Abstract
Cerebral cavernous malformations (CCM) are vascular lesions causing seizures and stroke. Mutations causing inactivation of one of three genes, ccm1, -2, or -3, are sufficient to induce vascular endothelial cell defects resulting in CCM. Herein, we show that loss of expression of the CCM1, -2, or -3 proteins causes a marked increase in expression of the GTPase RhoA. Live cell imaging with a RhoA-specific biosensor demonstrates increased RhoA activity with loss of CCM1, -2, or -3, with an especially pronounced RhoA activation in both the cytosol and the nucleus with loss of CCM1 expression. Increased RhoA activation was associated with Rho kinase-dependent phosphorylation of myosin light chain 2. Functionally, loss of CCM1, -2, or -3 inhibited endothelial cell vessel-like tube formation and extracellular matrix invasion, each of which is rescued by chemical inhibition or short hairpin RNA knockdown of Rho kinase. The findings, for the first time, define a signaling network for CCM1, -2, and -3 in CCM pathology, whereby loss of CCM1, -2, or -3 protein expression results in increased RhoA activity, with the activation of Rho kinase responsible for endothelial cell dysregulation. The results define Rho kinase as a therapeutic target to rescue endothelial cells from loss of CCM protein function.
Keywords: Brain, Cell Permeabilization, Drug Action, Extracellular Matrix, shRNA, Cerebral Cavernous Malformation, ROCK Inhibitor, Rho Kinase, RhoA, RhoA Biosensor
Introduction
Cerebral cavernous malformations (CCM)3 are clusters of leaky, dilated capillaries in the central nervous system that occur in ∼0.5% of the general population and up to 1.5% of the Hispanic population (1). CCM frequently lead to clinical sequelae such as hemorrhage, epilepsy, and neurological deficits (1). The disease is associated with both germline and somatic mutations in one of three genes, ccm1, -2, or -3 (2). The three CCM proteins form a common complex, indicating that they function coordinately (3, 4); each lacks defined catalytic domains, indicating that they are scaffold- or adaptor-like proteins for organization of protein complexes (3–5). The identical disease phenotype produced upon loss of any one of the three CCM proteins suggests that they coordinately regulate a common mechanism required for vascular integrity (3, 4, 6, 7).
It was recently shown that loss of endothelial cell expression of CCM2 resulted in activation of the GTPase RhoA (7, 8). Crose et al. (8) demonstrated that CCM2 knockdown in brain microvascular endothelial cells resulted in defective RhoA degradation because of the dysregulation of Smurf1, a CCM2 binding partner, and an ubiquitin-protein isopeptide ligase (E3) that controls RhoA degradation. RhoA overabundance induced by loss of CCM2 was shown to increase cytoskeletal stability, inhibit vessel-like tube formation, and increase endothelial cell monolayer permeability (7, 8). Herein, we show that loss of CCM1, -2, or -3 expression results in a common phenotype associated with RhoA overexpression and activation. We define ROCK as a critical RhoA effector whose increased activation dysregulates endothelial cell function. ROCK is activated by RhoA and phosphorylates several substrates, including myosin light chain, myosin light chain phosphatase, and LIM kinase for the regulation of actin cytoskeletal dynamics (9). ROCK has also been shown to regulate vascular permeability and has been a drug discovery target for regulation of vascular bed diseases (10). Our findings show that ROCK inhibition rescues extracellular matrix invasion and vessel-like tube formation, two endothelial cell functions disrupted by loss of CCM protein expression.
EXPERIMENTAL PROCEDURES
Establishment of Knockdown Cell Lines
Lentiviral gene-specific shRNAs in pLKO.1 were obtained from the University of North Carolina - Chapel Hill Lenti-shRNA Core Facility.
RhoA Biosensor
Imaging and image processing were performed as described (11).
Tube Formation Assay and Live Cell Imaging
7.0 × 10−4 cells were incubated for 15 h on Matrigel (BD Biosciences) and stained with rhodamine phalloidin as described previously (8). Imaging was performed on either a Pathway (BD Biosciences) or a Cellomics ArrayScan (Thermo Scientific). For live cell imaging, six fields of cells were imaged via transmitted light every 10 min for 15 h. Cellomics ArrayScan software was used to quantitate mean tube area.
Statistical Significance
Where indicated, statistical significance was calculated using the two-tailed Student's t test. See supplemental material for additional Experimental Procedures.
RESULTS
Knockdown of CCM1, -2, or -3 Induces RhoA Overexpression and Persistent RhoA Activity
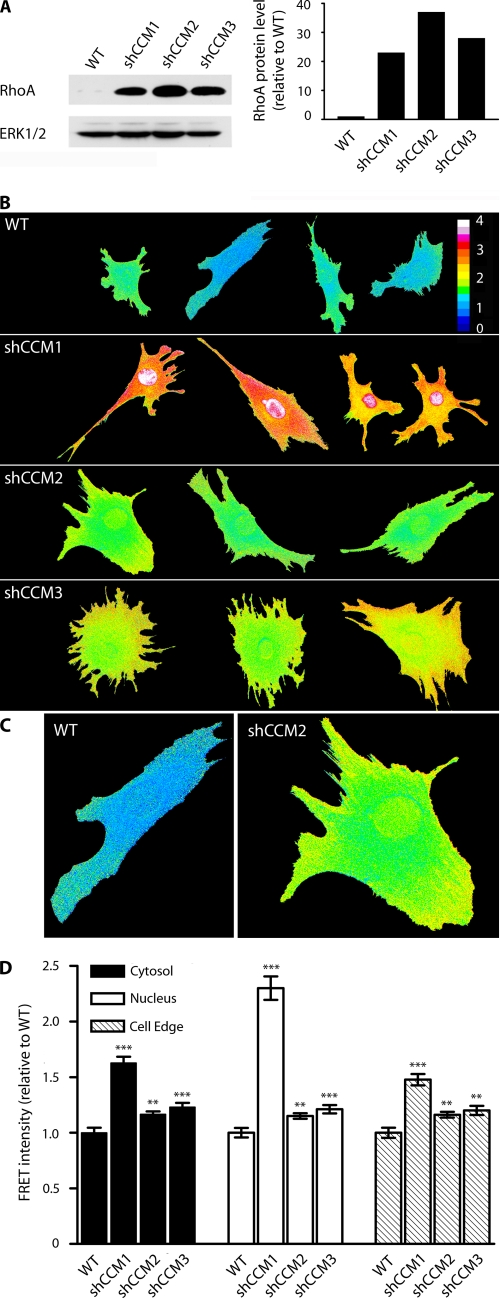
Expression of each of the three CCM proteins was selectively inhibited by shRNA knockdown in endothelial cells (supplemental Fig. 1, A and B). Stable knockdown of CCM1, -2, or -3 each resulted in 20–35-fold increases in RhoA expression relative to WT cells (Fig. 1A). The finding that inhibition of CCM1, -2, or -3 protein expression similarly caused marked increases in RhoA expression demonstrates for the first time that the three CCM proteins function coordinately to regulate RhoA protein levels; loss of any one CCM protein causes an increase in RhoA expression. Previously, we showed that CCM2 knockdown resulted in dysregulated Smurf1-mediated degradation of RhoA (8). The control of RhoA protein levels requiring all three CCM proteins is consistent with the requirement of a functional protein complex of CCM1, -2, and -3 for regulating the degradation of RhoA.
FIGURE 1.
RhoA abundance and activity are increased in shCCM1, -2, or -3 endothelial cells. A, Western blot showing that the abundance of RhoA in shCCM1, -2, or -3 cells is increased 23-, 37-, and 28-fold relative to WT cells. B, WT, shCCM1, -2, or -3 cells were infected with a FRET-based RhoA biosensor, where activation of RhoA leads to FRET. The measured FRET signal has been pseudocolored, where blue indicates low FRET and low RhoA activity, and red/white indicates high FRET and high RhoA activity. RhoA activity is increased at the cell edge (defined as 1.5-μm width at the edge of the cell), the cytoplasm, and the nucleus of shCCM1, -2, or -3 endothelial cells. C, enlarged view of a representative control and shCCM2 knockdown cell showing active RhoA at the cell edge, cytoplasm, and nucleus. D, bar graph showing the -fold change in FRET intensity for the cytoplasm, nucleus, and cell edge of shCCM1, -2, or -3 relative to WT control cells. Cytoplasm -fold FRET increase values are: shCCM1 cells = 1.62 (p value 4 × 10−11), shCCM2 cells = 1.16 (p value 0.001), shCCM3 cells = 1.23 (p value 0.0003). Nuclear -fold FRET increases are: shCCM1 cells = 2.33 (p value 4.8 × 10−11), shCCM2 cells = 1.16 (p value 0.002), shCCM3 cells = 1.23 (p value 0.0002). Cell edge -fold FRET increases are: shCCM1 cells = 1.48 (p value 5 × 10−9), shCCM2 cells = 1.16 (p value 0.002), shCCM3 cells = 1.20 (p value 0.002). Data represent the mean ± S.E. for a minimum of 25 cells in two independent experiments. Error bars, ***, p < 0.001, **, p < 0.02, *, p < 0.05.
A RhoA biosensor based on FRET, which has been extensively characterized for measurement of activated RhoA in live cells (11, 12), was used to measure the spatial dynamics of RhoA activity in control and CCM1, -2, or -3 knockdown endothelial cells. The overexpression of RhoA observed in CCM knockdown cells results in a persistent activation of RhoA (Fig. 1, B and C). In WT endothelial cells, RhoA activity is low and observed primarily at the cell edge as described previously for fibroblasts (11, 12). With shRNA knockdown of CCM2 or -3, there is a significant increase in RhoA activity not only at the cell edge but also in the cytoplasm and nucleus. When a population of ∼50 single cells is imaged and averaged for FRET intensity of the RhoA biosensor at the cell edge, cytoplasm, or nucleus, the CCM1, -2, and -3 knockdown cells each have a highly statistically significant increase in RhoA activation relative to control cells (Fig. 1D, see legend for p values). Strikingly, RhoA activity is extremely high in CCM1 knockdown cells relative to control or CCM2 or -3 knockdown cells (Fig. 1B). Statistical analysis of a population of individual CCM1 knockdown cells indicates an average of 1.62-, 2.33-, and 1.48-fold increase in FRET intensity relative to WT cells for the cytoplasm, nucleus, and cell edge, respectively (Fig. 1D).
These results show that regulation of RhoA protein expression is common to loss of any of the three CCM proteins. However, the pronounced RhoA activity in CCM1 knockdown cells when compared with CCM2 or -3 knockdown indicates that the CCM proteins are not simply in a common pathway but have specific functions. Although CCM2 binds Smurf1 for control of RhoA degradation, CCM1 appears to have a functional role as a negative regulator of RhoA activity, and loss of CCM1 has a pronounced effect on RhoA activity, which is different from the regulation of RhoA protein expression. Thus, the CCM proteins organize a signaling network, not simply a pathway for control of RhoA function.
ROCK2 Is Required for Increased Phosphorylation of Myosin Light Chain 2 in CCM1, -2, or -3 Knockdown Cells
ROCK1 and -2 are closely related kinases with overlapping but also distinct functions (13). Both ROCK1 and ROCK2 bind activated GTP-RhoA (13). MLC2 is phosphorylated by ROCK1/2, and phospho-MLC2 is a measure of ROCK1/2 activity in cells (9, 13). MLC2 is required for the control of actin cytoskeletal dynamics, induction of stress fiber formation, and cell contraction (9), whereby ROCK1/2 phosphorylation of MLC2 and MLC phosphatase leads to increased cytoskeletal stability (13, 14).
We previously showed that stress fiber-associated phospho-MLC2 was increased in brain microvascular endothelial cells upon knockdown of CCM2 (8). Given the increased RhoA activity observed in CCM1, -2, or -3 knockdown endothelial cells (Fig. 1), we predicted that phosphorylation of MLC2 would be increased with knockdown of each of the CCM proteins. Phospho-MLC2 is indeed increased with loss of expression of each of the three CCM proteins (Fig. 2). However, there is not a linear relationship with the RhoA activity measured in Fig. 1 with knockdown of CCM1, -2, or -3 and the relative levels of phospho-MLC2 measured by immunoblotting. This is consistent with dysregulation of a regulatory network differentially controlled by each CCM protein. Nonetheless, control of RhoA expression is a convergent regulatory function requiring each CCM protein.
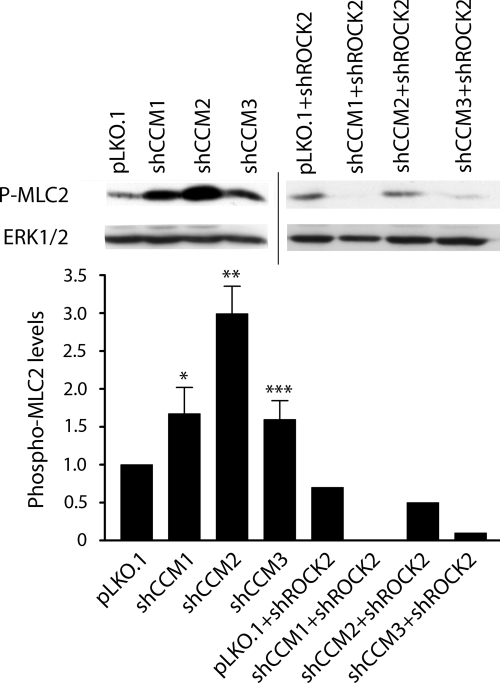
FIGURE 2.
Western blots showing an increase in phospho-MLC2 in shCCM1, -2, or -3 endothelial cells, which is lost upon shRNA knockdown of ROCK2. The bar graph shows the densitometric quantitation of band intensity. The abundance of phospho-MLC2 (P-MLC2) in shCCM1, -2, or -3 cells increases 1.7-, 3.0-, and 1.6-fold, respectively, relative to control pLKO.1 cells (the graph represents an average of three independent experiments). Upon shRNA knockdown of ROCK2, this abundance decreases below detectable levels, 50 and 90%, respectively, for shCCM1, -2, or -3 relative to pLKO.1. Error bars, ***, p < 0.001, **, p < 0.02, *, p < 0.05. Line indicates two separate blots.
In mouse embryonic endothelial cells, ROCK2 is the functionally predominant ROCK form and was targeted for shRNA knockdown. Loss of ROCK2 expression reversed the increase in phospho-MLC2 observed in each of the CCM protein knockdown cell lines, demonstrating that ROCK2 stimulates the phosphorylation of MLC2 in mouse embryonic endothelial cells (Fig. 2).
Knockdown of CCM1, -2, or -3 Inhibits Endothelial Cell Vessel-like Tube Formation and Invasion of Extracellular Matrix
Knockdown of CCM1, -2, or -3 in endothelial cells results in inhibition of extracellular matrix invasion and vessel-like tube formation (supplemental Fig. 2 and Fig. 3A). Even after 48 h, whereas WT cells organize into a well formed tubule network, CCM1, -2, and -3 knockdown cells fail to form vessel-like tubes (supplemental Fig. 3A). The findings define a common phenotype for loss of each of the three CCM proteins with inhibition of invasion and vessel-like tube formation, providing quantitative assays to define treatments to rescue the CCM pathology. Based on the common inhibition of invasion and vessel-like tube formation and the increased ROCK2-dependent increase in phospho-MLC2 with knockdown of each CCM protein, we reasoned that ROCK2 was a therapeutic target to reverse the CCM phenotype.
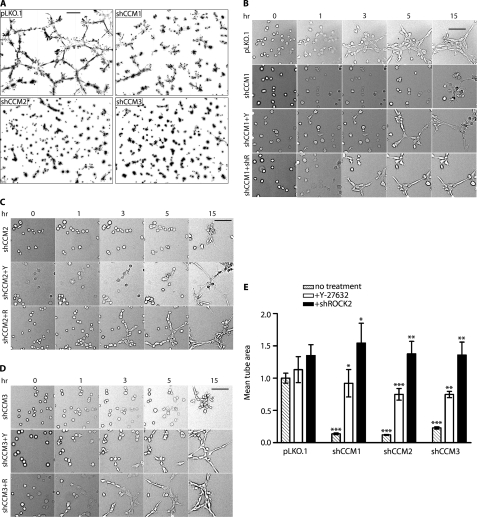
FIGURE 3.
ROCK inhibitor Y-27632 and ROCK2 shRNA rescue tube formation in shCCM1, -2, or -3 endothelial cells. A, knockdown of CCM1, -2, or -3 results in loss of tube formation. The image is a nine-panel montage of ×10 frames, and the bar represents 300 μm. B, tube formation in shCCM1 cells can be rescued with Y-27632 (+Y) or shROCK2 (+R). Frames are from supplemental Movie 1 taken at 0, 1, 3, 5, and 15 h after the start of treatment with Y-27632. The bar represents 100 μm. The image is a single ×10 field. C, tube formation rescue in shCCM2 cells by shROCK2 or Y-27632. D, tube formation rescue in shCCM3 cells by shROCK2 or Y-27632. E, the bar graph represents the mean tube area of pLKO.1 and shCCM1, -2, and -3 cells, normalized to pLKO.1. The data are the means ± S.E. of six frames from at least three independent experiments. Error bars, ***, p < 0.001, **, p < 0.02, *, p < 0.05.
CCM1, -2, or -3 Knockdown Pathology Is Rescued by Inhibition of ROCK
Relevant to rescue of the CCM pathology, we previously demonstrated that the small molecule ROCK inhibitor Y-27632 (Calbiochem) rescued inhibition of endothelial cell migration resulting from shRNA knockdown of CCM2 in a scrape wound-healing assay with brain microvascular endothelial cells (8). Y-27632 or shRNA knockdown of ROCK2 was used to test the role of ROCK in rescuing loss of endothelial cell invasion of extracellular matrix (supplemental Fig. 2). Importantly, knockdown of CCM1, -2, or -3 did not affect ROCK2 expression, nor did knockdown of ROCK2 affect expression of CCM1, -2, or -3 (supplemental Fig. 4). Inhibition of extracellular matrix invasion resulting from CCM1, -2, or -3 knockdown was rescued by either loss of ROCK2 expression or treatment of cells with Y-27632 (supplemental Fig. 2). CCM1 knockdown cells were only weakly rescued by Y-27632 but strongly rescued by shRNA knockdown of ROCK2. This may in part be due to the time-dependent reversal of the strong CCM1 knockdown phenotype with Y-27632 (Figs. 1 and 3B, and see below).
In vessel-like tube formation assays, time-lapse imaging of control versus CCM1, -2, or -3 knockdown endothelial cells showed that knockdown of ROCK2 or treatment of cells with Y-27632 to inhibit ROCK activity rescued vessel-like tube formation for each of the three CCM protein knockdown endothelial cell lines (supplemental Fig. 3B and Fig. 3, B–D). Analysis of multiple vessel-like tube formation assays indicated that rescue of the CCM pathology, as measured by the increase in mean tube area, was greater with shRNA knockdown of ROCK2 when compared with Y-27632 (Fig. 3E and supplemental Fig. 3B). Analysis of the time-lapse images indicated that the onset of vessel-like tube formation was delayed several hours with treatment of CCM1, -2, or -3 knockdown cells with Y-27632 when compared with vessel-like tube formation with control or ROCK2 knockdown cells (Fig. 3, B–D, and supplemental Movies 1–3). This finding is similar to the reduced rescue of extracellular matrix invasion in CCM1 knockdown cells treated with Y-27632 when compared with shROCK2 (supplemental Fig. 2) and is likely due to the time required for chemical inhibition of ROCK and reversal of the ROCK-mediated changes in cellular physiology.
Y-27632 Treatment Induces Endothelial Cell Shape Changes Required for Vessel-like Tube Formation in CCM1, -2, or -3 Knockdown Cells
Loss of ROCK2 expression by targeted shRNA knockdown inhibits the activated RhoA stimulation of MLC2 phosphorylation (Fig. 2). Hence, rescue of the in vitro CCM pathology caused by loss of one of the three CCM proteins, as measured by vessel-like tube formation and invasion of extracellular matrix, is essentially complete, and the cells behave similar to control cells. Y-27632 rescue of the CCM pathology is similar to shRNA knockdown of ROCK2. Pharmacological ROCK2 inhibition reverses pathways controlled by ROCK2 that contribute to the CCM1, -2, or -3 knockdown phenotype. In a subpopulation of CCM1, -2, or -3 knockdowns, incubation with Y-27632 induces a time-dependent change in cell shape involving cell flattening and the extension of multiple membrane protrusions (supplemental Fig. 5). The cell shape changes are evident after a 1-h treatment with Y-27632, and by 4 h, these cells are forming nucleation centers for the initiation of tube formation (supplemental Fig. 5, arrowhead). CCM knockdown cells in the absence of Y-27632 are unable to undergo significant cell shape change and extend only small filopodial-like structures (supplemental Fig. 5, arrows). In contrast, pLKO.1 knockdown cells do not undergo the shape changes induced by Y-27632 because ROCK2 is not activated and driving constitutive RhoA control of the actin cytoskeleton (13, 14). Consistent with these observations is the fact that loss of ROCK2 expression in both control cells and CCM protein knockdown cells causes formation of well defined vessel-like tubes 4 h after initiating the assay (Fig. 3, B–D, and supplemental Fig. 3B). The findings indicate that ROCK2 actively drives the CCM pathology characterized by inhibition of invasion of extracellular matrix and vessel-like tube formation.
DISCUSSION
The three ccm genes encode scaffold or adaptor proteins capable of forming a CCM1-2-3 protein complex for organization of proteins involved in regulating the cytoskeleton (3, 5). Thus, the molecular basis of CCM is fascinating in that loss of function of a scaffold or adaptor protein is sufficient to develop the pathology (5–7). The function of CCM proteins was further elucidated by the discovery that RhoA becomes overexpressed with loss of CCM2 expression (7, 8) and that CCM2 regulates RhoA protein level by controlling its degradation (8). We have now shown that CCM1 and -3 proteins in addition to CCM2 are required for regulation of RhoA protein levels, and loss of CCM1, -2, or -3 each results in the pronounced increase in expression of RhoA. This result supports the critical role of the CCM protein complex in controlling RhoA expression. The RhoA biosensor allowed measurement of live cell images for RhoA activity in control and CCM1, -2, and -3 knockdown cells. Clearly, RhoA activity is increased with loss of CCM1, -2, or -3 proteins, and the increased RhoA activity results in changes in regulation of ROCK and the cytoskeleton. The pronounced RhoA activity with CCM1 knockdown strongly suggests that CCM1 has a function controlling a Rho guanine nucleotide exchange factor or Rho GTPase-activating protein. This difference suggests a function specific for CCM1 relative to CCM2 or -3. However, the effect of CCM1 loss of expression was not generally different from what was observed with loss of CCM2 or -3 expression for changes in phospho-MLC2 levels or dysregulation of invasion or vessel-like tube formation. These findings indicate there is not simply a linear pathway of RhoA expression-ROCK activation-phosphorylation of MLC2. Rather, the CCM proteins represent a dynamic regulatory network where they are sometimes in a complex but other times found in different cellular locations (4, 5), consistent with common but also distinct functions. We have proposed that CCM proteins localize signaling complexes to specific cellular locations associated with actin reorganization (3, 8). This would suggest that CCM proteins spatially control RhoA degradation for regulating specific physiological functions. Interestingly, activation of Rho GTPases and ROCK has also been observed in Smith-Lemli-Opitz syndrome (15), suggesting that dysregulation of this signaling axis has major pathophysiological implications in different human diseases.
Surgical resection is currently the standard treatment for symptomatic CCM, a highly invasive procedure with significant risk to the patient (16). In addition, lesions located in critical areas such as the brainstem or basal ganglia are more likely to exhibit a poor natural history, yet no treatment exists because they are surgically inaccessible. The discovery that inhibition of ROCK, a kinase activated by RhoA, is able to rescue dysregulated endothelial cell physiology resulting from loss of CCM1, -2, or -3 expression provides, for the first time, a pharmacological approach using a small molecule kinase inhibitor for treatment of CCM. The function of RhoA and ROCK in promoting vascular permeability is consistent with the dysregulated RhoA-ROCK signaling axis being important in promoting CCM pathology. Thus, ROCK inhibitors are clearly potential therapeutics for the treatment of CCM. Studies have shown that two ROCK inhibitors, Fasudil and Y-27632, are reasonably well tolerated in animals (17). In fact, Fasudil has been used in Japan for treatment of cerebral vasospasm following subarachnoid hemorrhage since 1995 (18), indicating that ROCK inhibitors can be tolerated in humans.
Given that the RhoA-ROCK signaling network is clearly dysregulated in CCM, there is still much to learn about the RhoA activation and inactivation cycle and how it is dysregulated in CCM endothelial cells. Questions also remain regarding the CCM protein complex and control of signaling networks including the potential involvement of additional kinases that could contribute to the CCM pathology and be pharmacologically targeted for treatment of CCM. For example, MEKK3, a MAP3K that regulates ERK5, c-Jun N-terminal kinase (JNK), and NFκB, is in the CCM protein complex and is important for regulating responses to inflammatory cytokines such as interleukin-1 (IL-1) (3, 19). Significantly, it appears that a pharmacological small molecule treatment to control CCM is a real possibility.
Supplementary Material
Acknowledgments
We thank Dr. Klaus Hahn for the RhoA biosensor construct and Stephanie Hart for production of the RhoA biosensor adenovirus stock.
This work was supported, in whole or in part, by National Institutes of Health Grants GM068820 (to G. L. J.), GM068820-S1 and HL092338 (to S. B.), T32GM80079 (to C. F. D. and C. M. W.), and HL094020 (to C. M. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures, Figs. 1–5, and Movies 1–3.
- CCM
- cerebral cavernous malformations
- shRNA
- short hairpin RNA
- shCCM1
- -2, or -3, cells treated with shRNA for CCM1, -2, or -3
- ROCK
- Rho kinase
- MLC2
- myosin light chain 2
- FRET
- Förster resonance energy transfer
- WT
- wild type.
REFERENCES
- 1.Plummer N. W., Zawistowski J. S., Marchuk D. A. (2005) Curr. Neurol. Neurosci. Rep. 5, 391–396 [DOI] [PubMed] [Google Scholar]
- 2.Laberge-le Couteulx S., Jung H. H., Labauge P., Houtteville J. P., Lescoat C., Cecillon M., Marechal E., Joutel A., Bach J. F., Tournier-Lasserve E. (1999) Nat. Genet. 23, 189–193 [DOI] [PubMed] [Google Scholar]
- 3.Hilder T. L., Malone M. H., Bencharit S., Colicelli J., Haystead T. A., Johnson G. L., Wu C. C. (2007) J. Proteome Res. 6, 4343–4355 [DOI] [PubMed] [Google Scholar]
- 4.Zawistowski J. S., Stalheim L., Uhlik M. T., Abell A. N., Ancrile B. B., Johnson G. L., Marchuk D. A. (2005) Hum. Mol. Genet. 14, 2521–2531 [DOI] [PubMed] [Google Scholar]
- 5.Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. (2003) Nat. Cell Biol. 5, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 6.Kleaveland B., Zheng X., Liu J. J., Blum Y., Tung J. J., Zou Z., Sweeney S. M., Chen M., Guo L., Lu M. M., Zhou D., Kitajewski J., Affolter M., Ginsberg M. H., Kahn M. L. (2009) Nat. Med. 15, 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehead K. J., Chan A. C., Navankasattusas S., Koh W., London N. R., Ling J., Mayo A. H., Drakos S. G., Jones C. A., Zhu W., Marchuk D. A., Davis G. E., Li D. Y. (2009) Nat. Med. 15, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crose L. E., Hilder T. L., Sciaky N., Johnson G. L. (2009) J. Biol. Chem. 284, 13301–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 10.Chrissobolis S., Sobey C. G. (2006) Stroke 37, 2174–2180 [DOI] [PubMed] [Google Scholar]
- 11.Pertz O., Hodgson L., Klemke R. L., Hahn K. M. (2006) Nature 440, 1069–1072 [DOI] [PubMed] [Google Scholar]
- 12.Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G. L., Hahn K. M., Danuser G. (2009) Nature 461, 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao J. K., Seto M., Noma K. (2007) J. Cardiovasc. Pharmacol. 50, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaunas R., Nguyen P., Usami S., Chien S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 15895–15900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X. S., Wassif C. A., Backlund P. S., Song L., Holtzclaw L. A., Li Z., Yergey A. L., Porter F. D. (January12, 2010) Hum. Mol Genet. 10.1093/hmg/ddq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad I. A. (2005) Surg. Neurol. 63, 317–318 [DOI] [PubMed] [Google Scholar]
- 17.Inan S., Büyükafşar K. (2008) Br. J. Pharmacol. 155, 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki Y., Shibuya M., Satoh S., Sugiyama H., Seto M., Takakura K. (2008) Neurol. Med. Chir. (Tokyo) 48, 241–247; discussion 247–248 [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki K., Gohda J., Kanayama A., Miyamoto Y., Sakurai H., Yamamoto M., Akira S., Hayashi H., Su B., Inoue J. (2009) Sci. Signal. 2, ra66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.