Abstract
The BCL-2 family members BAK and BAX are required for apoptosis and trigger mitochondrial outer membrane permeabilization (MOMP). Here we identify a MOMP-independent function of BAK as a required factor for long-chain ceramide production in response to pro-apoptotic stress. UV-C irradiation of wild-type (WT) cells increased long-chain ceramides; blocking ceramide generation prevented caspase activation and cell death, demonstrating that long-chain ceramides play a key role in UV-C-induced apoptosis. In contrast, UV-C irradiation did not increase long-chain ceramides in BAK and BAX double knock-out cells. Notably, this was not specific to the cell type (baby mouse kidney cells, hematopoietic) nor the apoptotic stimulus employed (UV-C, cisplatin, and growth factor withdrawal). Importantly, long-chain ceramide generation was dependent on the presence of BAK, but not BAX. However, ceramide generation was independent of the known downstream actions of BAK in apoptosis (MOMP or caspase activation), suggesting a novel role for BAK in apoptosis. Finally, enzymatic assays identified ceramide synthase as the mechanism by which BAK regulates ceramide metabolism. There was no change in CerS expression at the message or protein level, indicating regulation at the post-translational level. Moreover, CerS activity in BAK KO microsomes can be reactivated upon addition of BAK-containing microsomes. The data presented indicate that ceramide-induced apoptosis is dependent upon BAK and identify a novel role for BAK during apoptosis. By establishing a unique role for BAK in long-chain ceramide metabolism, these studies further demonstrate that the seemingly redundant proteins BAK and BAX have distinct mechanisms of action during apoptosis induction.
Keywords: Apoptosis, Enzymes/Lipid, Lipid, Lipid/Sphingolipids, Microsomes, Mitochondria, BAK, BCL-2 proteins, CerS, Ceramide
Introduction
Programmed cell death (apoptosis) is a complex, multistep process essential for normal tissue homeostasis and development. A key event in apoptotic signaling is mitochondrial outer membrane permeabilization (MOMP),3 whereby pro-apoptotic factors are released from the mitochondrial intermembrane space into the cytosol resulting in the activation of caspases and triggering the execution phase of apoptosis. Multiple cell death pathways converge on MOMP, and it is considered a necessary step for the commitment of cells to apoptosis. Anti-apoptotic BCL-2 proteins like BCL-2 and BCL-XL can block MOMP and cell death when overexpressed. On the other hand, the pro-apoptotic BCL-2 proteins BAK and BAX are necessary for MOMP in response to a wide variety of death-inducing signals. In fact, cells lacking both BAK and BAX are highly resistant to apoptotic stimuli (1–4).
Sphingolipids have been extensively implicated in the apoptotic response. Ceramide, a central molecule of sphingolipid metabolism, has been shown to be involved in programmed cell death through several lines of evidence. First, cellular levels of ceramide are elevated by a variety of apoptotic stimuli. Second, blocking ceramide generation delays or abrogates cell death in response to many different signals (5–9). Moreover, cancer cells can be induced to undergo apoptosis when endogenous ceramide levels are elevated by targeting ceramide metabolism with chemical inhibitors (10, 11) or small interference RNA (12–16) or by adding ceramide analogues (8, 9, 12–16). Despite the widespread implication of ceramide in the control of apoptosis, its connections with other pro-apoptotic factors (e.g. BCL-2 family proteins, caspases, etc.) are still unclear. Furthermore, although several enzymes have been shown to regulate apoptotic stress-induced ceramide generation (e.g. sphingomyelinases, ceramide synthases, etc.), the upstream factors that regulate this generation are largely unknown.
One proposed mechanism of ceramide action in apoptosis is through the control of MOMP. Ceramide can induce MOMP in vitro through the formation of ceramide channels even in the absence of pro-apoptotic BCL-2 family members (17), suggesting that it may function independently or downstream of BAK/BAX. Cells lacking both BAK and BAX are resistant to many apoptotic stimuli known to increase endogenous ceramide levels (2, 4). Thus, in apoptosis, the actions of ceramide may depend on BAK and/or BAX. Alternatively, BAK and/or BAX could be required for the production of ceramide in response to these stresses.
Here, we report data consistent with the latter hypothesis: BAK and BAX double knock-out (DKO) cells were unable to generate long-chain ceramides in response to multiple apoptotic stimuli. Moreover, BAK, but not the closely related molecule BAX, was essential for long-chain ceramide production during apoptosis. This function was independent of the established role of BAK in the induction of MOMP and subsequent caspase activation. Rather, BAK controlled ceramide generation at least in part by regulating the activity of ceramide synthase (CerS). These results identify a novel role for BAK in the induction of apoptosis as a regulator of long-chain ceramide generation and establish a unique function of BAK that is not performed by the closely related and seemingly functionally redundant molecule BAX.
EXPERIMENTAL PROCEDURES
Reagents
The chemicals used were fumonisin B1 (FB1, Cayman Chemical); myriocin, cisplatin, and anti-actin (Sigma); z-VAD-fmk (R&D); 4′,6-diamidino-2-phenylindole, growth media, and fetal bovine serum (Invitrogen); C17-sphingosine, C16- and C24 fatty acyl-CoA (Avanti Polar Lipids); Bid BH3-R9 (AnaSpec); PI and Annexin V-FITC (BD Pharmingen); SDS-PAGE gels, SDS buffer, transfer buffer, skimmed milk, and nitrocellulose membrane (Bio-Rad); ECL (enhanced chemiluminescence) detection system (Pierce); anti-CerS2 and anti-CerS6 (Novus Biologicals); and anti-CerS4 and anti-CerS5 (Santa Cruz Biotechnology).
Culture and Treatment of Cells
BMK cells (kind gift from Dr. E. White, Rutgers University) were maintained in Dulbecco's modified Eagle's medium, high glucose, supplemented with 2 mm l-glutamine, 5% fetal bovine serum. 24–48 h after plating, fresh growth media was added, and cells were UV-C-irradiated (λmax = 253.7 nm, 10 mJ/cm2) or treated with cisplatin (freshly prepared, 25 μm). Where indicated, cells were pretreated for 2 h with either myriocin (100 nm), FB1 (25 μm), or z-VAD-fmk. Hematopoietic cells were maintained at 200,000–400,000 cells/ml in RPMI (Mediatech) supplemented with 10% fetal calf serum (HyClone), 350 pg/ml IL-3 (BD Pharmingen), 10 mm HEPES (Mediatech), 55 μm β-mercaptoethanol (Sigma), antibiotics, and l-glutamine (Mediatech). Where indicated cells were treated with cisplatin (10 μm) or placed in IL-3-deficient media.
Measurement of Cell Viability
Cell viability was measured using one of three methods: 1) annexin V-FITC and propidium iodide (PI) staining and flow cytometry (Medical University of South Carolina flow cytometry and cell sorting shared resource facility) using a commercially available kit (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions; 2) hematopoietic cell viability was determined using vital dye exclusion (4′,6-diamidino-2-phenylindole), and cells were analyzed on a BD LSR II flow cytometer; and 3) viability of cells treated with cell-permeable t-Bid BH3 peptide was determined using trypan blue exclusion.
Caspase Activity
Caspases 3/7 activity was determined in cell lysates using a commercially available kit (BioVision, Mountain View, CA) according to the manufacturer's instructions.
Mass Spectrometry
Following treatments, cells were washed three times with phosphate-buffered saline, and the cell pellet was snap frozen. MS was performed as previously described (18). Ceramides were normalized to lipid phosphates.
C17-sphingosine Labeling
Cells were labeled with C17-sphingosine (1 μm, 13 min, Avanti), washed three times with phosphate-buffered saline, and collected. The reaction was stopped by the addition of 2 ml of extraction solvent containing ethyl acetate/2-propanol/water (60/30/10, v/v) supplemented with internal standard for EI/LC/MS analysis. Lipids are extracted twice, dried under a stream of nitrogen, and resuspended into 150 μl of 1 mm NH4COOH in 0.2% HCOOH in methanol and analyzed by EI/LC/MS.
In Vitro Ceramide Synthase Activity
Cells at 70% confluence were washed twice with cold phosphate-buffered saline, harvested by scraping in lysis buffer (20 mm Hepes (pH 7.4), 2 mm KCl, 2 mm MgCl2 250 mm sucrose, protease inhibitors), and lysed via 10 passages through a 28-gauge insulin syringe. Intact cells and nuclei were removed via centrifugation at 1,000 × g for 10 min, and the mitochondrial enriched fraction was removed via centrifugation at 10,000 × g for 10 min. The resulting supernatant was centrifuged at 100,000 × g for 1 h to pellet the microsomes. Ceramide synthase activity was measured in the microsomes (15–17 μg as indicated) as previously described (19). Briefly, a reaction mix (100 μl of final volume) containing 15 μm C17-sphingosine and 50 μm C16 or C24 fatty acyl-CoA in 25 mm potassium phosphate buffer (pH 7.4) was pre-warmed at 37 °C for 5 min. The enzyme reaction was initiated via addition of the microsomes, and after 15 min at 37 °C was terminated via the addition of 2 ml of extraction solvent containing ethyl acetate/2-propanol/water (60/30/10, v/v) supplemented with internal standard for EI/LC/MS analysis. Lipids are extracted twice, dried under a stream of nitrogen, and resuspended into 150 μl of 1 mm NH4COOH in 0.2% HCOOH in methanol and analyzed by EI/LC/MS.
For reconstitution activity assays, different amounts of WT and BAK KO microsomes were combined so that the total microsomal protein was equal to 20 μg, and then the in vitro CerS activity was measured. C16 fatty acyl-CoA and C17-sphingosine were used as substrates. The predicted activity was calculated from the sum of the individual activities of the component parts.
Real-time Quantitative PCR Analysis
Total RNA was extracted using a Qiagen RNeasy Kit (Qiagen) according to the manufacturer's protocol. The concentration and quality of total RNA preparations were evaluated spectrophotometrically. Complementary DNA was synthesized from 1 μg of the total RNA using the Superscript II Kit for first-strand synthesis (Invitrogen). Real-time reverse transcription-PCR was performed on a Bio-Rad iCycler detection system using iQ SYBR Green Supermix (Bio-Rad). Standard reaction volume was 25 μl containing 12.5 μl of Supermix, 9.5 μl of dH2O (Sigma), 400 nm specific oligonucleotide primers, and 50 ng of cDNA template. β-Actin was used as reference gene. Primer sequences are listed in supplemental Table 1.
RESULTS
BAK/BAX and Ceramide Generation Are Necessary for UV-C Irradiation-induced Apoptosis
We first sought to determine if BAK/BAX and/or ceramide was necessary for genotoxic-stress-induced apoptosis in our model system. Using wild type (WT) and BAK/BAX double knock-out (DKO) baby mouse kidney cells (BMK cells) (20), we induced apoptosis using ultraviolet light (UV-C) and examined cell death by annexin V/PI staining (Fig. 1a). As expected, WT BMK cells are highly sensitive to UV-C irradiation with >50% of the cells positive for both annexin V-FITC and PI staining by 6 h (Fig. 1a). On the other hand, DKO BMK cells were highly resistant to UV-C (Fig. 1a). In addition, WT cells showed activation of caspases 3/7 following UV-C irradiation (Fig. 1b) consistent with apoptosis. However, the DKO cells do not have increased caspase 3/7 activity (Fig. 1b) even at 24 h post-UV-C irradiation when they show decreased viability. Loss of viability without activation of caspases is consistent with necrotic cell death and is in agreement with previous reports in DKO cells following treatment with DNA-damaging agents (2).
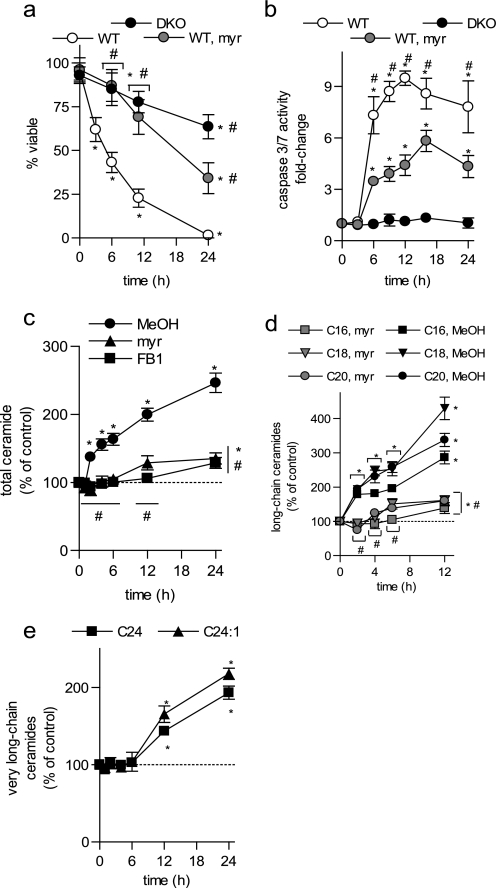
FIGURE 1.
BAK/BAX and ceramide are important mediators of UV-C irradiation-induced apoptosis. Wild-type (WT) and BAK and BAX double knock-out (DKO) BMK cells were untreated or UV-C irradiated (10 mJ/cm2). Where indicated, WT cells were pretreated with vehicle (MeOH), myriocin (myr, 100 nm, 2 h), or FB1 (25 μm, 2 h) and then left untreated or UV-C irradiated (10 mJ/cm2). a, cells negative for annexin V-FITC and PI by flow cytometry were deemed viable. b, caspase 3/7 activity was measured and expressed relative to control, non-irradiated cells. c, total ceramides; d, long-chain ceramides; and e, very long-chain ceramides were measured in WT BMK cells, normalized to total lipid phosphate, and expressed relative to untreated controls. a–e, data are the mean of three independent experiments. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding control and #, p ≤ 0.05 statistically significant difference between myr or DKO and the corresponding time-matched WT value.
UV-C irradiation is known to induce elevations in endogenous ceramides during the induction of apoptosis (21–23). We characterized UV-C-induced ceramide generation in WT BMK cells (Fig. 1c) and found that total ceramide was elevated as early as 2 h following irradiation and continued to increase up to 24 h. Because ceramides with specific chain lengths have been shown to contribute differently to cellular responses to stress stimuli (24, 25), we determined the specific ceramides elevated following UV-C irradiation. In WT cells, UV-C irradiation increased most significantly the long-chain ceramides (C16, C18, and C20; Fig. 1d and supplemental Fig. 1a) at time points prior to caspase 3/7 activation. The very long-chain ceramides (C24 and C24:1), although the most abundant, were only modestly elevated at 12-h post-irradiation (Fig. 1e and supplemental Fig. 1b) and masked changes in long-chain ceramides when total ceramides were examined (Fig. 1d).
Ceramide can be generated by multiple pathways in cells. To determine if ceramide was generated via either de novo synthesis or the salvage pathway, cells were preincubated with myriocin (myr), a potent inhibitor of serine palmitoyl transferase, the first and rate-limiting enzyme in de novo synthesis, or with FB1, an inhibitor of ceramide synthases (CerSs). Preincubation with myriocin (100 nm, 2 h) or FB1 (25 μm, 2 h) prevented UV-C-induced increases in total and long-chain ceramides (Fig. 1, c and d), indicating that ceramide was generated by the de novo pathway. Importantly, blocking ceramide generation with myriocin inhibited UV-C irradiation-induced cell death (Fig. 1a) and caspase 3/7 activation (Fig. 1b). Taken together, these results indicate that, in addition to BAK/BAX, ceramide generation plays a key role in UV-C irradiation-induced apoptosis of BMK cells.
BAK and BAX Double Knock-out Cells Fail to Generate Long-chain Ceramides in Response to UV-C Irradiation
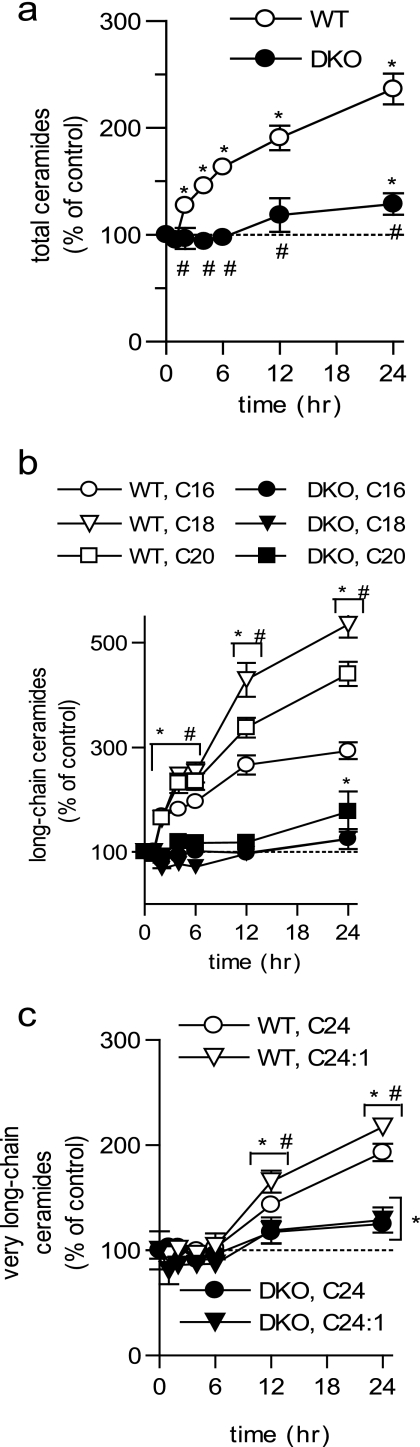
Although there is evidence for ceramide involvement in apoptosis both upstream and downstream of BAK/BAX (17, 26, 27), a causal relationship between the BAK/BAX and ceramide pathways remains unclear. DKO BMK cells are resistant to UV-C irradiation (Fig. 1, a and b), an apoptotic stimulus that induces elevations in long-chain ceramides in WT BMK cells (Fig. 1d). Thus, we hypothesized that long-chain ceramide generation is downstream of BAK/BAX, and that BAK/BAX are required for their generation following UV-C irradiation. Accordingly, the effects of UV-C irradiation on ceramide levels in DKO BMK cells were determined. Indeed, DKO cells were unable to elevate total and long-chain ceramides until 24 h post-UV-C irradiation (Fig. 2, a and b). Notably, only very long-chain ceramides (C24 and C24:1) were increased in DKO cells, albeit to a lesser extent than WT cells (Fig. 2c), whereas long-chain ceramides were not increased throughout the entire time course (Fig. 2b). This suggests that ceramide generation is dependent on BAK and/or BAX during UV-C induced apoptosis.
FIGURE 2.
Ceramide generation following UV-C irradiation is dependent on BAK/BAX. WT and DKO BMK cells were left untreated or UV-C irradiated (10 mJ/cm2). a, total ceramides; b, long-chain ceramides; and c, very long-chain ceramides were measured, normalized to total lipid phosphate, and expressed relative to their corresponding untreated control cells. Data are the mean of three independent experiments. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding control and #, p ≤ 0.05 statistically significant difference between DKO and the corresponding time-matched WT value.
BAK and BAX Double Knock-out Cells Fail to Generate Long-chain Ceramides in Response to Cisplatin
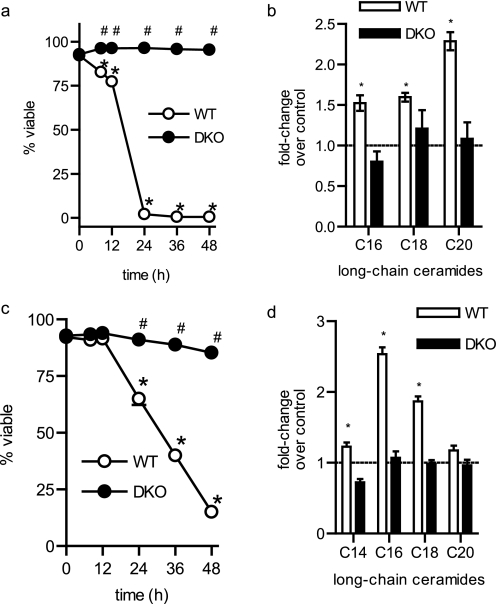
To determine if the effect of BAK/BAX knock-out on long-chain ceramide generation was stimulus specific, identical studies using another DNA-damaging agent, cisplatin, were performed. Cisplatin induces DNA damage that has been reported to lead to ceramide generation (28, 29). As with UV-C, WT BMK cells are sensitive to cisplatin, but DKO BMK cells are highly resistant (Fig. 3a). Similarly, cisplatin induced caspase activation at 9 h in WT cells, with no effect on caspase activity in DKO cells (Fig. 3b), and WT BMK cells preferentially generated C16-, C18-, and C20-ceramides following cisplatin treatment (Fig. 3c). As with UV-C irradiation, myriocin blocked ceramide elevation (Fig. 3c), delayed caspase activation (Fig. 3b), and abrogated cisplatin-induced cell death (Fig. 3a). More importantly, DKO cells treated with cisplatin showed no elevation of any ceramide species at any of the time points examined (Fig. 3d). Thus, the requirement of BAK and BAX for ceramide generation is not specific to UV-C irradiation. Furthermore, the requirement of BAK/BAX for ceramide generation following either UV-C irradiation or cisplatin treatment is not clone-specific (supplemental Fig. 2). This confirms that ceramide generation is dependent on BAK and BAX during DNA damage-induced apoptosis.
FIGURE 3.
BAK/BAX are required for ceramide generation following cisplatin treatment. Wild-type (WT) and Bak−/− Bax−/− (DKO) BMK cells were treated with vehicle (water) or cisplatin. a, viability; b, caspase 3/7 activity, and long-chain ceramide measurements in WT (c) and DKO (d) cells were performed as described in Fig. 1. c, where indicated WT BMK cells were preincubated (2 h) with myriocin (100 nm) prior to being treated with cisplatin. The average of three independent experiments is shown. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding control and #, p ≤ 0.05 statistically significant difference between WT and the time-matched myr and DKO value.
BAK and/or BAX Is Required for the Generation of Ceramide in Response to Other Apoptotic Stimuli and in Other Cell Lines
To determine if the requirement for BAK and/or BAX in stress-induced ceramide generation extends beyond the DNA damage response, we chose to utilize an alternative model of ceramide-mediated cell death: growth factor withdrawal-induced apoptosis (30). In this model, IL-3-dependent hematopoietic cell lines are derived from the bone marrow of mice (e.g. WT or DKO) and cultured (31). Depriving WT hematopoietic cells of the growth factor IL-3 leads to cell cycle arrest, atrophy, and the induction of apoptosis (Fig. 4a) (31). On the other hand, DKO cells fail to undergo apoptosis in response to growth factor withdrawal (Fig. 4a), although these cells do stop proliferating and undergo profound cellular atrophy demonstrating their strict dependence on IL-3 (31). We found that C16-, C18-, and C20-ceramides were elevated following withdrawal of IL-3 (Fig. 4b and supplemental Fig. 3a). However, this increase in long-chain ceramides was not observed in hematopoietic cells lacking BAK and BAX (Fig. 4b). Like BMK cells, murine hematopoietic cells also underwent programmed cell death (Fig. 4c) in response to cisplatin. Treatment of murine hematopoietic cells for 8 h with cisplatin induced elevations in C16- and C18-ceramide (Fig. 4d and supplemental Fig. 3b). However, no increase in ceramides occurred in DKO hematopoietic cells (Fig. 4d). These results indicate that the requirement for BAK/BAX for ceramide generation during apoptosis was not specific to the DNA damage response and was independent of cell type, suggesting that it is a general phenomenon of apoptosis.
FIGURE 4.
The requirement of BAK/BAX for ceramide generation during apoptosis is not specific to the apoptotic stimulus or cell type. a, viability of hematopoietic cells following the removal of IL-3. b, ceramide was measured in the cells in a 8 h following removal of IL-3. c, viability of hematopoietic cells treated with 10 μm cisplatin. d, ceramides were measured in the cells in c 8 h after cisplatin treatment. The average of three independent experiments is shown. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding untreated control and #, p ≤ 0.05 statistically significant difference between DKO and the corresponding time-matched WT value.
Ceramide Generation during Apoptosis Does Not Depend on MOMP or Activated Caspases
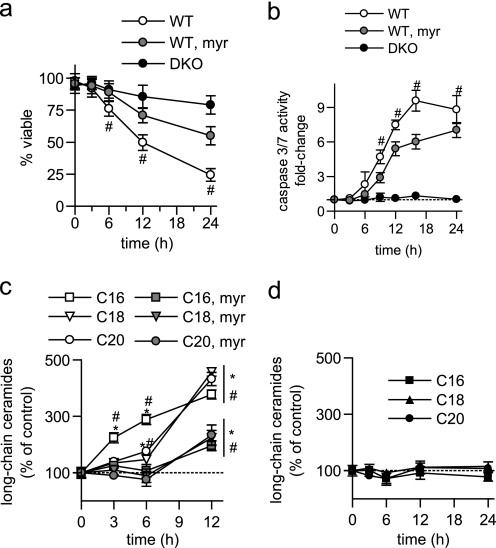
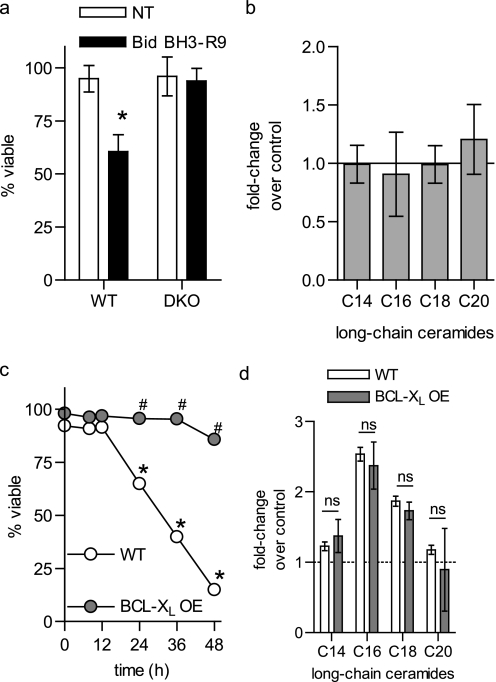
The data presented thus far suggest that ceramide generation during apoptosis occurs downstream of BAK and BAX. Classically, BAK and BAX are required for the induction of MOMP during apoptosis triggered by a variety of stimuli (1–4). To determine if the ability of BAK and BAX to trigger MOMP could account for their role in ceramide generation, we determined whether MOMP in the absence of an upstream apoptotic stimulus was sufficient to promote ceramide generation. To directly activate BAK and/or BAX and induce MOMP, the cell-permeable Bid BH3-R9 peptide was utilized. BH3-only proteins induce MOMP and cell death through a BAK/BAX-dependent mechanism (32). Consistent with this, Bid BH3-R9 killed WT but not DKO cells (Fig. 5a). Significantly, Bid BH3-R9 addition did not trigger long-chain ceramide generation in WT BMK cells, despite the loss in viability (Fig. 5b). Thus, activation of BAK/BAX in the absence of a death stimulus was insufficient to induce ceramide generation.
FIGURE 5.
Ceramide generation during apoptosis is independent of MOMP. a and b, WT BMK cells were treated with 30 μm R-t-Bid-BH3 for 6 h. Viability (a) was measured by trypan blue exclusion and (b) long-chain ceramides were measured. c, WT and BCL-XL-overexpressing (BCL-XL OE) hematopoietic cells were treated with cisplatin (10 μm) and (a) viability was measured at the indicated times via vital dye exclusion (4′,6-diamidino-2-phenylindole) and flow cytometry and (b) long-chain ceramides measured at 8 h. a, c, and d, data represent the average of three independent experiments. Error bars, ±S.D. b, data are the mean of duplicates from each of two independent experiments. Error bars, ±S.E. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding untreated control and #, p ≤ 0.05 statistically significant difference between DKO and the corresponding time-matched WT value. ns, no significant difference.
If ceramide generation is truly independent of MOMP, then it should still be generated when MOMP is blocked in cells containing BAK and BAX. Overexpression of BCL-XL protects cells from apoptotic stimuli via inhibiting MOMP. Accordingly, to further verify that ceramide generation is independent of MOMP, cells expressing the anti-apoptotic protein BCL-XL were utilized. As with BAK and BAX deletion (Fig. 4c), BCL-XL overexpression protected hematopoietic cells from cisplatin-induced death (Fig. 5c). However, overexpression of BCL-XL did not interfere with ceramide generation (Fig. 5d) further indicating that ceramide generation is independent of MOMP.
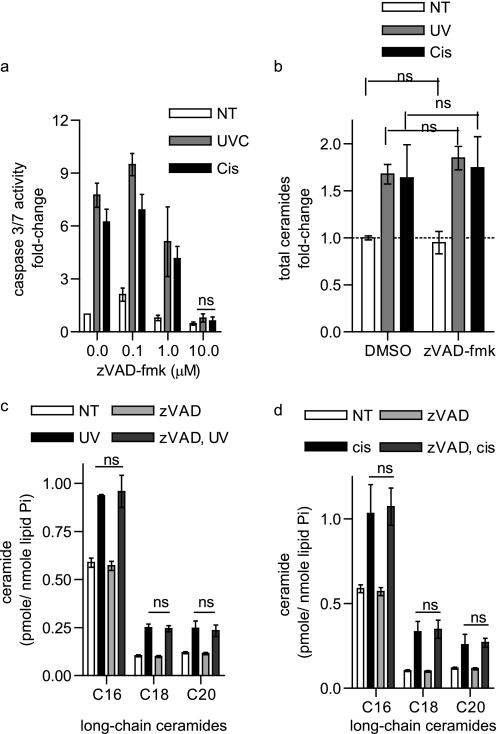
Cells lacking BAK and BAX do not activate caspases following death stimuli. Therefore, as a complementary approach, apoptosis was inhibited further downstream of MOMP at the level of caspase activation. The broad spectrum caspase inhibitor z-VAD-fmk completely inhibited caspase activation in response to either UV-C irradiation or cisplatin (10 μm, Fig. 6a). However, z-VAD-fmk did not interfere with total or long-chain ceramide generation in response to either these stimuli (Fig. 6, b–d). Taken together, these results with Bid BH3-R9, BCL-XL overexpression, and z-VAD-fmk indicate that the role of BAK and BAX in long-chain ceramide generation is not a result of their ability to trigger MOMP or caspase activation.
FIGURE 6.
Ceramide generation during apoptosis is independent of caspase activation. a, caspase 3/7 activity measured in WT BMK cells were pretreated for 2 h with vehicle (DMSO) or the indicated concentrations of z-VAD-fmk and then UV-C-irradiated or treated with cisplatin for 6 h. b, total ceramides measured in WT BMK cells pretreated for 2 h with vehicle (DMSO) or 10 μm z-VAD-fmk and then either UV-C-irradiated or treated with cisplatin for 6 h. c and d, long-chain ceramides measured in WT BMK cells pretreated for 2 h with vehicle (DMSO) or 10 μm z-VAD-fmk and then either UV-C irradiated (c) or treated with cisplatin (d) for 6 h. a–d, data are the mean of three independent experiments. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. ns, no significant difference.
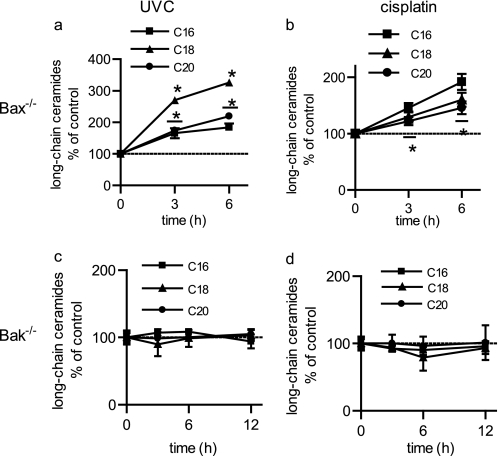
BAK Is Necessary for Generation of Long-chain Ceramides
Results indicate that ceramide generation is BAK/BAX-dependent, but is independent of their established roles in MOMP. Therefore, to determine the mechanism of action, it became important to determine which protein, BAK or BAX, modulates ceramide metabolism using single knock-out BMK cells. Importantly, cells containing BAK, but not BAX (BAX KO), were capable of generating long-chain ceramides in response to either UV-C irradiation (Fig. 7a) or cisplatin treatment (Fig. 7b). In contrast, cells containing BAX, but not BAK (BAK KO), could not generate ceramides following UV-C irradiation (Fig. 7c) or cisplatin treatment (Fig. 7d). Significantly, these results indicate that BAK, but not BAX, is required for ceramide generation during the induction of apoptosis.
FIGURE 7.
BAK, but not BAX, is required for the generation of long-chain ceramides during apoptosis. Ceramides were measured in BMK cells following either UV-C irradiation (a and c) or treatment with cisplatin (b and d). a and b, Bak+/+ Bax−/− BMK cells (Bax KO); c and d, Bak−/− Bax+/− (Bak KO) BMK cells. Data are the mean of three independent experiments. Error bars, ±S.D. For statistical analyses a two-way ANOVA was performed with a Bonferroni post-test. *, p ≤ 0.05 statistically significant difference between treatment and its corresponding untreated control.
BAK Regulates Ceramide Generation via a Ceramide Synthase
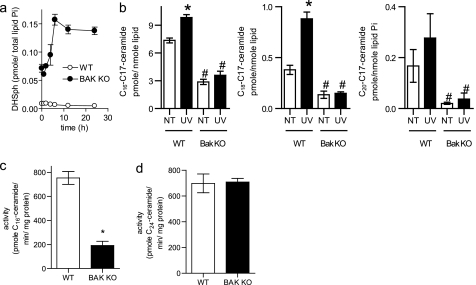
DNA damage triggers ceramide generation via the de novo pathway (Figs. 2 and 4). The acylation of sphinganine (dihydrosphingosine) by ceramide synthases (CerSs) is required to form dihydroceramide, which is subsequently converted to ceramide. Six CerS isoforms are present in mammalian cells, each with its own fatty acyl-CoA preference (33). Thus, specific CerS isoforms are responsible for the generation of ceramides of specific chain lengths. Cells lacking BAK have dramatically elevated dihydrosphingosine levels, both basally and following UV-C irradiation (Fig. 8a). In addition, only long-chain ceramides were acutely generated in response to the apoptotic stimuli utilized in this study (Figs. 1d, 2b, 3c, 4b, 4d, 5d, 6b, 6c, 6d, 7a, and 7b). Thus, we hypothesized that BAK might regulate ceramide generation at the level of CerS. To measure the flux of sphingosine into ceramide, we used in situ C17-sphingosine labeling. C17-sphingosine is one carbon shorter than naturally occurring C18-sphingosine, allowing its metabolism to ceramide to be followed in intact cells by MS. Notably, conversion of C17-sphingosine to long-chain C17-ceramides was reduced in Bak−/− cells relative to controls both basally and following UV-C irradiation (Fig. 8b), suggesting that BAK regulates CerS. To confirm this, in vitro CerS assays were performed on microsomes isolated from WT or Bak−/− BMK cells utilizing C16 fatty acyl-CoA and C17-sphingosine as substrates. Consistent with our intact cell labeling data (Fig. 8b), CerS activity in microsomes from Bak−/− cells was reduced by 75% relative to control cells (Fig. 8c). Importantly, when C24 fatty acyl-CoA was utilized as a substrate for the formation of C24-C17-ceramide in the in vitro CerS activity assay, there was no difference in the activity between WT and BAK KO cells. This suggests that BAK regulates specific CerS isoforms.
FIGURE 8.
BAK is required for ceramide synthase activity. a, WT and BAK KO BMK cells were left untreated or UV-C-irradiated, and dihydrosphingosine (DHSph, sphinganine) levels were measured and normalized to total lipid phosphate. b, WT and BAK KO BMK cells were left untreated or UV-C-irradiated for 2 h and labeled with C17-sphingosine. Long-chain C17-ceramides were measured by mass spectrometry. *, p ≤ 0.001 as measured via a two-way ANOVA with a Bonferroni post-test. c, long-chain ceramide synthase activity was measured in vitro in microsomes isolated from WT and Bak KO cells utilizing C16 fatty acyl-CoA and C17-sphingosine as substrates. *, p ≤ 0.001 according to Student's t test. d, very long-chain ceramide synthase activity was measured in vitro in microsomes isolated from WT and BAK KO cells utilizing C14 fatty acyl-CoA and C17 sphingosine as substrates. a–c, data are the mean of three independent experiments. Error bars, ±S.D. d, data are the mean of two independent experiments each performed in duplicate. Error bars, ±S.E.
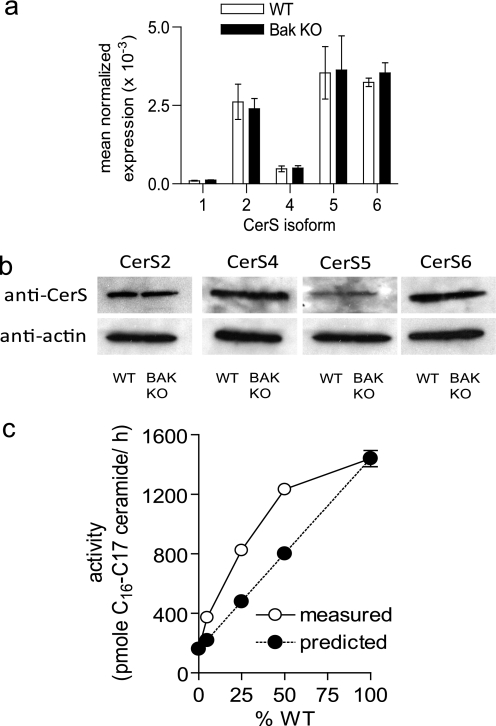
To determine if BAK regulates CerS activity via their expression, we performed quantitative reverse transcription-PCR and Western analysis. There was no difference in CerS isoform message or protein between WT and Bak KO cells (Fig. 9, a and b). Taken together, these data suggest that BAK regulates ceramide generation through post-translational regulation of specific CerS isoforms.
FIGURE 9.
BAK regulates CerS activity at the post-translational level. a, real-time reverse transcription-PCR was performed on RNA extracted from WT and Bak KO BMK cells. b, representative Western blot of CerS2, CerS4, CerS5, and CerS6 in WT and BAK KO cell lysates. c, WT and BAK KO microsomes were combined so that the total microsomal protein was 20 μg of microsomes (the percentage of the 20-μg amount that was the WT is indicated in the figure), and long-chain in vitro CerS activity was measured. The predicted activity was calculated from the sum of the measured individual component activities (see supplemental Table 2). a–c, data are the mean of three independent experiments. Error bars, ±S.D.
CerSs are present at equal levels in WT and BAK KO cells but are not active when BAK is absent. We wanted to determine if the CerS activity could be restored by adding back BAK. Unfortunately, BAK is an integral membrane protein that cannot be purified in the full-length form. However, to get around this problem, we performed reconstitution assays by adding small amounts of WT microsomes to BAK KO microsomes (Fig. 9c and supplemental Table 1). In vitro CerS activity assays were performed utilizing C16 fatty acyl-CoA and C17-sphingosine as substrates. 20 μg of total microsomes was utilized as the enzyme source using the following combinations of WT and BAK KO microsomes: 0% WT (20 μg of BAK KO), 5% WT (1 μg of WT, 19 μg of BAK KO), 25% WT (5 μg of WT, 15 μg of BAK KO), 50% WT (10 μg of WT and 10 μg of BAK KO), and 100% WT (20 μg of WT). In addition, the activities of the individual components for each of the above combinations were also measured and used to calculate the predicted activity for the combinations. In all cases, the addition of WT microsomes to the BAK KO microsomes resulted in higher activity than would be predicted by the sum of their component parts. This is consistent with BAK (or another factor present in the WT microsomes) reactivating the CerS from the BAK KO cells. The Hill coefficient for this reactivation is 1.09, which indicates a lack of cooperatively and suggests a 1:1 complex is formed between the “activation factor” and CerS. Taken together, the reconstitution assays confirm that CerS are present albeit inactive in BAK KO cells and that their activity can be restored via the addition of BAK containing microsomes.
DISCUSSION
In this study, we have identified a novel role for BAK as a regulator of ceramide production during the induction of apoptosis. This function was independent of both MOMP and caspase activation, previously established as events downstream of BAK activation. Importantly, BAK was necessary for ceramide generation in multiple cell types in response to diverse apoptotic stimuli, suggesting that the ubiquitously expressed BAK may be generally required for stress-induced ceramide generation. To our knowledge, this is the first study implicating BAK as a regulator of sphingolipid metabolism and the first to identify a protein that acts as a regulator of CerS activity during apoptosis. Importantly, because BAK is a well established regulator of apoptosis, these data provide a key mechanistic link between two parallel pathways to MOMP induction. More significantly, this study describes a unique function for BAK that cannot be performed by the closely related and seemingly functionally redundant protein BAX.
Recent studies have also identified functions for BAK and BAX in apoptosis that extend beyond MOMP, including ER calcium homeostasis and ER stress responses (34–36). Ceramide also regulates ER calcium levels and activates ER stress surveillance machinery (37–40). Moreover, ceramide generation occurs following the induction of ER stress with thapsigargin (38). The connection between BAK and ceramide generation established here raises the possibility that BAK may regulate ER calcium and ER stress responses by elevating cellular ceramide. Mechanistically, the actions of BAK are mediated, at least in part, through regulation of CerS activity. Like CerS, BAK is localized to both the ER and the mitochondrial membranes (41, 42). Thus, CerS and BAK may interact either directly or indirectly. The fact that only long-chain in vitro CerS activity was BAK-dependent and that this activity could be restored via the addition of BAK-containing microsomes is highly supportive of direct affects of BAK on specific CerS isoforms. BAK exists both as a monomer and homo- and hetero-oligomers in cells. The Hill coefficient for the reactivation of long-chain in vitro CerS activity was calculated to be 1 (1.09), which strongly suggests that a 1:1 complex is formed between the “CerS activation factor” (possibly BAK) from the WT microsomes and CerS in the BAK KO microsomes.
BAK and BAX are seemingly functionally redundant in the induction of apoptosis (4). However, the mechanism by which they induce apoptosis may differ. It has been previously shown that BAK, but not BAX, plays an important role in mitochondrial fragmentation, a morphological change that facilitates MOMP during apoptosis (35). Here we describe a second unique function for BAK in apoptosis, namely regulation of ceramide generation. Intriguingly, ceramides can also cause mitochondrial fragmentation (43, 44). Furthermore, ceramides induce negative curvature and lateral phase separation in giant liposomes leading to their fragmentation (45). Thus, ceramide generation may change membrane curvature to facilitate BAK-induced mitochondrial fragmentation in parallel with the inhibition of mitochondrial fusion machinery as has been previously described (35).
Connecting BAK to ceramide generation provides a novel link between apoptotic pathways previously thought to function independently. Ceramide can activate BAX during apoptosis (26, 27), and in some systems BAX activation is dependent upon the presence of BAK (44). Thus, BAK may induce BAX activation and apoptosis in part through ceramide generation. Ceramide has been implicated in both components of MOMP induction, namely mitochondrial fragmentation (43, 44) and formation of a pore in the mitochondrial outer membrane (17, 26, 27, 46). Thus, the data presented herein now point to a model where a BAK-mediated increase in ceramide facilitates MOMP induction via mitochondrial fragmentation and pore formation, leading to cytochrome c release, caspase activation, and the execution phase of apoptosis.
Supplementary Material
Acknowledgments
We are indebted to Dr. Eileen White of Rutgers University for donation of all kidney cell lines and to Drs. Craig Thompson and Tullia Lindsten for supplying the DKO hematopoietic cells. We also thank Drs. Jerry Chipuk and Stefka Spassieva for helpful discussions and critical reading of the manuscript and are grateful to the Flow Cytometry & Cell Sorting Shared Resource and the Lipidomics Shared Resource of the Hollings Cancer Center for their invaluable support.
This work was supported, in whole or in part, by National Institutes of Health Grants 1F30 ES016975-01 (to T. D. M.) from NIEHS, F31 CA126494 (to K. R. R.), K08 CA100526 (to A. L. E.), and R01 AG016583 from NIA and P01 CA097132 from NCI (to L. M. O.). This work was also supported by a Veterans Administration Career Development Award-2 (to L. J. S.), pilot project funding from a Veterans Administration REAP (to L. J. S. and L. M. O.), and American Cancer Society Institutional Research Grant IRG-97-219-11 (subaward to L. J. S.). The shared resources of the Hollings Cancer Center were supported in part by a Cancer Center Support Grant (P30 CA138313).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–3.
- MOMP
- mitochondrial outer membrane permeabilization
- BMK
- baby mouse kidney cell
- WT
- wild type
- DKO
- BAK and BAX double knock-out cell
- CerS
- ceramide synthase
- myr
- myriocin
- FB1
- fumonisin B1
- z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- FITC
- fluorescein isothiocyanate
- IL-3
- interleukin-3
- PI
- propidium iodide
- LC
- liquid chromatography
- MS
- mass spectrometry
- KO
- knock-out
- ER
- endoplasmic reticulum
- ANOVA
- analysis of variance
- El
- electrospray ionization.
REFERENCES
- 1.Zong W. X., Lindsten T., Ross A. J., MacGregor G. R., Thompson C. B. (2001) Genes Dev. 15, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Vela A., Opferman J. T., Cheng E. H., Korsmeyer S. J. (2005) EMBO Rep. 6, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsten T., Thompson C. B. (2006) Cell Death Diff. 13, 1272–1276 [DOI] [PubMed] [Google Scholar]
- 4.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alphonse G., Aloy M. T., Broquet P., Gerard J. P., Louisot P., Rousson R., Rodriguez-Lafrasse C. (2002) Int. J. Radiat. Biol. 78, 821–835 [DOI] [PubMed] [Google Scholar]
- 6.Chmura S. J., Nodzenski E., Beckett M. A., Kufe D. W., Quintans J., Weichselbaum R. R. (1997) Cancer Res. 57, 1270–1275 [PubMed] [Google Scholar]
- 7.Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 8.Sautin Y., Takamura N., Shklyaev S., Nagayama Y., Ohtsuru A., Namba H., Yamashita S. (2000) Thyroid 10, 733–740 [DOI] [PubMed] [Google Scholar]
- 9.Selzner M., Bielawska A., Morse M. A., Rüdiger H. A., Sindram D., Hannun Y. A., Clavien P. A. (2001) Cancer Res. 61, 1233–1240 [PubMed] [Google Scholar]
- 10.Rodriguez-Lafrasse C., Alphonse G., Aloy M. T., Ardail D., Gérard J. P., Louisot P., Rousson R. (2002) Int. J. Cancer 101, 589–598 [DOI] [PubMed] [Google Scholar]
- 11.Alphonse G., Bionda C., Aloy M. T., Ardail D., Rousson R., Rodriguez-Lafrasse C. (2004) Oncogene 23, 2703–2715 [DOI] [PubMed] [Google Scholar]
- 12.Lavieu G., Scarlatti F., Sala G., Carpentier S., Levade T., Ghidoni R., Botti J., Codogno P. (2006) J. Biol. Chem. 281, 8518–8527 [DOI] [PubMed] [Google Scholar]
- 13.Pchejetski D., Golzio M., Bonhoure E., Calvet C., Doumerc N., Garcia V., Mazerolles C., Rischmann P., Teissié J., Malavaud B., Cuvillier O. (2005) Cancer Res. 65, 11667–11675 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y. Y., Han T. Y., Yu J. Y., Bitterman A., Le A., Giuliano A. E., Cabot M. C. (2004) J. Lipid Res. 45, 933–940 [DOI] [PubMed] [Google Scholar]
- 15.Bektas M., Jolly P. S., Müller C., Eberle J., Spiegel S., Geilen C. C. (2005) Oncogene 24, 178–187 [DOI] [PubMed] [Google Scholar]
- 16.Taha T. A., Kitatani K., El-Alwani M., Bielawski J., Hannun Y. A., Obeid L. M. (2006) FASEB J. 20, 482–484 [DOI] [PubMed] [Google Scholar]
- 17.Siskind L. J., Feinstein L., Yu T., Davis J. S., Jones D., Choi J., Zuckerman J. E., Tan W., Hill R. B., Hardwick J. M., Colombini M. (2008) J. Biol. Chem. 283, 6622–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 19.Spassieva S., Seo J. G., Jiang J. C., Bielawski J., Alvarez-Vasquez F., Jazwinski S. M., Hannun Y. A., Obeid L. M. (2006) J. Biol. Chem. 281, 33931–33938 [DOI] [PubMed] [Google Scholar]
- 20.Degenhardt K., Sundararajan R., Lindsten T., Thompson C., White E. (2002) J. Biol. Chem. 277, 14127–14134 [DOI] [PubMed] [Google Scholar]
- 21.Dai Q., Liu J., Chen J., Durrant D., McIntyre T. M., Lee R. M. (2004) Oncogene 23, 3650–3658 [DOI] [PubMed] [Google Scholar]
- 22.Charruyer A., Jean C., Colomba A., Jaffrézou J. P., Quillet-Mary A., Laurent G., Bezombes C. (2007) Biochem. J. 405, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee M., Wu S. (2001) Mol. Cell. Biochem. 219, 21–27 [DOI] [PubMed] [Google Scholar]
- 24.Kroesen B. J., Jacobs S., Pettus B. J., Sietsma H., Kok J. W., Hannun Y. A., de Leij L. F. (2003) J. Biol. Chem. 278, 14723–14731 [DOI] [PubMed] [Google Scholar]
- 25.Senkal C. E., Ponnusamy S., Rossi M. J., Bialewski J., Sinha D., Jiang J. C., Jazwinski S. M., Hannun Y. A., Ogretmen B. (2007) Mol. Cancer Ther. 6, 712–722 [DOI] [PubMed] [Google Scholar]
- 26.Jin J., Hou Q., Mullen T. D., Zeidan Y. H., Bielawski J., Kraveka J. M., Bielawska A., Obeid L. M., Hannun Y. A., Hsu Y. T. (2008) J. Biol. Chem. 283, 26509–26517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashkar H., Wiegmann K., Yazdanpanah B., Haubert D., Krönke M. (2005) J. Biol. Chem. 280, 20804–20813 [DOI] [PubMed] [Google Scholar]
- 28.Min J., Mesika A., Sivaguru M., Van Veldhoven P. P., Alexander H., Futerman A. H., Alexander S. (2007) Mol. Cancer Res. 5, 801–812 [DOI] [PubMed] [Google Scholar]
- 29.Noda S., Yoshimura S., Sawada M., Naganawa T., Iwama T., Nakashima S., Sakai N. (2001) J. Neurooncol. 52, 11–21 [DOI] [PubMed] [Google Scholar]
- 30.Dbaibo G. S., Pushkareva M. Y., Jayadev S., Schwarz J. K., Horowitz J. M., Obeid L. M., Hannun Y. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lum J. J., Bauer D. E., Kong M., Harris M. H., Li C., Lindsten T., Thompson C. B. (2005) Cell 120, 237–248 [DOI] [PubMed] [Google Scholar]
- 32.Letai A., Bassik M. C., Walensky L. D., Sorcinelli M. D., Weiler S., Korsmeyer S. J. (2002) Cancer Cell 2, 183–192 [DOI] [PubMed] [Google Scholar]
- 33.Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) J. Biol. Chem. 281, 25001–25005 [DOI] [PubMed] [Google Scholar]
- 34.Klee M., Pallauf K., Alcalá S., Fleischer A., Pimentel-Muiños F. X. (2009) EMBO J. 28, 1757–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks C., Wei Q., Feng L., Dong G., Tao Y., Mei L., Xie Z. J., Dong Z. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11649–11654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetz C., Bernasconi P., Fisher J., Lee A. H., Bassik M. C., Antonsson B., Brandt G. S., Iwakoshi N. N., Schinzel A., Glimcher L. H., Korsmeyer S. J. (2006) Science 312, 572–576 [DOI] [PubMed] [Google Scholar]
- 37.Muriel M. P., Lambeng N., Darios F., Michel P. P., Hirsch E. C., Agid Y., Ruberg M. (2000) J. Comp. Neurol. 426, 297–315 [DOI] [PubMed] [Google Scholar]
- 38.Lei X., Zhang S., Bohrer A., Ramanadham S. (2008) J. Biol. Chem. 283, 34819–34832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park M. A., Zhang G., Martin A. P., Hamed H., Mitchell C., Hylemon P. B., Graf M., Rahmani M., Ryan K., Liu X., Spiegel S., Norris J., Fisher P. B., Grant S., Dent P. (2008) Cancer Biol. Ther. 7, 1648–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mousley C. J., Tyeryar K., Ile K. E., Schaaf G., Brost R. L., Boone C., Guan X., Wenk M. R., Bankaitis V. A. (2008) Mol. Biol. Cell 19, 4785–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heath-Engel H. M., Shore G. C. (2006) Cell Death Diff. 13, 1277–1280 [DOI] [PubMed] [Google Scholar]
- 42.Bionda C., Portoukalian J., Schmitt D., Rodriguez-Lafrasse C., Ardail D. (2004) Biochem. J. 382, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. (2001) EMBO J. 20, 2690–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parra V., Eisner V., Chiong M., Criollo A., Moraga F., Garcia A., Härtel S., Jaimovich E., Zorzano A., Hidalgo C., Lavandero S. (2008) Cardiovasc. Res. 77, 387–397 [DOI] [PubMed] [Google Scholar]
- 45.Holopainen J. M., Angelova M. I., Kinnunen P. K. (2000) Biophys. J. 78, 830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kepp O., Rajalingam K., Kimmig S., Rudel T. (2007) EMBO J. 26, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.