Abstract
The NF-κB transcription factor plays a pivotal role in breast cancer progression and therapy resistance. However, the mechanisms by which the tightly regulated NF-κB becomes constitutively activated during breast cancer pathogenesis remain obscure. Here, we report that PDZ-LIM domain-containing protein 2 (PDLIM2), an essential terminator of NF-κB activation, is repressed in both estrogen receptor-positive and estrogen receptor-negative breast cancer cells, suggesting one important mechanism for the constitutive activation of NF-κB. Indeed, PDLIM2 reexpression inhibited constitutive NF-κB activation and expression of NF-κB-targeted genes in those breast cancer cells. Importantly, PDLIM2, but not its mutants defective in NF-κB termination, could suppress in vitro anchorage-independent growth and in vivo tumor formation of those malignant breast cells. In addition, we have shown that PDLIM2 repression involves promoter methylation. Accordingly, treatment of the breast cancer cells with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine reverses the methylation of the PDLIM2 promoter, restored PDLIM2 expression, and suppressed tumorigenicities of human breast cancer cells both in vitro and in vivo. These studies thus provide important mechanistic insights into breast cancer pathogenesis. These studies also suggest a tumor suppression function of PDLIM2 and a therapeutic strategy for breast cancer.
Keywords: Breast Cancer, NF-κB Transcription Factor, NF-κB, Signal Transduction, Tumor, PDLIM2
Introduction
Breast cancer has the highest incidence among women in the Western world, affecting more than 12% of women and accounting for 15% of all cancer deaths in women (1). In addition, although breast cancer is relatively rare in men, both the incidence and the death rate of male breast cancer remain one percentile compared with those of female breast cancer. The limitation on better treatments for this malignancy is largely due to lack of knowledge on the molecular mechanism of how breast cancer proceeds to become an invasive tumor that is resistant to cancer therapies.
Recent studies have suggested that NF-κB, a transcription factor essential for inflammatory and immune response, plays a pivotal role in the biology and therapy resistance of breast cancer (2–4). For example, constitutive activation of its prototypic member RelA/p65 has been shown to be important for the development and progression of breast cancer and therefore resistant to cancer therapies (5, 6). However, NF-κB activation is tightly controlled under physiological conditions (7). In resting, nonstimulated cells, NF-κB is sequestered in the cytoplasm by specific inhibitors, the IκB proteins (mainly IκBα). In response to a variety of stimuli, including microbial infection and proinflammatory cytokines, the NF-κB-bound IκBα is quickly phosphorylated, ubiquitinated, and degraded, allowing liberated NF-κB to enter the nucleus for gene transcription. Besides induction of proinflammatory cytokines, and cell proliferation and survival genes, nuclear NF-κB also induces expression of IκBα, which in turn enters the nucleus and shuttles the activated NF-κB back to the cytoplasm to reconstitute the status quo ante (8). However, this feedback inhibitory mechanism is not sufficient or even necessary for the turnoff of NF-κB activation (9). Instead, ubiquitination-mediated proteasomal degradation of activated NF-κB directly in the nucleus provides a more rapid but also essential mechanism for NF-κB termination.
Both biochemical and genetic studies demonstrate that newly discovered PDZ-LIM domain-containing protein 2 (PDLIM2),3 also known as mystique or SLIM (26–28), specifically targets nuclear p65 for polyubiquitination-mediated proteasomal degradation and therefore terminates NF-κB activation (10). It was suggested that the C-terminal LIM domain of PDLIM2 is required for promoting ubiquitination of nuclear p65, whereas its N-terminal PDZ domain is involved in shuttling nuclear p65 along the nuclear framework into discrete intranuclear compartments for the proteasome-mediated degradation. Accordingly, PDLIM2 knock-out mice are more sensitive to lipopolysaccharide-induced shock due to enhanced p65 activation and subsequently augmented production of inflammatory cytokines (10).
Currently, the mechanism of constitutive NF-κB activation during breast cancer pathogenesis remains largely unknown. Here, we show that PDLIM2 is repressed in both estrogen receptor (ER)-positive and ER-negative breast cancer cells and that this repression involves DNA methylation. Reexpression of PDLIM2 inhibited NF-κB constitutive activation, in vitro anchorage-independent growth, and in vivo tumor formation of these malignant cells. On the other hand, PDLIM2 mutants defective in NF-κB termination lose this breast cancer suppression ability. These studies suggest one important mechanism for the constitutive activation of NF-κB in breast tumorigenesis and a novel breast cancer suppression function of PDLIM2.
MATERIALS AND METHODS
Expression Vectors and Reagents
Myc-PDLIM2 and its PDZ or LIM domain deletion mutants, κb-TATA firefly and thymidine kinase Renilla luciferase reporter constructs, anti-PDLIM2 and anti-Hsp90 antibodies have been described before (11, 12). The anti-Myc antibody was generated from the 9E10 hybridoma as described (13). Nucleoside analog 5-aza-2′-deoxycytidine (5-aza-dC), calcein AM, puromycin, and blasticidin were purchased from Sigma. Sixty-day slow release pellets of 25 mg of estrogen were purchased from Innovative Research of America.
Cell Lines
The human ER-positive breast cancer cell lines MCF-7, HMT3522 T4-2, MDA-MB-361, HCC70, and T-47D, the human ER-negative breast cancer cell lines MDA-MB-231, MDA-MB-468, SK-BR-3, HS578t, the human ER mutation breast cancer cell line BT-20, and the human embryonic kidney cell line HEK293T were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The human nontumorigenic breast epithelial cell lines HMT3522 S-1 (ER-positive) and MCF10A (ER-negative) were cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium supplemented with 20 ng/ml human epidermal growth factor, 100 ng/ml cholera toxin, 10 ng/ml bovine insulin, and 500 ng/ml hydrocortisone and 10% fetal bovine serum. All of the ER-positive breast cells were also supplied with estrogen.
Immunoblotting Analysis
Cells were lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 0.25% (w/v) sodium deoxycholate, 1% (v/v) Nonidet P-40, 1 mm dithiothreitol) supplemented with 1 mm phenylmethylsulfonyl fluoride. The whole cell lysates were then subjected to SDS-PAGE and immunoblotting using the indicated antibodies as described previously (14, 15).
Real-time PCR Analysis
Total RNA was prepared with TRIzol reagent, and cDNA was generated with SuperScript II reverse transcriptase (Invitrogen) followed by regular RT-PCR and real-time PCR assays as described (16, 17). Primer pairs used for RT-PCR were: glyceraldehyde-3-phosphate dehydrogenase forward, 5′-CACAGTCCATGCCATCACTG, and reverse, 5′-CTTACTCCTTGGAGGCCATG; PDLIM2 forward, 5′-GACAGCCAGTCTTCCCAGAG, and reverse, 5′-TCTCACAGGTGTGGAGCTTG. Primer pairs used for real-time PCR were: β-actin forward, 5′-ATCAAGATCATTGCTCCTCCTGAG, and reverse, 5′-AGCGAGGCCAGGATGGA; Bcl-2 forward, 5′-ATGTGTGTGGAGAGCGTCAACC, and reverse, 5′-TGAGCAGAGTCTTCAGAGACAGCC; cyclin D1 forward, 5′-CCGTCCATGCGGAAGATC, and reverse, 5′-ATGGCCAGCGGGAAGAC; PDLIM2 forward, 5′-GCCCATCATGGTGACTAAGG, and reverse, 5′-ATGGCCACGATTATGTCTCC.
Bisulfite Genomic DNA Sequencing
Genomic DNA from 5-aza-dC-treated or mock-treated cells was isolated using the PureLink Genomic DNA Purification kit (Invitrogen). Genomic DNA aliquots were then treated with sodium bisulfite using the EZ DNA Methylation-Gold kit (Zymo Research) followed by PCR to amplify the PDLIM2 promoter using Hot-Start Taq enzyme (Qiagen). Primers designed to recognize the bisulfite-modified regions (−1084 to −800) of the PDLIM2 promoter were: forward, 5′-AGAGGAGTTTATATATATTTAGG and reverse, 5′-TACCTAACAACCCTCTCTCC. The PCR products were then used for DNA sequencing to determine the methylation status of the CpG dinucleotides with the PDLIM2 promoter as we described (18).
Subcellular Fractionation Assays
Based on previous published methods (10, 11), soluble and insoluble nuclear extracts were prepared, respectively, using hypertonic buffer (20 mm HEPES, pH 8.0, 1 mm EDTA, 20% (v/v) glycerol, 0.1% (v/v) Triton X-100, and 400 mm NaCl) and insoluble buffer (20 mm Tris, pH 8.0, 150 mm NaCl, 1% (w/v) SDS, 1% (v/v) Nonidet P-40, and 10 mm iodoacetamide) after the cytoplasmic fraction was extracted by the hypotonic buffer (20 mm HEPES, pH 8.0, 10 mm KCl, 1 mm MgCl2, 0.1% (v/v) Triton X-100, and 20% (v/v) glycerol). The purity of the obtained fractions was confirmed by checking expression of Hsp90 (cytoplasm), Sp1 (soluble nuclear fraction), or lamin B (insoluble nuclear fraction) in different fractions.
Luciferase Gene Reporter Assays
Cells were transfected with the κb-TATA firefly and thymidine kinase Renilla luciferase reporter in the presence or absence of PDLIM2. At 40 h after transfection, luciferase activity was measured as described before (11).
Cell Growth Assays
Cells were seeded into 12-well plates at a density of 5000 cells/well followed by 5 μm 5-aza-dC or vehicle treatment. The drug-containing medium was replenished each day. Cell density at the indicated time points was determined by replacing the medium with 2 μm calcein AM in 1× dissociation solution (Trevigen). After a 1-h incubation, diesterase activity (relative fluorescence units) was measured with a Tecan Infinite 200 Microplate Reader, using an excitation wavelength of 485 nm and emission wavelength of 520 nm (18).
Retroviral Transduction and Generation of Stable Transfectants
HEK293T cells were transfected with retroviral vector pQCXIP or pMX using FuGENE 6 (Roche Applied Science) followed by viral supernatant collection and infection of MCF-7 and MDA-MB-231 cells as described (20). Stable transfectants were obtained by selection with puromycin (for pQCXIP) or blasticidin (for pMX).
Colony Formation Assays
Cell suspension in culture medium containing 0.6% SeaPlaque low melting agarose (Biowhittaker Molecular Applications) was added to 6-well plates coated with an initial underlay of 1% agarose in culture medium as described before (21). For the colony inhibition studies, cells were treated with 5 μm 5-aza-dC or vehicle for 48 h before being plated. After plating, drug or vehicle diluted in culture medium (0.5 ml) was added on the top of agarose every 3 days. Colony growth was scored after 21 days.
In Vivo Tumorigenicity Assays
Five-week-old female SCID mice (Charles River Laboratories) were challenged in the mammary fat pads with the indicated breast cancer cells or their cell lines stably expressing PDLIM2 or PDLIM2 mutants. For the MCF-7 cell inoculation, the mice were subcutaneously implanted with a slow release pellet of 25 mg estrogen at least 3–4 days before the cell injection. The recipient mice were monitored daily and killed and dissected for tumor evaluation at day 20 (for MCF-7 cells) or day 30 (for MDA-MB-231 cells) after injection, or the mice were also injected intraperitoneally with 5-aza-dC (5 mg/kg body weight) or vehicle every 48 h for a total of five times after cell inoculation. All experiments involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh.
Statistical Analysis
Data were reported as mean ± S.D. Student's t test (two-tailed) was used to assess the significance of differences between two groups, and p values ≥ 0.05 and 0.01 were considered statistically significant and highly statistically significant, respectively.
RESULTS
PDLIM2 Expression Is Repressed in Both ER-positive and ER-negative Breast Cancer Cells
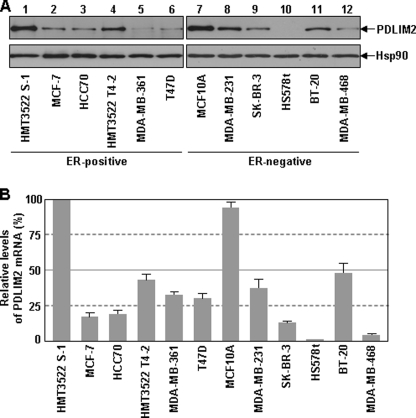
Given the causative role of constitutive activation of NF-κB in breast cancer pathogenesis and the involvement of PDLIM2 in the termination of NF-κB activation, we hypothesized that PDLIM2 is involved in the pathogenesis of breast cancer. To test this hypothesis, we examined the protein level of PDLIM2 in a large panel of human breast cancer cell lines, including MCF-7 and MDA-MB-231, the two most used model cell lines of human ER-positive and ER-negative breast cancers, respectively. Regardless of ER expression status, all of the breast cancer cell lines expressed much lower levels of PDLIM2 protein, compared with the nontumorigenic breast epithelial cells HMT3522 S-1 (ER-positive) and MCF10A (ER-negative) (Fig. 1A). These data suggest that PDLIM2 protein expression is repressed in both ER-positive and ER-negative breast cancer cells.
FIGURE 1.
Repression of PDLIM2 expression in ER-positive or ER-negative breast cancer cells. A, Protein repression of PDLIM2 in breast cancer cells. Protein expressions of PDLIM2 in the indicated nontumorigenic breast epithelial cell lines, ER-positive breast cancer cell lines and ER-negative breast cancer cell lines were analyzed by immunoblotting using PDLIM2 specific antibody. Hsp90 was used as a loading control. B, RNA repression of PDLIM2 in breast cancer cells. The relative levels of PDLIM2 mRNAs in the indicated breast cancer cells were analyzed by real-time PCR using β-actin mRNA as a control and represented as percentile of that in HMT3522 S-1 cells (set as 100). The data presented are the mean ± S.D. (error bars) (n ≥3).
Our recent studies have shown that repression of PDLIM2 is actually at the mRNA level in colon cancer or by the human T cell leukemia virus type I, the etiological agent of adult T cell leukemia (11, 16, 18). Thus, we performed quantitative real-time RT-PCR to examine the mRNA expression of PDLIM2 in those breast cancer cells as well as the control HMT3522 S-1 and MCF10A cells. In correlation with the protein down-regulation, PDLIM2 mRNA levels were also significantly decreased in all the breast cancer cell lines compared with normal control cells (Fig. 1B). These data indicate that PDLIM2 repression occurs at the mRNA level in both ER-positive and ER-negative breast cancer cells.
PDLIM2 Inhibits NF-κB Constitutive Activation in Breast Cancer Cells
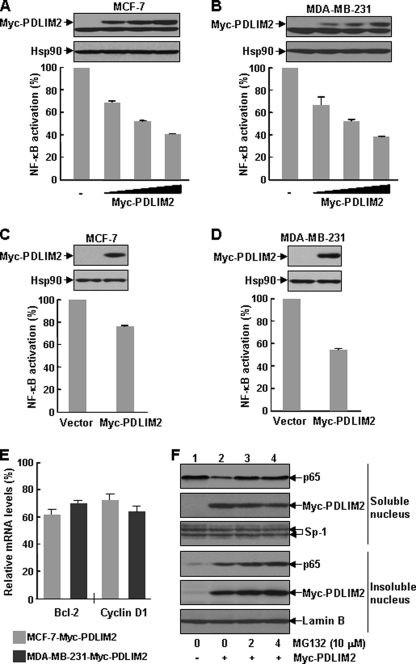
The data above also suggested that PDLIM2 repression may contribute to the constitutive activation of NF-κB in breast cancer cells. To address this important issue, we performed luciferase gene reporter assays to examine the impact of PDLIM2 restoration on the constitutive activation of NF-κB in MCF-7 and MDA-MB-231 cells. Indeed, transient expression of exogenous PDLIM2 resulted in a dose-dependent suppression of NF-κB activation in both MCF-7 and MDA-MB-231 cells (Fig. 2, A and B). Consistently, stable expression of PDLIM2 also inhibited the constitutive activation of NF-κB and expression of certain NF-κB-targeted genes including Bcl2 and cyclin D1, two well known tumor-associated genes, in these breast cancer cells (Fig. 2, C–E).
FIGURE 2.
PDLIM2 inhibition of NF-κB constitutive activation in breast cancer cells. A and B, dose-dependent inhibition of NF-κB constitutive activation in breast cancer cells by transiently expressed PDLIM2. MCF-7 and MDA-MB-231 cells were transfected with κb-TATA driven firefly luciferase reporter and thymidine kinase-driven Renilla luciferase reporter in the presence of increasing amounts of Myc-PDLIM2 followed by measure of luciferase activity. The luciferase activities were presented as the percentile of that in cells without PDLIM2 transfection (denoted as 100). The data presented are the mean ± S.D. (error bars) (n = 3). The protein expression levels of transfected myc-tagged PDLIM2 and endogenous Hsp90 were detected by direct immunoblotting using Myc antibody. C and D, inhibition of NF-κB constitutive activation in breast cancer cells by stably infected PDLIM2. MCF-7 and MDA-MB-231 cells stably expressing Myc-PDLIM2 or an empty vector were transfected with κb-TATA-driven luciferase reporter followed by measure of luciferase activity. The luciferase activities were presented as the percentile of that in the vector-expressing cells (denoted as 100). The data presented are the mean ± S.D. (error bars) (n = 3). The protein levels of stably expressed PDLIM2 were also examined by immunoblotting. E, inhibition of NF-κB-targeted genes by PDLIM2. The MCF-7 and MDA-MB-231 stably expressing cells described in D were also used for real-time PCR analysis to measure the expression levels of Bcl-2 and cyclin D1, which were represented as percentile of that in vector control cells (set as 100). The data presented are the mean ± S.D. (error bars) (n = 3). F, PDLIM2 inhibition of p65 in breast cancer cells. The transfected MDA-MB-231 cells were treated with 10 μm MG132 for the indicated time periods followed by subcellular fractionation and immunoblotting assays to determine p65 expression in the soluble nuclear fraction and insoluble nuclear fraction. Expression of Myc-PDLIM2, Sp1, and lamin B was also detected.
Because it was reported that PDLIM2 prevents persistent NF-κB activation by shuttling nuclear p65 into particular intranuclear compartments for proteasomal degradation (10), we next examined the effect of PDLIM2 on p65 expression in soluble and insoluble nuclear fractions of MDA-MB-231 cells, which show high NF-κB activity. Consistent with previous observations (10), overexpression of PDLIM2 decreased p65 in the soluble nuclear fraction but increased p65 in the insoluble nuclear fraction (Fig. 2F). Treatment of these cells with the proteasome inhibitor MG132 resulted in accumulation of p65 in the insoluble nuclear fractions, although a partial restoration of p65 in the soluble nuclear fraction was also observed. Interestingly, the proteasome inhibition also resulted in a dramatic decrease of the co-expressed PDLIM2 in the soluble nuclear fraction but an increase in the insoluble nuclear fraction. Given the roles of PDLIM2 in protein shuttling and subsequent degradation, it was plausible that after delivering p65 into the insoluble nuclear compartments from the soluble nucleoplasm, PDLIM2 could not be released until p65 was degraded by the proteasome. Collectively, these data suggest that PDLIM2 repression is one important mechanism of the constitutive activation of NF-κB in human breast cancer cells.
PDLIM2 Suppresses Tumorigenicities of Breast Cancer Cells Both in Vitro and in Vivo
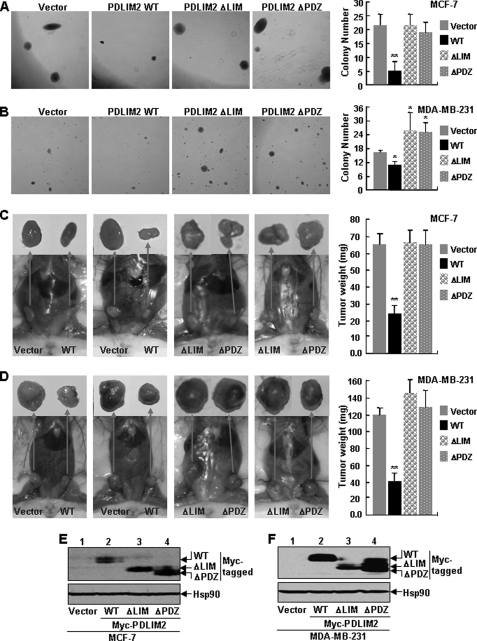
To investigate the significance of PDLIM2 repression in breast cancer directly, we performed the colony formation assay using the MCF-7 and MDA-MB-231 cells stably expressing PDLIM2 or an empty vector. As shown in Fig. 3A, MCF cells stably expressing PDLIM2 formed much fewer and smaller colonies in soft agar compared with the control cells expressing empty vectors alone. Similar results were obtained when the MDA-MB-231 stable cells were used for the assays (Fig. 3B). These data suggest that PDLIM2 suppresses the anchorage-independent growth of both MCF-7 and MDA-MB-231 cells.
FIGURE 3.
Suppression of tumorigenicities of breast cancer cells by PDLIM2, but not its mutants defective in NF-κB termination. A and B, suppression of the in vitro anchorage-independent growth of human breast cancer cells by PDLIM2 but not its mutants defective in NF-κB termination. MCF-7 and MDA-MB-231 cells stably expressing empty vector, PDLIM2, or its LIM or PDZ deletion mutant were plated in soft agar, and colony numbers were counted at day 21 after plating. The data presented are the mean ± S.D. (error bars) (n ≥ 6; *, p < 0.05; **, p < 0.01). WT, wild type. C and D, suppression of the in vivo tumor formation of human breast cancer cells by PDLIM2 but not its mutants defective in NF-κB termination. The MCF-7 and MDA-MB-231 stable cell lines were subcutaneously inoculated into the mammary fat pads of the SCID mice for the tumor formation. For the MCF-7 cell inoculation, the mice were subcutaneously implanted with a slow release pellet of 25 mg of estrogen at least 3–4 days before the cell injection. The data presented are the mean ± S.D. (error bars) (n ≥ 4; **, p < 0.01). E and F, expression levels of stably infected PDLIM2 and its mutants. The indicated stable cell lines were examined by immunoblotting using anti-Myc antibody. Expression of endogenous Hsp90 protein was used as a loading control.
To validate the in vitro studies in vivo, we separately implanted the control vector and PDLIM2-expressing breast cancer cells into the right and left mammary fat pads of SCID mice. As shown in Fig. 3, C and D, both control vector and PDLIM2-expressing cells of MCF-7 and MDA-MB-231 developed tumors in the mammary glands of the mice. However, the tumors formed by the PDLIM2 stable cancer cell lines were significantly smaller than those formed by the vector control cell lines. These data indicate that PDLIM2 suppresses tumorigenicity of MCF-7 and MDA-MB-231 cells in vivo.
PDLIM2 Mutants Defective in NF-κB Termination Fail to Suppress Tumorigenicities of Breast Cancer Cells
To examine the role of PDLIM2-mediated suppression of NF-κB in breast cancer pathogenesis, we also generated MCF-7 and MDA-MB-231 cells stably expressing a PDLIM2 LIM or PDZ deletion mutant, which loses the ability to suppress NF-κB activation (10). We then examined the soft agar colony formation ability of the PDLIM2 mutant stable cell lines. In contrast to those cells stably expressing wild type PDLIM2, the MCF-7 and MDA-MB-231 cells expressing the PDLIM2 LIM or PDZ deletion mutant retained the ability to form colonies in an efficiency similar to the vector control cells (Fig. 3, A and B).
In consistent with the in vitro studies, the SCID mouse xenograft studies indicated that expression of the PDLIM2 LIM or PDZ deletion mutant had no obvious effect on the tumor formation ability of both MCF-7 and MDA-MB-231 cells (Fig. 3, C and D). It was noteworthy that the inability of the LIM and PDZ deletion mutants in suppressing the tumorigenicity of MCF-7 and MDA-MB-231 cells was not due to their inefficient expression because their protein levels in these stable cell lines were comparable with that of PDLIM2 (Fig. 3, E and F). These data indicate that both LIM and PDZ domains of PDLIM2 are indispensable for its role in suppressing the tumorigenicity of MCF-7 and MDA-MB-231 cells. Altogether, these studies suggest that the repression of PDLIM2 contributes to constitutive activation of NF-κB and subsequent breast cancer pathogenesis.
PDLIM2 Repression in Breast Cancer Cells Involves DNA Methylation
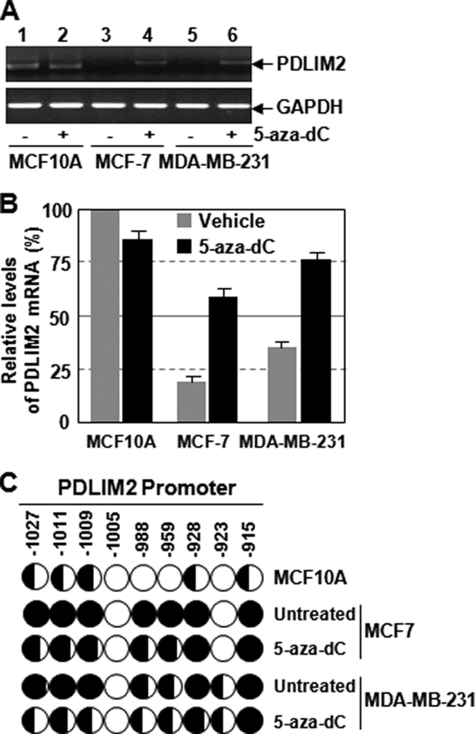
To investigate the mechanism of PDLIM2 repression in breast cancer cells, we examined the potential role of DNA methylation, a major mechanism responsible for repression of tumor suppressor genes in neoplastic cells (22). In agreement with our recent finding that PDLIM2 repression in adult T cell leukemia or colon cancer is mediated by DNA methylation (16, 18), treatment of the hypomethylating agent 5-aza-dC led to almost complete restoration of PDLIM2 expression in both MCF-7 and MDA-MB-231 cells (Fig. 4, A and B). These data suggest that PDLIM2 repression in human breast cancer cells also involves DNA methylation.
FIGURE 4.
DNA methylation-mediated repression of PDLIM2 in breast cancer cells. A, 5-aza-dC-mediated recovery of PDLIM2 expression in human breast cancer cells by RT-PCR analysis. MCF10A, MCF-7, and MDA-MB-231 cells were treated with 5 μm 5-aza-dC or vehicle for 48 h, followed by RT-PCR to determine mRNA levels of PDLIM2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B, 5-aza-dC-mediated recovery of PDLIM2 expression in human breast cancer cells by real time PCR analysis. PDLIM2 mRNA levels in the 5-aza-dC- or mock-treated cells were also analyzed by real-time PCR. The PDLIM2 inductions by 5-aza-dC are represented as percentile of that in mock-treated MCF10A cells (set as 100). The data presented are the mean ± S.D. (error bars) (n = 3). C, methylation of the PDLIM2 promoter in breast cancer cells. The indicated cell lines were treated with 5 μm 5-aza-dC or vehicle for 5 days, followed by the bisulfite genomic DNA sequencing. Each circle represents a CpG site; open circles represent unmethylated CpG dinucleotides, and filled circles represent methylated CpG sites. The ratios of the filled area in circles represent percentiles of the methylation in the CpG sites. The position of each CpG nucleotide relative to the PDLIM2 transcription initiation site (+1) is indicated at the top.
To investigate whether the PDLIM2 promoter is methylated in breast cancer cells and whether 5-aza-dC-mediated recovery of PDLIM2 expression involves reverse of the methylation of the PDLIM2 promoter, we performed the bisulfite genomic DNA sequencing. As shown in Fig. 4C, the PDLIM2 promoter was hypermethylated in both MCF-7 and MDA-MB-231 cells compared with the normal control cell line. As expected, treatment of 5-aza-dC induced a dramatic decrease in methylation of the PDLIM2 promoter. These data strongly suggest that promoter methylation directly controls the expression of PDLIM2 in breast cancer cells, similar to adult T cell leukemia cells and colorectal cancer cells.
5-Aza-dC Administration Suppresses Tumorigenicities of Breast Cancer Cells Both in Vitro and in Vivo
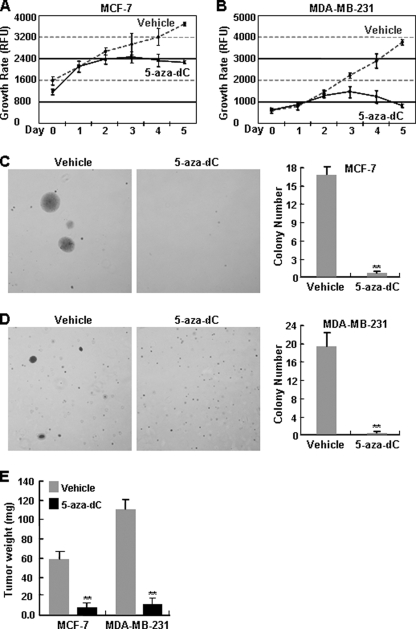
In association with PDLIM2 recovery, the 5-aza-dC treatment resulted in growth inhibition of these breast cancer cells in culture (Fig. 5, A and B). In contrast, MCF10A cells were largely resistant to the 5-aza-dC treatment (18). These data were in agreement with the fact that 5-aza-dC is toxic to cancer cells but not normal cells both in vitro and in vivo (16, 23, 24). Moreover, 5-aza-dC could almost completely suppress colony formation in soft agar and tumor formation in SCID of both MCF-7 and MDA-MB-231 cells (Fig. 5, C–E), suggesting that the hypomethylating agent prevents the tumorigenicities of those malignant breast cancer cells both in vitro and in vivo. These studies further substantiate the PDLIM2 epigenetic repression and its important role in breast cancer pathogenesis. These studies also suggested an immediate therapeutic approach for both ER-positive and ER-negative cancers.
FIGURE 5.
In vitro and in vivo suppression of breast cancer cell tumorigenicities by 5-aza-dC. A and B, growth suppression of breast cancer cells by 5-aza-dC. MCF-7 and MDA-MB-231 cells were treated with 5 μm 5-aza-dC or vehicle for the indicated time points, followed by cell growth assay. The data presented are the mean ± S.D. (error bars) (n = 3). C and D, in vitro tumor suppression of breast cancer cells by 5-aza-dC. MCF-7 and MDA-MB-231 cells were treated with 5-aza-dC or vehicle for 48 h before being plated in soft agar. After plating, drug or vehicle diluted in culture medium was added on the top of agarose every 3 days. Colony growth was scored after 21 days. The data presented are the mean ± S.D. (error bars) (n = 3; **, p < 0.01). E, in vivo tumor suppression of breast cancer cells by 5-aza-dC. Female SCID mice were inoculated with MCF-7 or MDA-MB-231 at the mammary fat pads. The receiving mice were injected intraperitoneally with 5-aza-dC (5 mg/kg body weight) or vehicle upon cell inoculation. The 5-aza-dC administrations were repeated 48 h later for four more times. Of note, the mice inoculated with MCF-7 cell were subcutaneously implanted with a slow release pellet of 25 mg of estrogen prior to the cell injection. The data presented are the mean ± S.D. (error bars) (n ≥ 3; **, p < 0.01).
DISCUSSION
A key role of NF-κB in cancer development and cancer therapy resistance has been well demonstrated. However, it remains largely unknown how the tightly regulated NF-κB becomes constitutively activated for the pathogenesis of human cancers including breast cancer. Here, we have shown that the NF-κB terminator PDLIM2 is repressed in human breast cancer cells, regardless of their ER expression status. Using the ER-positive breast cancer cell MCF-7 and ER-negative breast cancer cell MDA-MB-231 as models, we have further demonstrated that the repression of PDLIM2 in breast cancer cells is mediated by promoter methylation. Interestingly, treatment of the hypomethylating agent 5-aza-dC not only reverses the methylation of the PDLIM2 promoter and restores the expression of PDLIM2 but also suppresses the tumorigenicities of human breast cancer cells both in vitro and in vivo. These findings suggest a potential prognostic and therapeutic strategy for human breast cancer.
Although many targets other than PDLIM2 of 5-aza-dC may contribute to the breast cancer suppression of this antitumor drug (25), PDLIM2 restoration seems to play a dominant role. PDLIM2 reexpression alone is able to suppress colony formation in soft agar and tumor formation in SCID mice of both MCF-7 and MDA-MB-231 cells, although to a lesser extent, compared with the 5-aza-dC administration. These data suggest a novel role of PDLIM2 in breast cancer suppression.
In line with the role of PDLIM2 in NF-κB termination (10), PDLIM2 reexpression is sufficient to block the constitutive NF-κB activation and expression of NF-κB-targeted genes in both MCF-7 and MDA-MB-231 cells. On the other hand, PDLIM2 mutants defective in NF-κB termination fail to suppress the tumorigenicity of those breast cancer cells. These findings thus provide mechanistic insights into how NF-κB is constitutively activated in breast cancer and how PDLIM2 acts a novel breast cancer suppressor.
In addition to breast cancer, our recent published and unpublished data suggest that PDLIM2 is epigenetically repressed in certain other cancer types such as colon cancer, and adult T cell leukemia caused by the human T cell leukemia virus type I oncogenic retrovirus (11, 16, 18). It is noteworthy that the pathogenesis of these PDLIM2 repression-associated cancers has already been linked to NF-κB constitutive activation (19), suggesting a common tumor suppressor function of PDLIM2. Based on these findings, it is conceivable to speculate that during cancer pathogenesis, PDLIM2 is repressed due to DNA methylation of its promoter, which in turn contributes to NF-κB constitutive activation and subsequent cancer development and progression.
Acknowledgments
We thank M. J. Bissell, L. F. Chen, Y. Huang, and D. C. Radisky for various breast cancer cell lines.
This study was supported, in whole or in part, by NCI/National Institutes of Health Grants R01 CA116616, R01 CA102011, and R01 CA130966. This work was also supported by American Cancer Society Grants RSG-06-066-01-MGO and RSG-CSM-107144, grants from the Pennsylvania Department of Health, and a Hillman Innovative Research Scholar and a Hillman Innovative Cancer Research Award (to G. X. and S. Y. C.).
- PDLIM2
- PDZ-LIM domain-containing protein 2
- ER
- estrogen receptor
- 5-aza-dC
- 5-aza-2′-deoxycytidine
- RT
- reverse transcription.
REFERENCES
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M. J. (2009) CA Cancer J. Clin. 59, 225–249 [DOI] [PubMed] [Google Scholar]
- 2.Cao Y., Karin M. (2003) J. Mammary Gland Biol. Neoplasia 8, 215–223 [DOI] [PubMed] [Google Scholar]
- 3.Wu J. T., Kral J. G. (2005) J. Surg. Res. 123, 158–169 [DOI] [PubMed] [Google Scholar]
- 4.Biswas D. K., Iglehart J. D. (2006) J. Cell. Physiol. 209, 645–652 [DOI] [PubMed] [Google Scholar]
- 5.Nakshatri H., Bhat-Nakshatri P., Martin D. A., Goulet R. J., Jr., Sledge G. W., Jr. (1997) Mol. Cell. Biol. 17, 3629–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratt M. A., Tibbo E., Robertson S. J., Jansson D., Hurst K., Perez-Iratxeta C., Lau R., Niu M. Y. (2009) Oncogene 28, 2710–2722 [DOI] [PubMed] [Google Scholar]
- 7.Xiao G. (2007) Cytokine Growth Factor Rev. 18, 233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao G., Rabson A. B., Young W., Qing G., Qu Z. (2006) Cytokine Growth Factor Rev. 17, 281–293 [DOI] [PubMed] [Google Scholar]
- 9.Saccani S., Marazzi I., Beg A. A., Natoli G. (2004) J. Exp. Med. 200, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T., Grusby M. J., Kaisho T. (2007) Nat. Immunol. 8, 584–591 [DOI] [PubMed] [Google Scholar]
- 11.Yan P., Fu J., Qu Z., Li S., Tanaka T., Grusby M. J., Xiao G. (2009) Blood 113, 4370–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qing G., Yan P., Qu Z., Liu H., Xiao G. (2007) Cell Res. 17, 520–530 [DOI] [PubMed] [Google Scholar]
- 13.Qing G., Qu Z., Xiao G. (2005) J. Biol. Chem. 280, 40578–40582 [DOI] [PubMed] [Google Scholar]
- 14.Qu Z., Qing G., Rabson A., Xiao G. (2004) J. Biol. Chem. 279, 44563–44572 [DOI] [PubMed] [Google Scholar]
- 15.Qing G., Qu Z., Xiao G. (2005) J. Biol. Chem. 280, 18–27 [DOI] [PubMed] [Google Scholar]
- 16.Yan P., Qu Z., Ishikawa C., Mori N., Xiao G. (2009) Neoplasia 11, 1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qing G., Yan P., Xiao G. (2006) Cell Res. 16, 895–901 [DOI] [PubMed] [Google Scholar]
- 18.Qu Z., Yan P., Fu J., Jiang J., Grusby M. J., Smithgall T. E., Xiao G. (2010) Cancer Res. 70, 1766–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S. C., Xiao G. (2003) Cancer Metastasis Rev. 22, 405–422 [DOI] [PubMed] [Google Scholar]
- 20.Cvijic M. E., Xiao G., Sun S. C. (2003) J. Immunol. Methods 278, 293–304 [DOI] [PubMed] [Google Scholar]
- 21.Qing G., Qu Z., Xiao G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5324–5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luczak M., Jagodziñski P. P. (2006) Folia Histochem. Cytobiol. 44, 143–154 [PubMed] [Google Scholar]
- 23.Garcia-Manero G. (2008) Curr. Opin. Oncol. 20, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samlowski W. E., Leachman S. A., Wade M., Cassidy P., Porter-Gill P., Busby L., Wheeler R., Boucher K., Fitzpatrick F., Jones D. A., Karpf A. R. (2005) J. Clin. Oncol. 23, 3897–3905 [DOI] [PubMed] [Google Scholar]
- 25.Lo P. K., Sukumar S. (2008) Pharmacogenomics 9, 1879–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrado M., Senatorov V. V., Trivedi R., Fariss R. N., Tomarev S. I. (2004) Invest. Ophthalmol Vis. Sci. 45, 3955–3963 [DOI] [PubMed] [Google Scholar]
- 27.Loughran G., Healy N. C., Kiely P. A., Huigsloot M., Kedersha N. L., O'Connor R. (2005) Mol. Biol. Cell 16, 1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T., Soriano M. A., Grusby M. J. (2005) Immunity 22, 729–736 [DOI] [PubMed] [Google Scholar]