Likewise, in Higgins' regulatory focus framework, promotion and prevention refer to the differing means by which individuals engage in goal pursuit (Higgins et al., 2001). Individuals high in promotion focus will engage in maximal self-regulation towards the achievement of positive outcomes, whereas individuals high in prevention focus will tend to engage self-regulatory strategies toward the avoidance of negative outcomes. Thus, these individual differences in motivational style or orientation may have a significant moderating impact on how affect impacts cognitive control through experimentally induced changes in motivational state. In prior work, we and others have shown that personality traits such as BIS/BAS can have a significant moderating effect on brain activity and behavior during the performance of tasks that have a high cognitive control demand, such as high-load working memory paradigms (Lieberman, 2000; Gray & Braver, 2002; Gray et al., 2005).
Thus, it is reasonable to postulate that the functional impact of emotion on cognition and behavior may be mediated via motivationally-oriented action dispositions that are induced by changes in affective state, and which may interact with stable individual differences. Prior studies have demonstrated that manipulations of affective variables (e.g., via mood inductions or affectively valenced stimuli) can influence cognitive control in a variety of domains, including response inhibition (Chiu, 2008; Verbruggen & De Houwer, 2007), working memory (J. R. Gray, 2001; Kensinger, 2003; Levens, 2008), and selective attention (Gable, 2008; Rowe, 2007; Williams et al., 1996). A key question examined in the current study is whether direct manipulations of motivational variables may be a particularly effective way of inducing these functional changes in cognitive control and behavioral performance.
The integral links between emotion, motivation and cognitive control have become better appreciated in recent cognitive and neuroscience research (reviewed in Pessoa, 2009). Positive affect and rewarding incentives have been found to provide performance benefits during cognitive tasks involving classification learning (Maddox et al., 2006; Markman et al., 2005), working memory (Krawczyk, 2007; Taylor, 2004; Heitz et al., 2007) and attention-switching (Locke, 2008; Dreisbach, 2006; Dreisbach 2008; Muller et al., 2007). Negative reinforcement has also been shown to modulate affective states and cognitive performance in humans, as a few studies have shown enhanced cognitive performance following negatively reinforcing cues (Engelmann & Pessoa, 2007; Small et al., 2005). Individual differences in stable approach (reward-focused; see Maddox, 2006; Markman, 2005; Locke, 2008) and avoidance (penalty-focused; Locke, 2008; Braver et al., 2009) motivational states have also been shown to interact with these positive and negative reinforcement effects. It has even been shown that literal physical approach and avoidance movements can impact cognitive control processes (Koch et al., 2009; Koch et al., 2008), presumably through implicit changes in motivational state. However, presently it remains unclear whether the cognitive processes impacted by negative reinforcement are common or distinct from those involved when positive reinforcers are used.
The current investigation focused on addressing two related questions regarding the interaction of motivation and cognitive control. First, do manipulations of motivational state have selective effects on cognitive control processes, and are they similar to what has been observed for manipulations of affective state? Second, are the cognitive control effects of positive (approach) and negative (avoidance) motivational states similar or distinct in terms of behavioral signatures and modulation by individual differences? The first question was examined in Experiment 1, which used monetary rewards to induce positive affect and approach motivation during task-switching performance. Task-switching paradigms may be especially appropriate in this domain, because they can provide sensitive and selective behavioral indices of cognitive control function, in terms of task-switch costs. Moreover, in a test of the failure to engage hypothesis (De Jong, 2000), it has been demonstrated that reward manipulations can enhance cognitive control (i.e., reducing switch costs) during task-switching (Nieuwenhuis & Monsell, 2002). The current study represented a significant extension of the prior work, by more directly establishing the specificity of the motivational effect through a within-subject design. Experiment 2 addressed the relationship between positive and negative affective states induced through motivational manipulations during working memory task performance as well as examining the influence of motivation-related individual difference effects. A high-load working memory task was employed in Study 2 both because of prior work showing motivational effects in this paradigm (Heitz et al., 2007), and because comparisons between Experiment 1 and 2 provided the opportunity to test whether the effects might generalize across different task domains. Primary reinforcers (directly delivered liquids) were employed as an ecologically valid means of manipulating qualitatively distinct affective/motivational states associated with approach towards rewarding stimuli versus avoidance of aversive stimuli.
Experiment 1
This experiment investigated the impact of motivation on cognitive control using a cued task-switching paradigm. Task-switching paradigms have been popular in studies of cognitive control and have important advantages that make them well-suited for examining motivational effects, as they have a structure that explicitly involves the changing prioritization of task sets or goals (Meiran et al., 2000; Monsell, 2003). Because of this, there is a natural parallel to motivation, as we hypothesize that motivational variables help set the priority level of these task goals and enhance cognitive control generally. Further, the reprioritization that occurs during task-switching does not occur perfectly, as demonstrated by switching costs in performance that are reliably associated with changing task sets (Meiran et al., 2000; Monsell, 2003). These switch costs can be taken as clear and robust behavioral markers of the efficacy of cognitive control. Thus, switch costs provide an index of the extent to which motivation impacts cognitive control performance through comparisons of single task (low cognitive control load) vs. mixed task (high cognitive control load) blocks, and through switch and repeat trials within the mixed block. An additional manipulation of interest, utilized in the current study, is the preparation time available after task cues prior to target onset. This manipulation permitted an examination of whether long vs. short preparatory times enhance the cognitive control performance benefits carried by the incentive cues, and whether these benefits are seen preferentially when cognitive control demands are high (mixed task).
Method
Participants
Twenty-six young adults (mean age = 20.13 years, 12 female) were recruited from Washington University to participate in the study. Written informed consent was obtained, in accordance with the Washington University Medical Center Human Subjects Committee. Two participants were excluded from analysis because of poor accuracy when performing the task (hit rates of 75% or lower in the experimental task). All participants were right-handed, native English speakers, had corrected to normal vision, and were free from psychiatric or neurological disorders. Participants received reimbursement for participation ($10 / hour or introductory psychology class credit) plus an additional monetary bonus due to the reward incentives. Although participants were not informed of this until the end of the experiment, the bonus was a fixed amount ($5, slightly larger than maximum possible reward), independent of task performance.
Task and Materials
Visual stimuli were presented using PsyScope software running on an Apple PowerMac G4 (Cohen et al., 1993). The target stimuli were bivalent, displaying pictures of faces with words superimposed on them. These stimuli were used for two different classification tasks: gender judgments (male or female) for the faces and syllable judgments (two-syllable or not) for the words. The tasks and stimuli were adapted from those used in Yeung, 2006. Stimulus combinations were created randomly from a bank of 144 faces (male and female), 76 two-syllable words, 38 one-syllable words, and 38 three-syllable words. The faces were stripped of hair and distorted with noise to equate perceptual difficulty between the words and faces. Responses were indicated and recorded via button presses on the PsyScope Button Box. The same two buttons were used for each task. Because the two different tasks involved both bivalent stimuli and overlapping response buttons, the resulting stimulus-response ambiguity necessitated the demand for cognitive control (Meiran, 2000).
Procedure
Participants engaged in a cued-task switching paradigm (trial structure shown in Figure 1A). Prior to each target stimulus, the participants were told to maintain their gaze on a fixation cross in the middle of the screen. A cue was then given, indicating that the participant was to attend to and perform one of the two tasks (“Attend Face” or “Attend Word”) on the subsequent target stimulus. Each response on a trial resulted in visual feedback. On correct response trials, the stimulus randomly changed either color or size, while incorrect responses were followed with the word “Incorrect” appearing in the middle of the screen. Participants were instructed to respond as quickly and accurately as possible. Participants performed four initial blocks of the task in the absence of incentives (and with no knowledge regarding the future potential for incentives; baseline block) to provide stable estimates of performance. The baseline blocks consisted of two single-task (1 face, 1 word) and two mixed-task blocks. All of the baseline blocks contained 48 trials and had a cue-to-target-interval (CTI) of 1500 msec.
Figure 1. Task Structure.
A) Experiment 1: Task Switching. Each trial consisted of a task cue indicating the relevant attentional dimension (word or face) and incentive value of the trial ($$=reward; XX=no reward), a short (500 msec) or long (200 msec) cue-to-target-interval (CTI) during which task preparation could occur, target presentation requiring either face gender or word syllable discrimination, and a feedback period indicating whether reward was obtained. B) Experiment 2: Working Memory. Each trial consisted of a reward cue indicating the incentive value and valence of the trial (indicated by a jug icon for liquid trials or square for no-incentive trials), presentation of the memory set (5-item word list), a short delay serving as a retention interval, a probe (requiring target judgment), and a feedback period during which liquid was delivered (on incentive trials).
Following the baseline block, participants were informed that they would be performing additional experimental blocks with the potential to earn monetary incentives based on their performance. The performance criteria for monetary incentives were based on each participant's own median reaction time on correct response trials collapsed across the single and mixed-task baseline blocks. These performance-linked bonuses were awarded on incentive trials if performance on that trial was both accurate and faster than the participant's median reaction time on baseline block trials. This criterion ensured that optimal performance was required to achieve high rates of reward. Incentive trials were indicated by an incentive cue accompanying the task cue. To match the presentation of task cues, two different cues were used with incentive trials indicated by $$ surrounding the task cue, and non-incentive trials indicated by XX surrounding the task cue. The symbols denoting incentive and non-incentive trials were also used alongside the task cues in the baseline blocks, but in these blocks participants were told that these symbols were irrelevant. This was done to rule out the possibility that subsequent performance in the incentive blocks could be affected by novelty of the incentive cues or differences in low-level perceptual salience. As with task-cues, the incentive cues were randomized on every trial within each experimental block, with 50% of trials containing reward cues and 50% containing non-reward cues. Thus, there was random trial-by-trial variation in the incentive value of trials within these blocks.
Participants performed eight incentive task blocks of 48 trials each, divided into four mixed-task blocks and four single-task blocks (two face and two word). Task-switch and task-repeat trials were equally likely during task switch blocks. Further, half of the mixed-task and single-task blocks were performed with a short CTI (500 msec) and the other half were performed with a long CTI (2000 msec). The RCI was held at a constant 2500 msec across blocks, a duration for which there are no observed differences in task switch cost between random or a fixed RCIs when using equal proportions of task switch and repeat trials, and one in which the impact of passive decay to switch costs is minimal (~1 msec per 100 msec of additional RCI) (Meiran, 2000). Thus, the long fixed RCI minimized the residual effects of previous trials on performance, and enabled better isolation of the effects of preparatory time on task switching performance.
Responses were again followed by informative visual feedback. On incentive trials, responses that met the incentive criteria (accurate and faster than baseline median reaction time) were followed by feedback in the form of a large green dollar sign. Correct responses that did not meet incentive criteria were followed by the words “Next Trial” as feedback. On non-incentive trials, regardless of reaction time, correct responses were followed by a change in color or size to the target stimulus. Incorrect responses in both incentive conditions were followed by the word “Incorrect”. Participants were again instructed to respond as quickly as possible while still maintaining accuracy on all trials of the task. The presentation of all blocks and response configurations were counterbalanced across subjects.
Results and Discussion
Descriptive data from all conditions are provided in Table 1
Table 1. RT, accuracy, and task-switching costs for Experiment 1.
Task Switching Behavioral Performance
| Baseline (No knowledge of Future Incentives) | |||||
|---|---|---|---|---|---|
| Single-Task | Mixed-Task |
Mixing Costs (Repeat-Single) | Local Switch Costs (Switch-Repeat) | ||
| Task-Repeat | Task-Switch | ||||
| Hit Rate | 0.91 (.04) | 0.88 (.07) | 0.84 (.05) | 0.05 (.03) | 0.04 (.03) |
| Response Time (msec) | 963 (209) | 973 (222) | 1013 (248) | 10 (17) | 50 (25) |
| Non-Incentive Trials Within Incentive Blocks | |||||
|---|---|---|---|---|---|
| Short CTI (500 msec) | Single-Task | Mixed-Task |
Mixing Costs (Repeat-Single) | Local Switch Costs (Switch-Repeat) | |
| Task-Repeat | Task-Switch | ||||
| Hit Rate | 0.88 (.07) | 0.86 (.07) | 0.83 (.03) | 0.02 (.01) | 0.03 (.02) |
| ResDonse Time (msec) |
700 (133) | 713 (144) | 755 (162) | 18 (11) | 38 (21) |
| Long CTI (2000 msec) |
|||||
| Hit Rate | 0.88 (.07) | 0.86 (.06) | 0.84 (.03) | 0.02 (.01) | 0.02 (.01) |
| Response Time (msec) | 676 (102) | 730 (151) | 786 (139) | 54 (18) | 56(14) |
| Incentive Trials | |||||
|---|---|---|---|---|---|
| Short CTI (500 msec) | Single-Task | Mixed-Task |
Mixing Costs (Repeat-Single) | Local Switch Costs (Switch-Repeat) | |
| Task-Repeat | Task-Switch | ||||
| Hit Rate | 0.9 (.07) | 0.88 (.06) | 0.86 (.07) | 0.02 (.02) | 0.02 (.01) |
| ResDonse Time (msec) |
639 (127) | 680 (136) | 697 (115) | 41 (13) | 17 (15) |
| Lona CT1 (2000 msec) |
|||||
| Hit Rate | 0.91 (.05) | 0.91 (.04) | 0.89 (.05) | 0 (.01) | 0.02 (.01) |
| Response Time (msec) | 613 (109) | 614 (103) | 602 (97) | 1 (18) | −12 (17) |
** Data refer to group means (excluding error trials) with standard deviation in parentheses
Global incentive effects
Globally, the incentive manipulation was successful in improving performance, as participants achieved above-criteria (i.e., rewarded) performance on 88% of incentive trials (range: 76% to 95%), even though the criteria was individually set based on baseline performance to achieve an expected rate of 50% reward.
Incentive cue effect
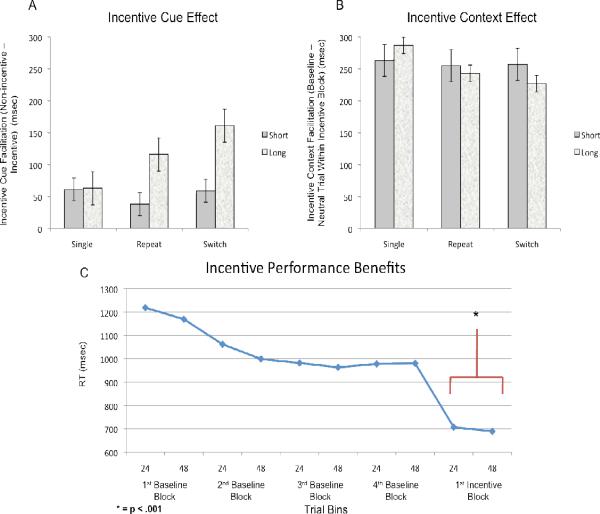
To further quantify the effects of incentive manipulation on behavioral performance during incentive blocks, a 2×2×3 repeated-measures analysis of variance (ANOVA) with within-subjects factors of trial type (single, repeat, switch), CTI duration (short, long), and incentive cue (incentive, non-incentive) was conducted. A significant main effect of incentive was found for both errors and RT, with 4.28% fewer errors (F(1, 23) = 4.3, p < .05) and 82 msec faster RTs (F(1,23) = 31.6, p < .001) on incentive relative to non-incentive trials. For RT, these main effects were qualified by significant interactions with trial type and CTI duration (see Figure 2A; incentive x trial type: (F(2,22) = 5.293, p < .05); incentive x CTI: (F(1,23) =7.4, p < .05); incentive x trial type x CTI: (F(2,22) = 7.732, p <. 01). The cue facilitation effects increased in repeat and switch trials relative to single task blocks, but only on trials with long CTI. This finding suggests that the facilitative effects of incentive are greatest when the demands on cognitive control are highest, but that this facilitation primarily occurs when there is a sufficient preparatory period prior to exertion of control.
Figure 2. Incentive Modulation of Cognitive Control.
A) Data indicate the magnitude of reaction time facilitation on incentive vs. no-incentive trials broken down by trial type (single, repeat, and switch) and CTI. There were incentive benefits on all incentive cued task trials. However, on repeat and switch trials, the greatest facilitation was observed with long CTI. B) Incentive Context Effect. Data indicate the magnitude of reaction time facilitation on no-incentive trials vs. baseline trials broken down by trial type (single, repeat, and switch) and CTI. Across all trial types and CTI durations, the incentive context effect was present, and large in magnitude. C) Onset of Incentive Benefits. Data indicate the task trials performed sequentially by participants, collapsed across participants and placed in 24 trial bins (first and second half of blocks) for the four baseline blocks and the first incentive block. Performance in the baseline blocks begins to asymptote during the second baseline block, and the incentive context effect begins at the beginning of the onset of the first incentive block..
Incentive context effect
Surprisingly, in addition to the direct facilitative effects of the incentive cue, we observed that even the non-incentive trials performed during the incentive block were strongly facilitated compared to baseline task performance. Even when restricting the analysis to the non-incentive trials within incentive blocks, which were identical to the baseline trials, RTs were ~250 msec faster during the incentive blocks. This effect was present for both short and long CTI's in the single-task (Long: 263 msec, t(46) =,5.206 p < .001; Short: 287 msec, t(46) = 5.94, p < .001), repeat, (Long: 255 msec, t(46) = 5.03, p < .001; Short: 243 msec, t(46) = 4.26, p < .001), and switch trials (Long: 257 msec, t(46) = 5.11, p < .001; Short: 227 msec, t(46) = 2.94, p < .005), leading to an insignificant interaction with CTI and trial type (both F < 1; Figure 2B). The incentive context effect did not induce a speed-accuracy tradeoff, as hit rates for non-incentive trials were not significantly impacted during incentive blocks (single task= 2.17% more errors, t(46) = .971, n.s.; mixed task = 2.01% more errors, (t(46) = .936, n.s.). This pattern also did not interact with trial type (F(2,22) = 1.192, n.s.).
One potential confound with regard to the incentive context effect is the fact that the baseline condition was always performed prior to the incentive blocks. This raises the issue of whether some of the putative context effects occurring during the incentive blocks were actually related to changes occurring during the baseline block. The data do not support such an alternative interpretation. First, although the same manipulation of incentive cues (XX vs. $$) was present during the baseline block, participants clearly did not attend to this manipulation (consistent with the task instructions which indicated that it was task-irrelevant), as there were no significant differences in hit rate (0.4%; F < 1) or RT (9 msec; F <1) as a function of cue type during the baseline block. Second, performance clearly reached asymptotic levels during the baseline condition, as indicated by the stable RTs beginning at the second half of the second baseline block, through the last block of baseline trials (Figure 2C). Thus, the striking reduction of RTs at the start of the first incentive block clearly represents a discontinuity in performance that cannot be attributed to a practice (or time-on-task) effect.
Task switching effects
We reanalyzed the data from the incentive blocks in terms of mixing costs (repeat– single task trials) and local switching costs (switch – repeat trials), a standard form used in the task-switching literature (Table 1). For non-incentive trials, both of these switching costs were significant at the long duration CTI (Mixing: (t(46) = 2.5 p < .05); Local Switch: (t(46) = 2.8 p < .01), reflecting the reliability of these effects in the literature. However, on incentive trials, particularly at the long delay, switching costs were abolished (Mixing: (t(46) = .031, n.s.; Local Switch: (t(46) = −.457, n.s.). The abolishment of these switch costs did not reflect a speed-accuracy tradeoff, as accuracy switch costs were also reduced on incentive trials (though this effect was not statistically significant). Thus, the nature of the effect is consistent with an optimization of cognitive control under incentive conditions.
Interestingly, although the switch costs in the incentive condition were not significantly different from zero, they actually showed a numerical trend towards a reverse switch cost. The numerically faster reaction times on incentive switch trials compared to repeat trials may have reflected an enhanced use of incentive information when cognitive control demands are the highest (i.e., on switch trials). However, this interpretation must be treated with caution since the effect was not statistically reliable.
Experiment 2
Experiment 1 provides evidence that motivational incentives can enhance performance in a cognitive control task. In Experiment 2, we compared the effects of positive versus negative motivational states by varying whether incentives were rewarding or aversive. Further, primary reinforcers (i.e., tasted liquids) were used as incentives rather than money, not only because of their continuity with well-established animal literature (in which primary reinforcers are exclusively utilized as motivational incentives), but also because of their increased ecological validity. Specifically, it is likely that the motivational/affective state induced by the consumption of an aversive liquid is qualitatively distinct, and not merely opposite to, that induced by the consumption of a pleasurable liquid. The same may not be true for monetary incentives (i.e., rewards and penalties). Two different concentrations of incentive liquid were used in order to test for intensity graded incentive effects.
Motivational incentive effects were examined within the context of performance of a demanding working memory task. We utilized a working memory rather than the task-switching paradigm of Experiment 1, because motivational effects have been shown most robustly within the domain of working memory (Krawczyk et al., 2007, Taylor et al., 2004, Pochon et al., 2002, Gilbert & Fiez, 2004). Indeed, in a recent study by Heitz and colleagues (Heitz et al., 2007), working memory capacity was found to be reliably increased under motivational incentives to an equivalent degree in low and high-span individuals. Likewise, although it is well-accepted that high-load working memory tasks place significant demands on cognitive control, these are likely to be at least somewhat distinct from those engaged in task-switching situations. Thus, investigating motivational manipulations within working memory as well as task-switching permits an examination of the extent to which the motivational effects are domain general.
Finally, individual differences in personality and motivational orientation were assessed to investigate how such individual differences might moderate the observed effects. In order to increase performance variability across participants, incentives were also dependent on a more stringent performance criterion than that used in Experiment 1 (i.e., keeping reward and penalty rates off the floor & ceiling).
Methods
Participants
Thirty young adults (mean age 20.77 years, 17 female) were recruited from Washington University to participate in return for payment ($10 / hour). Written informed consent was obtained, in accordance with the Washington University Medical Center Human Subjects Committee. Participants were required to be able to refrain from drinking liquids for three hours prior to the experiment without any adverse health effects or excessive discomfort, to be free from food allergies to the apple juice, and not to be on a salt restricted diet. All participants were right-handed, native English speakers, had corrected to normal vision, and were free from psychiatric or neurological disorders.
Task and Materials
Participants engaged in a delayed item recognition task of working memory (Sternberg, 1966). Visual stimuli were presented using E-Prime version 2.0 software running on a Windows PC laptop (Schneider, 2002). Participants saw a set of five English words on the screen, followed after a delay by a single recognition probe word, which they then had to identify as either part of the original memory set or not part of the set (Figure 1B). Eleven hundred stimulus words were taken from the English Lexicon Project at Washington University (http://elexicon.wustl.edu; (Balota, 2007), were each 1 to 2-syllables and 4–6 letters in length, and were classified as nouns, adjectives, or verbs. The mean frequency of the words was approximately log 10 based on the Hyperspace Analogue to Language (HAL) corpus (Lund & Burgess, 1996). Adverbs, plurals, and emotion-provoking words were not included. Each word was presented only once during the course of the experiment. Participants were instructed to press the target key when the probe word was part of the original memory set, and to press the non-target key when the probe word was not part of the original memory set. Responses were indicated and recorded via key presses on the laptop keyboard.
Procedure
Task performance was conducted under three different, blocked incentive conditions of 40 trials each: baseline, reward, and punishment. In the baseline condition, no incentives were provided (and participants were naive regarding the potential for incentives in upcoming blocks). In the reward condition, trials in which the performance criteria were met ended with a squirt of apple juice (rewarding liquid) delivered directly to the mouth; trials in which performance was below criteria were followed instead by a tasteless neutral liquid designed to resemble saliva based on a mixture of KCl and NaHCO (O'Doherty et al., 2001). In the punishment condition, trials in which the performance criteria were met ended with delivery of the neutral liquid, whereas below criteria trials were followed by delivery of saltwater (punishing liquid). All liquids were administered in 1.5mL portions. Participants performed the baseline block first without any knowledge that future blocks would be performed for incentives.
All three conditions shared a common trial structure (Figure 1B). First, a cue was presented (1000 msec), indicating the incentive available on the trial. Reward incentives were indicated with a light-colored “jug” icon, and punishment incentives were indicated with a dark-colored icon. On non-incentive and baseline trials a blue square was presented instead. Next, a memory set of five words was presented on the screen (2500 msec, followed by a delay period (retention interval; 3500 msec). After the delay, a probe word appeared and the participant responded to indicate whether or not the probe was included in the previously shown memory set. Half of the probes were targets (in the memory set) and half were new. Following the response, feedback was provided. The receipt of an incentive was based on baseline performance: incentives were gained if the given response was correct and faster then the 30th percentile (ordered from fastest to slowest) of the participants' correct baseline RTs. Participants were asked to refrain from drinking any liquids for three hours prior to the experiment, such that they would be thirsty and motivated to work for liquid rewards.
Each incentive block included three types of randomly intermixed trials, occurring with equal frequency: no incentive (identical to those of the baseline condition), high incentive, and low incentive trials. High and low incentives were differentiated by the number of incentive cue icons presented prior to trial onset (3 for high incentive, 1 for low incentive). High and low incentive trials differed in the concentration of liquids to be gained or avoided. During high reward incentive trials, a squirt of pure apple juice was delivered while during low reward incentive trials, a squirt of diluted apple juice (half apple juice/half water) was delivered. During high penalty incentive trials, a 0.4M concentration saltwater was delivered while on low penalty incentive trials, a 0.1M concentration saltwater was delivered. During non-incentive trials of the incentive blocks, participants did not receive any liquid, but instead only received a visual message, indicating whether the response was correct or not.
Individual Difference Measures
After the task was completed, participants completed several personality measures. Of particular interest were well-established measures from the literature previously used to measure traits related to reward and punishment sensitivity: the BIS/BAS scales (Carver & White; Gray, 1981), the Regulatory Focus Questionnaire (Higgins et al., 2001), and the Generalized Reward and Punishment Expectancy Scales (Ball & Zuckerman, 1990). The BIS/BAS scales measure emotional reactivity to cues of punishment (BIS) and reward (BAS). High BAS individuals tend to exert significant to achieve rewards and high BIS individuals exert more effort in identifying and avoiding potential threats. The GRAPES (reward subscale) is thought to tap into a slightly different aspect of reward processing: the extent to which people believe that they are likely to obtain rewards available in the environment. In contrast, the punishment subscale indicates the extent to which individuals believe it is likely that they will be the recipient of punishments and penalties in the environment. GRAPES and BIS/BAS are conceptually related because it would be difficult to be highly excited by reward cues, or worried about punishment cues, if a person felt there was slim to no chance of obtaining that outcome. The RFQ is a higher-level, broad construct. It assesses individuals' tendencies to focus on promotion goals (achieving intrinsically desired goals to move closer to an “ideal self”) vs. prevention goals (achieving extrinsically demanded goals to move closer to an “ought self”) (Higgins, 1999). Promotion-focused individuals direct more attention towards stimuli that indicate possible rewards, and look to maximize outcomes. Prevention-focused individuals direct attention towards possible punishments, and work to minimize damaging outcomes.
Results and Discussion
Descriptive data for all conditions are provided in Table 2.
Table 2. RT and accuracy rates for Experiment 2.
EXPERIMENT 2 RT and Hit Rate Differences Across Blocks (Exp. 2)
| Block Condition | Incentive Cue Type | |||
|---|---|---|---|---|
| non-incentive | low | high | Overall Incentive (low + high) | |
| Baseline |
RT M = 708 msec SD = 222 Accuracy Rate M = .95 SD = .10 |
--- | --- | --- |
| Reward |
RT M = 624 msec SD = 172 Accuracy Rate M = .86 SD = .10 |
RT M = 593 msec SD = 164 Accuracy Rate M = .87 SD = .16 |
RT M = 556 msec SD = 160 Accuracy Rate M = .90 SD = .10 |
RT M = 575 msec SD = 157 Accuracy Rate M = .89 SD = .10 |
| Punishment |
RT M = 643 msec SD = 161 Accuracy Rate M = .84 SD = .10 |
RT M = 577 msec SD = 147 Accuracy Rate M = .86 SD = .08 |
RT M = 559 msec SD = 155 Accuracy Rate M = .85 SD = .09 |
RT M = 568 msec SD = 146 Accuracy Rate M = .85 SD = .07 |
| Overall Across Incentive Blocks: |
RT M= 590 msec SD = 143 |
Accuracy Rate M = .84 SD = .07 |
||
Global incentive effects
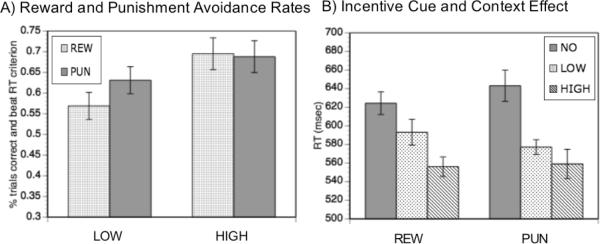
Across incentive valences, the incentive motivation successfully enhanced performance, as participants received a reward or avoided a punishment (REW = 63.2%; PUN = 66%) on a high proportion of trials relative to the rates suggested by the incentive criterion (30%). Nevertheless, these values were not at ceiling levels, indicating a more difficult task. Additionally, there was a high degree of between-subject variability in both conditions (REW range: 25–100% ; PUN range: 32 – 100%), making them suitable for individual difference analyses. On high incentive trials, rewards were more often achieved or punishments avoided than on low incentive trials (F(1,29) = 11.66, p < 0.01). Reward rates did not differ from punishment avoidance rates (F(1,29) = 0.64, p = 0.429) and there was no further interaction with incentive value (F(1,29) = 1.61, p = 0.215) (Figure 3A).
Figure 3.
A) Reward and punishment avoidance rates: proportion of trials correct and faster than criterion RT. Y – axis starts at the value expected by the RT criterion. B) Response times for blocked and item incentive effects (REW = reward block, PUN = punishment block, NO = no incentive trials, LOW = low concentration incentive trials, HIGH = high concentration ince1ntive trials).
Incentive cue effects
The trial and valence-specific effects of incentive were examined by analyzing performance in incentive blocks as a function of the incentive cue. A two-way (2 × 3) ANOVA including the factors valence (REW vs. PUN) and value (high vs. low vs. no trials) revealed a significant main effect of incentive value (F(2,28) = 16.12, p < 0.001, Figure 3B), with response latencies 62 msec faster on average for incentive trials compared to non-incentive trials. This incentive cue effect indicates that performance improvement occurred on a trial-by-trial basis. Additionally, faster RTs in high incentive compared to low incentive trials were observed (t(29) = 2.23, p = 0.05). There were no main effects or interaction with valence (main effect: F(1,29) = 0.012, p = 0.95; interaction: F(2,28) = 1.60, p = 0.21). For error rates, the effects of value, category, and their interaction were all insignificant (p's > .09), indicating the absence of a speed-accuracy tradeoff and providing evidence that WM performance was improved on incentive trials. Additionally, the absence of valence effects suggests that both approach and avoidance motivation equivalently enhanced performance.
Incentive context effect
The tonic incentive context effect found during task-switching was also observed in the working memory task, as no-incentive trials performed in the context of the incentive block were on-average 74 msec faster than the same trials performed in the context of the baseline block (REW: t(29) = 3.31, p < 0.01; PUN: t(29) = 2.28, p < 0.05). However, while overall accuracy in the task was high (89.4% correct averaged for all conditions) the incentive context was also associated with an increase in errors as confirmed by a comparison of no-incentive trials against baseline in both reward (t(29) = 5.18, p < 0.0001) and punishment (t(29) = 4.31, p < 0.0001). These decreases in overall accuracy may be explained by the increasing working memory interference that builds after each subsequent working memory trial. However, the RT facilitation effects associated with incentive context cannot be accounted for by the increasing interference account, and are unlikely to be explained merely by practice (see Experiment 1, Figure 2C).
Individual differences
The three scales for reward and punishment sensitivity were used to test for individual difference effects. The following data were obtained for the different measures: BIS (mean = 20.43, range = 20), BAS (mean = 13.70, range = 7), GRAPES-PUN (mean = 6.44, range = 12), GRAPES-REW (mean = 8.87, range = 12), RFQ-prevention (mean = 17.46, range = 18), RFQ-promotion (mean = 23.31, range = 16). The distribution of scores fell within normal ranges for each measure (Ball & Zuckerman, 1990; Carver & White, 1994; Higgins et al., 2001). In order to increase the psychometric robustness of the measures, we averaged (after z-score normalization) BIS, GRAPES-PUN, and RFQ-promotion, as well as BAS, GRAPES-REW, and RFQ-prevention to create composite indices of reward sensitivity and punishment sensitivity (Zelenski & Larsen, 1999).
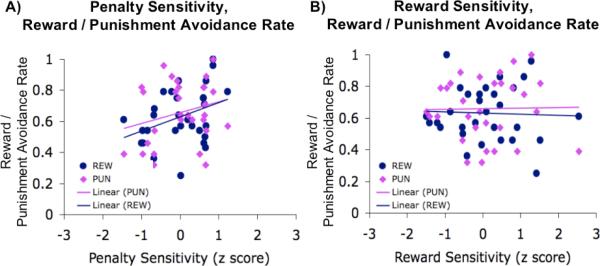
These indices were used in correlation analyses to test the individual variations in task performance. We observed that higher punishment sensitivity correlated positively with punishment avoidance rate (r = .33, p = .07), but also with reward rate (r = 0.40, p < 0.05, Figure 4A). These values did not change in when partial correlations were also computed controlling for reward sensitivity. On the contrary, reward sensitivity was not correlated with either reward or punishment avoidance rate in the reward nor the punishment block (Figure 4B). Thus punishment sensitivity explained a substantial part of performance variance, whereas reward sensitivity did not.
Figure 4. Correlations.
A) Correlation between penalty sensitivity (z-score) and reward or penalty avoidance rate in each task condition. REW= reward; PUN=punishment. Linear regression lines show the significant positive correlations in each condition. B) Correlation between reward sensitivity (z score) and reward / penalty avoidance rates in each task condition. REW= reward; PUN=punishment. Linear regression lines show the lack of correlation in either condition.
Because the reward and punishment avoidance rates comprised both RT and error measures, we looked to see if the individual difference measures were differentially strong with either. For the punishment condition, punishment sensitivity predicted error rates (r = −0.43, p < 0.05) and RT improvement (relative to baseline; r =.32, p =.08). The error rate effect remained reliable in partial correlations controlling for reward sensitivity, but the RT effect dropped below significance (r =.27, p =.16). In the reward condition, neither reward sensitivity nor punishment sensitivity predicted error or RT measures.
General Discussion
The current study consisted of two separate experiments that examined how manipulating motivational incentives, and the subsequently induced affective states, impacted performance in tasks involving cognitive control. The results of these studies support four main conclusions. First, observations from both experiments supported the primary hypothesis that performance would be enhanced with motivational incentives, but further suggest two dissociable behavioral changes: a trial-by-trial effect (incentive cue) and a global state-related effect (incentive context). Second, cognitive enhancement effects were not only observed in relationship to approach motivation and positive affect for reward incentives, but also from avoidance motivation and negative affect for punishment incentives. Third, individual differences in penalty/punishment sensitivity appeared to be specifically linked to incentive-related enhancements in performance, even when reward incentives were used. Finally, the cognitive enhancement effects occurred with both primary (liquid) and secondary (money) reinforcers, operating through the same incentive cue and context effects. These findings extend our knowledge of how motivation and affect influence cognitive performance.
Motivational Effects on Cognitive Control
The results of the present study provide evidence that motivational incentives can enhance cognitive control during task performance. Experiment 1 provided the most direct evidence for this claim. Incentive trials were associated with both reaction time facilitation and reduced errors, but were significantly stronger in mixed (i.e., task-switching) blocks that had high cognitive control demands, relative to low control single-task blocks. It should be noted that because a long RCI was employed (2500 msec), the switch costs in this task were relatively small in absolute magnitude at baseline. Nevertheless, these baseline costs were statistically robust, and under incentive conditions, these switch costs were statistically eliminated. Moreover, the incentive effects appeared to be related to preparatory control processes, as they were only found in trials with long preparatory intervals. This suggests cognitive control was optimal (quick RT and high accuracy) when incentive value was high, but only when sufficient preparatory time was available to integrate the incentive and task cue information into an effective task-set updating strategy. As such, the results suggest a tight link between motivational salience and preparatory task control.
A second important finding of this study was the identification of two distinct types of motivational effects. In addition to the trial-by-trial effects of incentive value modulation (the incentive cue effect), we also observed an additional global sustained effect of incentives (the incentive context effect). This effect is defined by changes in performance observed on no-incentive trials within incentive blocks, compared to these same trials performed in baseline (i.e., non-incentive) blocks. The performance changes observed due to incentive context appeared to be more global, potentially due to increased affective salience of the incentive blocks, and less specifically tied to enhanced cognitive control. Thus, in Experiment 1, the incentive context effect was equivalent across single, repeat, and switch blocks, and did not attenuate switch costs (an index for the need for cognitive control). Moreover, in Experiment 2, the incentive context effect provided benefits for RT, but not working memory accuracy (a key component of cognitive control).
It is important to note that the results also argue against a potential alternative explanation of the incentive context effect as merely being due to task practice. Specifically, we found that baseline RTs tended to asymptote well before the beginning of incentive blocks (Figure 2C). Thus, the dramatic RT facilitation effects observed at the onset of incentive block performance reveal a clear discontinuity that seems unlikely to reflect the types of gradual performance changes that are associated with increased task practice. Additionally, in other studies we have found similar context effects, even after both increasing the duration of the baseline blocks and comparing against post-incentive baselines (Savine & Braver, submitted).
Avoidance Motivation, Individual Differences, and Cognitive Performance
In Experiment 2, we found that the presence of both rewarding and punishing incentives led to improved performance in the Sternberg working memory task. This was observed via both block and trial-specific incentive effects. As such, the results provide a clear demonstration that avoidance motivation is as effective as approach motivation in leading to cognitive enhancement. This is an important demonstration, since to our knowledge there have been very few studies examining the effect of punishment incentives on cognitive processing, and fewer still that have directly compared reward and punishment conditions under matched conditions (Small et al., 2005).
Nevertheless, the finding of statistically equivalent effects of both punishment and reward incentives was somewhat surprising, given theoretical frameworks that suggest distinct effects of approach and avoidance motivation on cognitive control (Crowe & Higgins, 1997; Higgins, 1999). For example, in prior work, Gray and Braver (Gray & Braver, 2002; Gray et al., 2002) hypothesized and then confirmed that approach states would differentially enhance verbal or visuospatial working memory, whereas avoidance states would produce the opposite effects. The observed pattern of equivalent effects instead suggest an interpretation in which the incentive conditions led to changes in affect and a generalized increase in motivational drive, related to the increased salience of task performance, rather than a valence-specific effect (Roesch & Olson, 2004).
The strength of avoidance motivation was further ascertained by the results that performance under incentive conditions was significantly moderated by trait differences in a composite measure of the affective orientation of punishment sensitivity. Although the associations were most robustly observed in the punishment condition as might be expected, punishment sensitivity was also positively associated with higher rates of reward in the reward condition, whereas none of the conditions correlated with reward sensitivity. One interpretation of this pattern of results is that they indicate punishment sensitivity serves as a more powerful influence on cognitive processing than reward sensitivity. Since there is always a relative punishment possible as an outcome in Experiment 2 (neutral solution relative to juice in reward blocks; salt water relative to neutral solution in punishment blocks), the negative dimensions of incentives may have been more salient the positive ones. This interpretation is consistent with a standard view in the emotion literature that individuals are more sensitive to negative than positive information and emotions across a wide range of psychological phenomena (Baumeister et al., 2001). It is also consistent with the general economic principle of loss aversion (Kahneman & Tversky, 1979), which posits as a central assumption that people's preferences and behavior tend to be more strongly influenced by losses and disadvantages than by gains and advantages. Thus, it may have been the case that even in the rewarding incentive conditions, participants may have been focusing on negative reinforcing factors such as the loss of future rewards, the thought of “failing” at a “simple” task, or avoiding obtaining the neutral liquid.
Effects of Incentive Category and Valence
The two experiments differed in the nature of the motivational incentives used. Experiment 1 used money, a secondary incentive, while Experiment 2 used liquids, a primary incentive. Money served as a more abstract reward, as its delivery was delayed until after the completion of the task, whereas the liquid was delivered immediately as a direct behavioral consequence of performance. We believe that this is one of the first demonstrations in humans that primary consumed reinforcers can modulate affect and performance in cognitive tasks. As such, these results point to the continued use of such reinforcers in human cognitive research, so as to be able to draw closer connections to animal studies, which regularly use this same category of incentive.
Explorations of direct manipulations of incentive category also are an attractive target for future research. In a recent neuroimaging study directly comparing the effects of monetary and liquid incentives, we found that although behavioral performance effects were equivalent, strong distinctions in brain activation dynamics were also present across incentive categories (Beck et al., in preparation). We interpreted these patterns as reflecting the distinction between immediately consumed and purely symbolic rewards, which may translate into a motivational and affective distinction between implicit conditioned learning effects (for primary consumed rewards) and more consciously accessed representations of incentive value (for symbolic rewards). Further work will be needed to determine if such distinctions might also impact the modulation of cognitive control processes during task performance.
It is also worth investigating whether the emergence of distinct behavioral patterns is tied to the saliency of the incentive. We observed strong trial-by-trial and contextual effects using both the negative and positive reinforcing liquids, suggesting that the incentives incentive effects were similar both qualitatively and quantitatively to the performance effects observed with monetary incentives in Experiment 1. This observation was also made in a prior study using matched monetary rewards and penalties in the same working memory task employed here (Locke, 2008). Nonetheless, an important direction for future research will be to directly manipulate and assess motivational incentive effects in cognitive performance in terms of subjective value ratings, as a key hypothesis of the motivational framework is that the more highly an incentive is valued, the stronger an impact it will have on affect and subsequent behavioral performance.
A final important direction for future study would be to modify the task used in Experiment 2 to better detect subtle differences in cognitive control functions under approach vs. avoidance motivation states. For example, in a working memory task such as the one used, motivational valence might show interactions with working memory load, such that load-related changes in performance might differ in reward and punishment conditions. Alternatively, effects related to other working memory control processes may be detected, such as manipulation of maintained content or interference resolution. To detect such effects, it would be necessary to include task conditions that appropriately vary the demands on such processes (e.g., internal manipulations of load, interference, manipulation etc.). Thus, it may premature to conclude that reward and punishment incentives impact cognitive control in an equivalent fashion before testing for differences more carefully.
Motivation versus Affect
In comparison with studies directly utilizing motivational manipulations, there is a much larger literature examining the influence of mood induction on cognitive performance (Dreisbach & Goschke, 2004; Easterbrook, 1959; Isen, 1993; Oaksford et al., 1996; Rowe, 2007). These investigations have revealed that mood inductions may have diffuse impacts on performance, including the broadening of attentional focus with positive affect vs. narrowing of attentional focus with negative affect (Gasper & Clore, 2002; Rowe, 2007), and selective enhancement of verbal working memory with positive mood induction vs. enhancement of visual working memory with negative mood induction (Gray, 2001). How the impact of these inductions may resemble or differ from affective experience induced via the use of motivational incentives, such as those used in the present study, remains unclear. It may be useful to examine the impact of motivational incentives in tasks that more explicitly parallel those used in mood induction studies (e.g., tasks where attentional broadening/narrowing under reward and punishment can be examined). Likewise, in future studies, it will be important to more directly assess the relationship between motivational manipulations and affective ones, through additional measures, such as self-report scales and well-established psychophysiological indices (e.g., heart rate, skin conductance, pupil dilation, startle responses, etc).
References
- Ball SA, Zuckerman M. Sensation seeking, Eysenck's personality dimensions and reinforcement sensitivity in concept formation. Personality and Individual Differences. 1990;11:343–353. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchinson KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The english lexicon project. Behavioral Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5(4):323–370. [Google Scholar]
- Beck SM, Jimura K, Savine AC, Locke HS, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol Rev. 2007;114(2):376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of physiology. Cambridge University Press; Cambridge: 2000. pp. 602–642. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106(18):7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Ruge H. Functional neuroimaging of executive functions. In: Cabeza R, Kingstone A, editors. Functional neuroimaging of cognition. MIT Press; Cambridge, MA: 2006. pp. 307–347. [Google Scholar]
- Carver CS, Scheier MF. Origins and functions of positive and negative affect: A control-process view. Psychological Review. 1990;97:19–35. [Google Scholar]
- Carver CS, White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The bis/bas scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chiu PH, Holmes AJ, Pizzagalli DA. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage. 2008;42:988–997. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. Psyscope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using macintosh computers. Behavior Research Methods, Instruments, and computers. 1993;25(2):257–271. [Google Scholar]
- Crowe E, Higgins ET. Regulatory focus and strategic inclinations: Promotion and prevention in decision-making. Organizational Behavior and Human Decision Processes. 1997;69(2):117–132. [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. MIT Press; Cambridge, MA: 1995. pp. 361–387. [Google Scholar]
- Davidson RJ, Ekman P, Saron C, Senulis J, Friesen WV. Approach/withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- De Jong R. An intention-activation account of residual switch costs. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance xvii. The MIT Press; Cambridge: 2000. pp. 357–376. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dreisbach G. How positive affect modulates cognitive control: The costs and benefits of reduced maintenance capability. Brain and Cognition. 2006;60(1):11–19. doi: 10.1016/j.bandc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: Reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn. 2004;30(2):343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66(3):183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7(3):668–674. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Eysenck HJ. The biological basis of personality. Thomas; Springfield, IL: 1967. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, et al. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8(3):239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Frijda N. The emotions. Cambridge University Press; London: 1986. [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychological Science. 2008;19:476–482. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global versus local processing of visual information. Psychol Sci. 2002;13(1):34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Gilbert AM, Fiez JA. Integrating reward and cognition in the frontal cortex. Cognitive, Affective, and Behavioral Neuroscience. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- Gray JA. A critique of eysenck's theory of personality. In: Eysenck HJ, editor. A model for personality. Springer; New York: 1981. pp. 246–276. [Google Scholar]
- Gray JA. Personality dimensions and emotion systems. In: Ekman P, Davidson RJ, editors. The nature of emotion. Oxford; New York: 1994. pp. 329–331. [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS. Integration of emotion and cognitive control: A neurocomputational hypothesis of dynamic goal regulation. In: Moore SC, Oaksford MR, editors. Emotional cognition. John Benjamins; Amsterdam: 2002. pp. 289–316. [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fmri. Cogn Affect Behav Neurosci. 2005;5(2):182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Schrock JC, Payne TW, Engle RW. Effects of incentive on working memory capacity: Behavioral and pupillometric data. Psychophysiology. 2007;45(1):119–129. doi: 10.1111/j.1469-8986.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition and Emotion. 1997;11:637–661. [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52(12):1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Promotion and prevention as a motivational duality: Implications for evaluative processes. In: Trope SCY, editor. Dual-process theories in social psychology. Guilford Press; New York, NY: 1999. pp. 503–525. [Google Scholar]
- Higgins ET, Friedman RS, Harlow RE, Idson LC, Ayduk ON, Taylor A. Achievement orientations from subjective histories of success: Promotion pride versus prevention pride. European Journal Of Social Psychology. 2001;31:3–23. [Google Scholar]
- Humphreys MS, Revelle W. Personality, motivation, and performance: A theory of the relationship between individual differences and information processing. Psychological Review. 1984;91(2):153–184. [PubMed] [Google Scholar]
- Isen AM. Positive affect and decision making. In: Lewis M, Haviland J, editors. Handbook of emotion. Guilford; New York: 1993. pp. 261–277. [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- Kensinger EA, Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3:378–393. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Koch S, Holland RW, Hengstler M, van Knippenberg A. Body locomotion as regulatory process: Stepping backward enhances cognitive control. Psychoogical Science. 2009;20(5):549–550. doi: 10.1111/j.1467-9280.2009.02342.x. [DOI] [PubMed] [Google Scholar]
- Koch S, Holland RW, van Knippenberg A. Regulating cognitive control through approach-avoidance motor actions. Cognition. 2008;109(1):133–142. doi: 10.1016/j.cognition.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Research. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar E. Personality and susceptibility to positive and negative emotional states. Journal of Personality and Social Psychology. 1991;61:132–140. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Emotion processing effects on interference resolution in working memory. Emotion. 2008;8:267–280. doi: 10.1037/1528-3542.8.2.267. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Introversion and working memory: Central executive differences. Personality and Individual Differences. 2000;28:479–486. [Google Scholar]
- Locke HS. Unpublished Ph.D. Dissertation. Saint Louis; Washington University: 2008. Motivational and personality influences on cognitive control and task performance. [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments & Computers. 1996;28(2):203–208. [Google Scholar]
- Maddox WT, Baldwin GC, Markman AB. A test of the regulatory fit hypothesis in perceptual classification learning. Mem Cognit. 2006;34(7):1377–1397. doi: 10.3758/bf03195904. [DOI] [PubMed] [Google Scholar]
- Markman AB, Baldwin GC, Maddox WT. The interaction of payoff structure and regulatory focus in classification. Psychol Sci. 2005;16(11):852–855. doi: 10.1111/j.1467-9280.2005.01625.x. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of stimulus task sets and response task sets during task switching. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance xviii. The MIT Press; Cambridge: 2000. pp. 377–400. [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cognitive Psychology. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Monsell S, Driver J. Control of cognitive processes. Vol. XVIII. MIT Press; Cambridge, MA: 2000. [Google Scholar]
- Muller J, Dreisbach G, Goschke T, Hensch T, Lesch K, Brocke B. Dopamine and cognitive control: The prospect of monetary gains influences the balance between flexibility and stability in a set-shifting paradigm. European Journal of Neuroscience. 2007;26(12):3661–3668. doi: 10.1111/j.1460-9568.2007.05949.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Monsell S. Residual costs in task switching: Testing the failure-to-engage hypothesis. Psychon Bull Rev. 2002;9(1):86–92. doi: 10.3758/bf03196259. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314(5796):91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- Oaksford M, Morris F, Grainger B, Williams JMG. Mood, reasoning, and central executive processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22(2):476–492. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, et al. The neural system that bridges reward and cognition in humans: An fmri study. Proc Natl Acad Sci U S A. 2002;99(8):5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences, United States of America. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivational enhancement of cognitive control through performance incentives. submitted. [Google Scholar]
- Schneider W, Eshman A, Zuccolotto A. E-prime: A user's guide. Pittsburgh, PA: 2002. [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex. 2005;15(12):1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21(3):1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition and Emotion. 1998;12:387–420. [Google Scholar]
- Verbruggen F, De Houwer J. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cognition & Emotion. 2007;21(2):391–403. [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional stroop task and psychopathology. Psychological Bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Zelenski JM, Larsen RJ. Susceptibility to affect: A comparison of three personality taxonomies. Journal of Personality. 1999;67:761–791. doi: 10.1111/1467-6494.00072. [DOI] [PubMed] [Google Scholar]