Abstract
Connexin43 (Cx43) is widely expressed in embryonic brain, and its expression becomes restricted mainly to astrocytes as the central nervous system matures. Recent studies have indicated that Cx43 plays important, nonchannel, roles during central nervous system development by affecting neuronal cell migration. Here, we evaluated the effects of Cx43 on neuronal differentiation. For that we used an in vitro model of neural cell development (neurospheres) to evaluate, through immunocytochemistry, electrophysiology, and molecular biology, the degree of neuronal maturation from neurospheres derived from wild-type (WT) and Cx43-null mice. Our results indicate that Cx43 is a negative modulator of neuronal differentiation. The percent neurospheres containing differentiated neurons and the number of cells displaying inward currents were significantly higher in Cx43-null than in WT littermate neurospheres. Knockdown of Cx43 with small interfering RNA increased the number of WT neurospheres generating differentiated neurons. Blockade of gap junctional communication with carbenoxolone did not induce neuronal differentiation in WT neurospheres. Transfection of Cx43-null neurospheres with Cx43 mutants revealed that Cx43 carboxyl terminus prevents neuronal maturation. In agreement with these in vitro data, in situ analysis of embryonic day 16 brains revealed increased β-III-tubulin expression in germinal zones of Cx43-null compared with that of WT littermates. These results indicate that Cx43, and specifically its carboxyl terminus, is crucial for signaling mechanisms preventing premature neuronal differentiation during embryonic brain development.
Keywords: Connexin, Development, Gap Junctions, Neural Stem Cell, Neuroprogenitor Cell, Connexin43, Development, Neural Progenitor, Neuronal Differentiation, Neurosphere
Introduction
Multiple distinct mechanisms contribute to neocortical development. Diffusible and nondiffusible molecules have been implicated in neural cell proliferation, migration, and differentiation during brain development (1–4). Connexins (Cx),2 the proteins forming gap junctions, are prominent in neural progenitor cells and play important roles in neural cell proliferation, migration, and differentiation (5–9). Among the central nervous system gap junction proteins, Cx43 and Cx26 are the two most abundantly expressed in neural stem cells, although transcripts for other connexins (Cx29, Cx30, Cx30.2, Cx32, Cx36, and Cx47) are also present in embryonic states (10–12). The expression pattern of connexin proteins dramatically changes as the central nervous system matures, becoming restricted with respect to cell type. For instance, following postnatal development, Cx43 together with Cx30 are mainly found in astrocytes, whereas Cx29, Cx32, and Cx47 form the oligodendrocyte group of gap junction proteins, and Cx36, Cx45, and Cx30.2 are expressed in neurons (for review, see Ref. 13).
The dramatic decrease in Cx43 expression during central nervous system development, specifically during neuroblast maturation, has raised the possibility that concurrent loss of gap junctional communication may play an important role during neuronal differentiation. Indeed, initial studies (14, 15) indicating an inverse relation between the degree of electrical and dye coupling and neuronal differentiation suggested that coupling among neuroblasts, by favoring homogeneity among their intracellular compartment, was sufficient to maintain the neural progenitors in an undifferentiated state (16). However, in contrast with the idea that reduced coupling favors neuronal differentiation, treatment of P19 and NT2 “stem cell lines” with the gap junction channel blockers carbenoxolone or 18-α-glycerrhetinic acid was shown to reduce the expression of neuronal differentiation markers induced by retinoic acid treatment (17, 18). The fact, however, that expression levels of Cx43 in NT2 cells dramatically decreases as cells differentiate (17, 18) and that carbenoxolone and 18-α-glycerrhetinic were shown to reduce the expression of Cx43 in NT2 cells (19) raises the possibility that the expression of Cx43 itself rather than the reduced gap junctional communication may modulate neuronal differentiation.
In the present study, we investigated the mechanisms by which Cx43 modulates neuronal differentiation using neural stem cells derived from wild-type (WT) and Cx43-null mice. We show here for the first time that, at embryonic day (E) 16, there is an early onset of neuronal differentiation in brains of mice lacking Cx43. Moreover, using an in vitro model of neural cell development we show that this early onset of neuronal differentiation in the Cx43-null brain is unrelated to gap junctional coupling but can be prevented by exogenous expression of the carboxyl-terminal domain of the Cx43 molecule.
EXPERIMENTAL PROCEDURES
Embryonic Brain Sections
Pregnant Cx43 heterozygote C57BL/6J mice (Gja1Tm1/Kdr, initially purchased from Jackson Laboratory and maintained for 12 years in our animal facility) were anesthetized, and E16 embryos were removed. After decapitation, the embryonic brains were fixed in 4% p-formaldehyde overnight and then transferred to a cryoprotective solution (30% sucrose in phosphate-buffered saline). Coronal sections (14 μm thick) of WT and Cx43-null brains were cut in a cryostat (Leica-CM1510S; Leica Microsystems) and processed for immunohistochemitry (see below). The Albert Einstein College of Medicine Animal Care and Use Committee approved all experimental procedures performed.
Neurosphere Cultures
Neurospheres were prepared as described previously (20, 21). Briefly, neural progenitor cells were obtained by aspiration of forebrain tissues of E14 wild-type and Cx43-null C57BL/6J mouse embryos and mechanically dissociated into single cells in ice-cold Hanks' balanced solution (Ca2+- and Mg2+-free). Cells were transferred to tissue culture dishes containing Dulbecco's modified Eagle's medium nutrient mixture F12 (DMEM-F12; Invitrogen) supplemented with 5% B27 (Invitrogen), 1% antibiotics, and 20 ng/ml human recombinant epidermal growth factor (Sigma) and allowed to grow into floating neurospheres. Culture medium was changed twice a week, and neurospheres were mechanically dissociated into smaller neurospheres once a week. Neurosphere cultures were maintained for no longer than 2 months. For in vitro cell differentiation, floating neurospheres were plated on glass bottom microwells (MatTek Co., Ashland, MA) coated with poly-d-lysine- (10 μg/ml; Sigma) and fibronectin (10 μg/ml; Sigma-Aldrich) and bathed in DMEM-F12 in the absence of epidermal growth factor. About 10–15 neurospheres were seeded/MatTek dish.
Electrophysiology
Whole cell patch clamp recordings were performed at room temperature on progenitor cells emigrated from 2–3-day adherent neurospheres bathed in a solution containing 140 mm NaCl, 2 mm CsCl, 2 mm CaCl2, 1 mm MgCl2, 5 mm Hepes, 4 mm KCl, 5 mm glucose, 2 mm sodium pyruvate, and 1 mm BaCl2, pH 7.4 (using NaOH). The pipette solution contained 130 mm CsCl, 10 mm EGTA, 0.5 mm CaCl2, 3 mm MgATP, 2 mm Na2ATP, and 10 mm Hepes, pH 7.3 (CsOH). Whole cell membrane currents were measured using the patch clamp technique by applying 10-mV voltage steps from a holding potential of −100 mV to final values of +80 mV. All data were recorded through an Axopatch 200B amplifier and digitized using an Axon Instruments digitizer (Molecular Devices). Clampex 6.0 was used for recording and the Clampfit 9.0 program for analysis.
Immunostaining
Cryosections of E16 WT and Cx43-null brains (see above) were washed three times with 0.3% Triton X-100 in phosphate-buffered saline (PBST) and incubated with phosphate-buffered saline containing 10% normal goat serum for 30 min at room temperature. Brain sections were incubated with mouse anti-β-III-tubulin (1:500; Chemicon, Millipore) at 4 °C for 72 h after which they were washed several times with PBST prior to incubation with goat anti-mouse Alexa Fluor 488-conjugated secondary antibodies. Following several washes, slides were mounted in VectaShield containing DAPI (Vector Laboratories, Inc., Burlingame, CA).
2–3-day adherent neurospheres were fixed for 5 min with 4% p-formaldehyde and then treated for 30 min with a solution containing 0.4% Triton X-100 and 10% goat serum prior to overnight incubation with mouse anti-β-III-tubulin (1:500; Chemicon, Millipore). After several washes, cells were incubated with goat anti-mouse-conjugated Alexa Fluor 488 secondary antibodies and mounted using VectaShield containing DAPI.
Morphometric Analyses of Neurite Extensions
Confocal z-series (0.5-μm optical section) images of β-III-tubulin-positive cells derived from 2–3-day adherent WT and Cx43-null neurospheres were acquired using an upright confocal microscope (Olympus BX61WI) equipped with an argon/krypton laser and 20× dry objective. Images were captured using Fluoview 4.3 software (Olympus). Neurite lengths of reconstructed images were calculated using NeuronJ (an ImageJ plugin) image software.
Transfection and Carbenoxolone Treatments
Floating Cx43-null neurospheres were transfected with 6 μg/ml cDNA encoding either full-length Cx43, Cx43 truncated at position 257 (Met257), or with Cx43CT-(255–382) using Lipofectamine2000 reagent (Invitrogen). After 18–19 h, transfection reagents were removed, and neurospheres were plated in polylysine/fibronectin-coated MatTek dishes containing DMEM-F12 for 24–30 h. Cx43 constructs were originally obtained from Dr. Eric Beyer (full-length Cx43) and Dr. Mario Delmar (Cx43M257 and Cx43CT). In some experiments, WT neurospheres were transfected with Cx43 siRNA (sequence CAATTCCTCCTGCCGCAAT) (22, 24). Floating wild-type neurospheres were treated with the gap junction channel blocker carbenoxolone (CBX; 100 μm) for 18–19 h and then seeded on poly-d-lysine/fibronectin-coated MatTek dishes containing DMEM-F12 for 24–30 h in the continuous presence of CBX. Quantification of neuronal differentiation from transfectants and CBX-treated neurospheres was performed after immunostaining by measuring the percent neurospheres displaying at least four β-III-tubulin-positive cells with neurite projections extending >60 μm out of the neurospheres (referred to as neurospheres with differentiated neurons).
Statistical Analyses
All data are expressed as mean ± S.E. GraphPad Prism version 5 was used for statistical analysis, consisting of one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. Unpaired t test comparison was also employed in some cases.
RESULTS
Disruption of Cerebral Cortex Layer Formation in the Cx43-null Mice
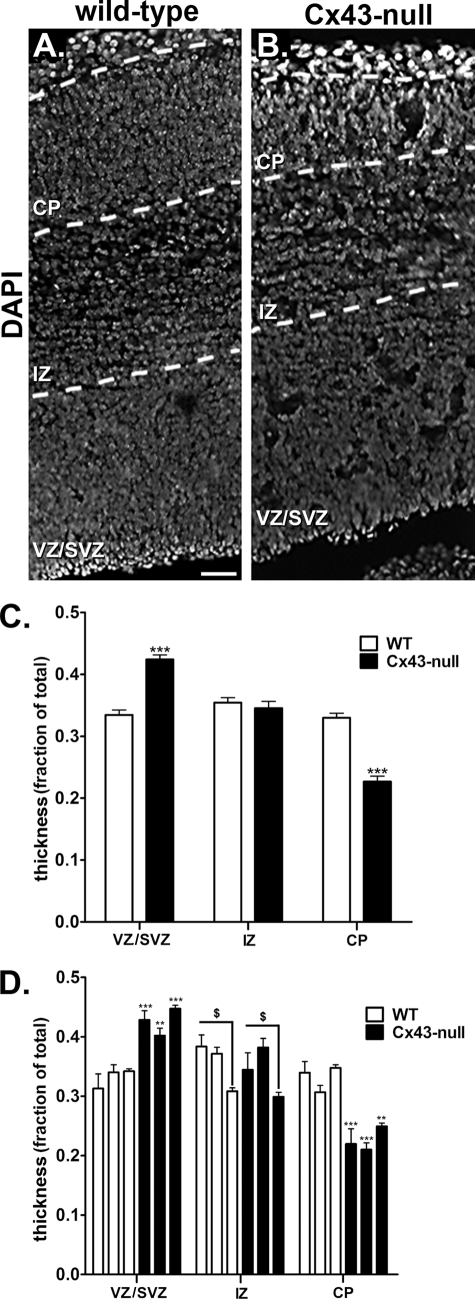
To evaluate whether global deletion of Cx43 affects the lamination of the cerebral cortex during the development of the nervous system, we stained brain coronal sections of E16 WT and Cx43-null mice with the nuclear marker DAPI. The anatomical dispersion of cells was the criterion to identify different sublayers of the neocortical wall (CP, IZ, and VZ/SVZ), and the thickness of each layer was measured and normalized to the total cortical thickness (referred to as fractional thickness). For that, 3 WT and 3 Cx43-null E16 brains derived from three separate litters were employed and measurements performed on 20 and 17 coronal sections of WT and Cx43-null forebrains, respectively. Fig. 1A shows the profile of cortical lamination of a WT animal stained with DAPI. In the Cx43-null mice (Fig. 1B), the fractional thickness of sublayer VZ/SVZ is evidently larger (0.40 ± 0.01, n = 43 measurements from 3 animals) compared with that of WT animals (0.33 ± 0.01, n = 58 measurements from 3 animals; p < 0.0001, unpaired t test; Fig. 1C). Moreover, we found a significant difference between the fractional CP thickness of the WT (0.33 ± 0.01, n = 58 measurements) and Cx43-null mice (0.23 ± 0.01, n = 43 measurements; p < 0.0001, unpaired t test; Fig. 1C). In contrast to the substantial and reciprocal thickness of CP and VZ/SVZ sublayers, the fractional thickness of the IZ sublayer was the same in the two genotypes (WTIZ: 0.36 ± 0.01, n = 58 measurements; Cx43-nullIZ: 0.35 ± 0.01, n = 43 measurements; p = 0.72, unpaired t test; Fig. 1C). Similar results in terms of fractional VZ/SVZ and CP sublayer thicknesses were obtained when analyses were performed for each animal individually (Fig. 1D). Interestingly, we found that one set of WT and Cx43-null brains had a thinner IZ sublayer compared with the other two sets of animals (within the same genotype) (Fig. 1D), but they did not significantly differ when compared with each other (between genotypes).
FIGURE 1.
Altered sublayer thickness of embryonic forebrains from Cx43-null mice. A and B, epifluorescence images of embryonic (day 16) forebrain cryosections (14 μm) showing the distribution of nuclei of progenitors stained with DAPI in three cortical layers (CP, cortical plate; IZ, intermediate zone; VZ/SVZ, ventricular zone/subventricular zone) of wild-type (A) and Cx43-null (B) mice. Scale bar, 16 μm. C, quantitative analyses of layer thickness (fraction of total), based on DAPI staining, shown as bar histograms of mean ± S.E. (error bars) values that were obtained from 58–43 hemicoronal sections of 3 WT and 3 Cx43-null animals, respectively. The fractional thickness of VZ/SVZ of Cx43-null brains was larger whereas the CP was smaller than those of WT littermates. D, mean ± S.E. (error bars) fractional thickness values obtained for the three cortical layers measured from each of the 3 wild-type (white bars) and Cx43-null (black bars) animals individually. Images were acquired using a cooled-CCD HQ2 camera (Photometrics) attached to an epifluorescence inverted microscope (TE2000-E; Nikon) equipped with a 10× dry objective and UV filter set. Three different litters were used.
These data showing reduction of the CP sublayer thickness in the Cx43-null E16 brains are in agreement with previous reports (23, 24) of reduced neuronal progenitor cells in the cortical plate of E18 and E21 brains, which was attributed to delayed neuronal cell migration in the absence of Cx43. However, in contrast to the reported accumulation of cells at the IZ sublayer of E21 brains following knockdown of Cx43 by shRNA (23) and in E18 brains of Cx43f/f:nestin-Cre mice (24), we found an enlargement of the VZ/SVZ sublayers in E16 brains of the global Cx43-null mice.
The Deletion of Cx43 Induces Early Neuronal Differentiation
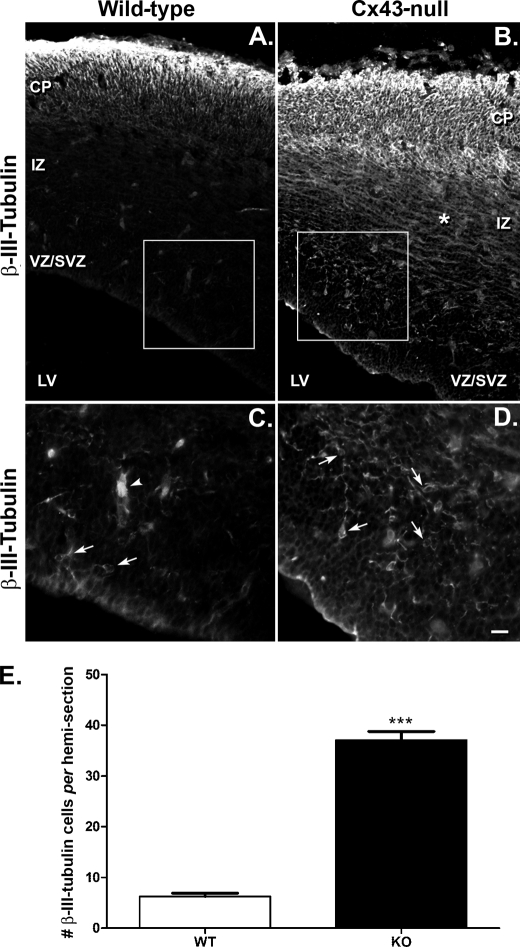
To determine whether the accumulation of cells in the proliferative zones could result in premature neuronal differentiation, we used the neuronal microtubule marker β-III-tubulin to analyze the neuronal phenotype in the E16 neocortex of WT and Cx43-null mice. In the WT mice, we observed high expression of β-III-tubulin in the CP and a few cells and/or cell processes in the IZ (Fig. 2A). Also, there was a rare occurrence of β-III-tubulin-positive cells in the proliferative sublayers VZ/SVZ at this embryonic age (Fig. 2, A and C). By contrast, the expression of β-III-tubulin was markedly higher in the Cx43-null mice and spread through all neocortical layers (Fig. 2B). Of note is the higher incidence of expression of β-III-tubulin in the VZ/SVZ sublayer of the Cx43-null mice (Fig. 2, B and D) as well as more intense β-III-tubulin immunoreactivity in the IZ (compare Fig. 2, A and B). Quantification of β-III-tubulin-positive cells in the germinal zone (VZ/SVZ) indicates a 6-fold increase in the number of neurons in Cx43-null E16 brains compared with those of WT (Fig. 2E; Cx43-null = 37.05 ± 1.7 cells, n = 20 hemicoronal sections from 3 animals; WT = 6.24 ± 0.6 cells, n = 25 hemicoronal sections from 3 animals).
FIGURE 2.
Premature neuronal differentiation in embryonic brains of mice lacking Cx43. A and B, confocal images of embryonic (day 16) forebrains of WT (A) and Cx43-null (B) mice showing the distribution of β-III-tubulin-positive neuronal cells in the three cortical layers (VZ/SVZ, IZ, and CP); LV, lateral ventricle. Immunohistochemstry revealed increased number of cells expressing anti-β-III-tubulin in the VZ/SVZ and IZ layers of the forebrains of Cx43-null mice compared with those of WT littermates. Note the tangential distribution of β-III-tubulin-positive fibers (*) in the IZ of Cx43-null brain. C and D, confocal images of WT and Cx43-null VZ/SVZ layers amplified 2× from regions delimited by the squares in A and B, respectively. Arrows indicate cell bodies of β-III-tubulin-positive cells, and arrowhead in C shows part of a blood vessel. Images were acquired using a laser scanning confocal inverted microscope (Zeiss Duo 510 Meta) equipped with a 20× dry objective and fluorescein isothiocyanate filter sets using an argon ion laser (488 nm line). Antibodies used were mouse anti-β-III-tubulin (1:500; Chemicon) and goat anti-mouse Alexa Fluor-conjugated 488 nm antibodies (1:2000; Invitrogen). E, bar histograms showing mean ± S.E. (error bars) number of β-III-tubulin-positive cells/hemicoronal section present in the VZ/SVZ of WT and Cx43-null (KO) E16 brains. DAPI stain of sections shown in this figure that were used to define sublayers boundaries are displayed in supplemental Fig. S1.
These results suggest that global deletion of Cx43 promotes early neuronal differentiation in the E16 mouse neocortex. The appearance of β-III-tubulin-positive cells at the VZ/SVZ also suggests that the early postmitotic neuroblasts differentiate prior to their migration into the cortical plate and that their accumulation in the VZ/SVZ thereby promotes the thickening of this sublayer (Fig. 1; for DAPI staining and demarcation of sublayer boundaries, see supplemental Fig. 1S). The presence of more β-III-tubulin-positive tangential processes in the IZ (Fig. 2) also suggests an early differentiation of the tangentially migrating neurons originating from the ganglionic eminence.
Early Neuronal Differentiation from Cx43-null Neurospheres
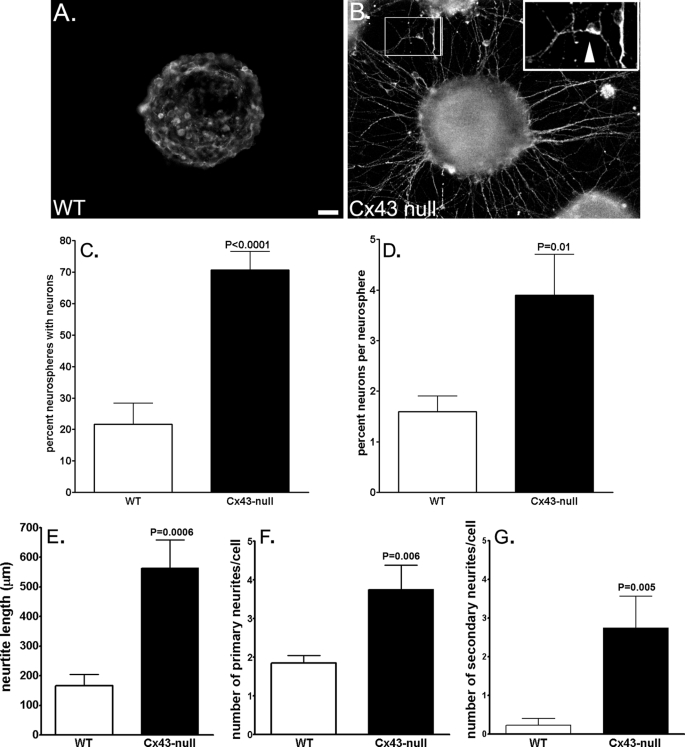
To evaluate the mechanisms by which Cx43 modulates neuronal differentiation, we used the neurosphere assay as an in vitro model of cell development. Neural progenitor cells derived from WT and Cx43-null E14 brains grown into embryonic bodies (neurospheres) were seeded in poly-d-lysine and fibronectin-coated dishes for 2–3 days to allow cell differentiation and then immunostained for β-III-tubulin. Quantification of percent Cx43-null neurospheres that after 3 days of adhesion to the substrate generated differentiated neurons (β-III-tubulin-positive cells) revealed that 70.7 ± 5.9% (n = 70 neurospheres from 16 animals derived from 7 litters) of Cx43-null neurospheres displayed neurons with neurites whereas only 21.7 ± 6.6% (n = 99 neurospheres from 20 animals derived from 9 litters) of WT neurospheres generated such differentiated β-III-tubulin-positive cells (Fig. 3, A–C). Although the percent neurospheres with differentiated neurons from Cx43-null brains was high, the absolute number of β-III-tubulin-positive cells generated from each neurosphere was quite small; nevertheless, there was a significantly higher percentage of cells expressing the neuronal marker in Cx43-null neurospheres compared with those of WT littermates (WT: 1.6 ± 0.3%, n = 14 neurospheres from 5 animals derived from 3 litters; Cx43-null: 3.9±.0.8%; n = 14 neurospheres from 5 animals derived from 3 litters; p = 0.011, unpaired t test; Fig. 3D).
FIGURE 3.
More substantial neuronal differentiation from Cx43-null-derived neurospheres than WT. A and B, examples of epifluorescence images of WT (A) and Cx43-null (B) neurospheres showing the expression of the neuronal marker β-III-tubulin in 3-day adherent progenitors. Note the extensive neurite projections from Cx43-null neurospheres and their complete absence in that of a WT littermate. Insets in B illustrate a β-III-tubulin-positive cell with three neurites (arrowhead) emanating from the cell body. Inset on the right was magnified twice that of the left. Scale bar, 50 μm. C and D, bar histograms of the mean ± S.E. (error bars) values of percent neurospheres with differentiated neurons (β-III-tubulin-positive cells with neurite projections) (C) and of percent neurons/ neurosphere (D) obtained from WT (white bars) and Cx43-null (black bars) mice. E–G, bar histograms of the mean ± S.E. (error bars) values of neurite length (E) and of the number of primary (F) and secondary (G) neurites measured from β-III-tubulin-positive progenitor cells derived from WT (white bars) and Cx43-null (black bars) neurospheres. Morphometric analyses of neurites from β-III-tubulin-positive cells were performed on reconstructed images of confocal z-sections (supplemental Fig. 2S) using NeuronJ software.
Besides this increased number, Cx43-null-derived β-III-tubulin-positive cells displayed a more mature phenotype than those from WT neurospheres. Quantification of neurite length and number of primary and of secondary branches were obtained from confocal images of neurospheres containing β-III-tubulin-positive cells using NeuronJ morphometry software. These analyses revealed that all three parameters of morphological complexity were higher in neurons derived from Cx43-null than those derived from WT neurospheres (Fig. 3, E–G and supplemental Fig. 2S). β-III-Tubulin-positive cells derived from Cx43-null neurospheres displayed neurites that were three times longer than those of WT cells (Cx43-null: 563.1 ± 95.8 μm; WT: 166.3 ± 37.4 μm; n = 12–13 cells from 3 WT and 2 Cx43-null animals derived from the same litter; Fig. 3E); furthermore, each Cx43-null-derived neuron had twice as many primary neurites (3.75 ± 2.18, n = 12 cells; Fig. 3F) as the WT-derived cells (1.85 ± 0.19, n = 13 cells) and 12 times more secondary neurites (2.75 ± 0.82, n = 12 cells; Fig. 3G) than WT-derived neurons (0.23 ± 0.17, n = 13 cells).
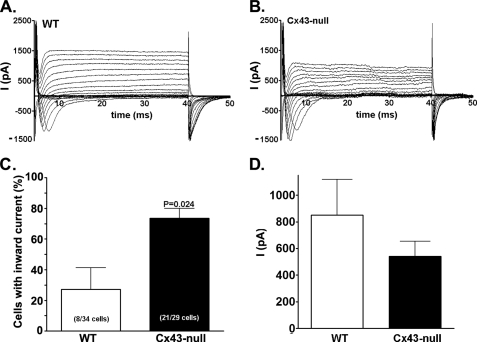
To extend the analysis of neuronal maturation to functional features, we performed whole cell patch clamp recordings on cells emigrated from WT and Cx43-null adherent neurospheres to investigate the presence and characteristics of their active conductances. In agreement with the higher number and advanced morphology of β-III-tubulin-positive cells found in Cx43-null neurospheres, electrophysiological recordings (Fig. 4, A and B) also evidenced a higher number of cells displaying neuronal-like inward currents in Cx43-null-derived cells than in WT littermates (Cx43-null: 73.5 ± 6.4%, WT: 27.2 ± 14.1%; n = 29–34 cells from 5 or 6 animals derived from 4 litters; p = 0.02, unpaired t test; Fig. 4C). Analyses of inward current amplitudes recorded from WT and Cx43-null cells in which currents were present indicated that both displayed similar current amplitudes (Cx43-null: 542.2 ± 111.7 pA, n = 14 cells; WT: 849.9 ± 269.7 pA, n = 8 cells, p > 0.05 unpaired t test; Fig. 4D). None of the cells displaying inward currents was electrically coupled to neighboring cells, as measured by dual-whole cell voltage clamp recordings (data not shown). Thus, our morphological and electrophysiological findings strongly indicate that deletion of Cx43 favors neuronal differentiation. No significant difference in ratio of nestin to glial fibrillary acidic protein expression levels was observed by Western blot analyses of 1–7-day adherent WT and Cx43-null neurospheres, suggesting that these markers are not affected by Cx43 expression (supplemental Fig. 3S).
FIGURE 4.
Electrical excitability of WT and Cx43-null progenitor cells. A and B, whole cell recordings obtained from WT (A) and Cx43-null (B) progenitors showing the presence of (neuronal-like) inward currents in cells from both genotypes. C and D, quantitative analyses of number of cells displaying inward currents (C) revealed a significantly higher number of cells with neuronal-like currents (21 of 29 cells) in the Cx43-null genotype compared with those of WT (8 of 34) progenitors. The amplitudes (D) of inward currents recorded from excitable WT and Cx43-null cells were not significantly different (p = 0.1469, unpaired t test). None of the cells displaying inward currents was electrically coupled to nearby cells, as evaluated by dual whole cell voltage clamp recordings.
Neuronal Differentiation Is Independent of Gap Junctional Communication Blockade
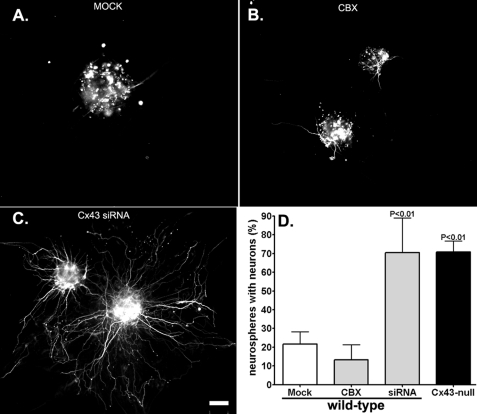
To evaluate whether lack of Cx43 gap junctional communication interferes with neuronal maturation, two strategies were employed, namely, pharmacological blockade of gap junction channels and acute down-regulation of Cx43 using siRNA. Continuous treatment (48 h) of WT neurospheres with the gap junction channel blocker CBX (100 μm) did not increase the number of WT neurospheres with neurons (β-III-tubulin-positive cells with neurite extensions) (WTCTRL: 26.5 ± 7.4%, n = 99 neurospheres from 9 animals derived from 7 litters; WTCBX: 13.2 ± 8.1, n = 43 neurospheres from 4 animals derived from 2 litters; p > 0.5 ANOVA followed by Newman-Keuls test; Fig. 5). That CBX treatment effectively decreased dye coupling among cells was tested using the Lucifer Yellow microinjection technique. Lucifer Yellow microinjection into one cell derived from 3-day adherent WT neurosphere resulted in dye diffusion to 8.3 ± 1.4 cells (n = 7 experiments; 3 animals); this spread was reduced to 2.7 ± 1.2 cells (n = 3 experiments, 2 animals; p < 0.05 ANOVA followed by Newman-Keuls test) after 48-h treatment with CBX, a value similar to that measured from Cx43-null-derived cells (2.9 ± 0.6, n = 7 experiments, 3 animals; p > 0.05 ANOVA followed by Newman-Keuls test). Differently from CBX, however, knockdown of Cx43 with siRNA significantly increased the percentage of neurospheres with neurons compared with those mock-treated WT neurospheres (WTMock: 21.1 ± 6.6%, n = 55 neurospheres from 7 litters; WTCx43siRNA: 70.4 ± 8+18.5%, n = 61 neurospheres from 5 animals derived from 3 litters; p < 0.05 ANOVA followed by Newman-Keuls test; Fig. 5). Examples of efficient Cx43 knockdown by siRNA are illustrated in supplemental Figs. 4S and 5S. Thus, these results indicate that early onset of neuronal differentiation seen in Cx43-null neurospheres and in Cx43-null brains is independent of gap junctional communication but dependent on the expression of Cx43 itself.
FIGURE 5.
Neuronal differentiation is independent of gap junction-mediated coupling. A–C, epifluorescence images showing β-III-tubulin-positive cells in mock transfected (A), CBX-treated (B), and Cx43 siRNA-transfected WT neurospheres (C). Note the presence of extensive neuronal neurite projections after knockdown of Cx43 with siRNA from WT neurospheres compared with CBX- and mock-treated neurospheres. Scale bar, 50 μm. D, bar histograms showing the mean ± S.E. (error bars) values obtained for the percent WT neurospheres with differentiated neurons (β-III-tubulin-positive cells with neurites) after 48-h treatment with transfection reagents (Mock), gap junction channel blocker CBX (100 μm), and after transfection with Cx43 siRNA. Knockdown of Cx43 with siRNA significantly increased the number of neurospheres with differentiated neurons to levels similar to those found in Cx43-null neurospheres; neither mock transfection nor CBX treatment altered the number of WT neurospheres with differentiated neurons. For knockdown efficiency, see supplemental Figs. 4S and 5S.
The Cytoplasmic C-terminal Domain of Cx43 Prevents Neuronal Differentiation
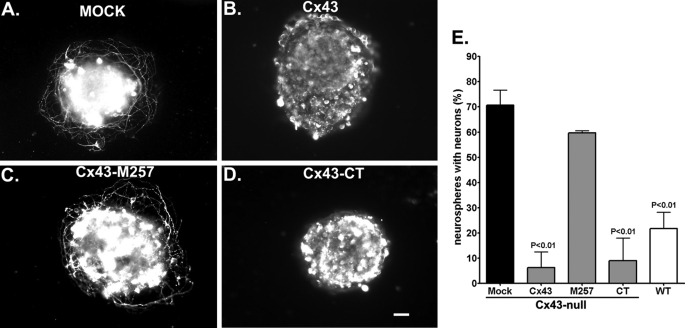
To test whether specific domains of Cx43 modulate neuronal differentiation, we transfected Cx43-null neurospheres with three Cx43 constructs: full-length Cx43 (Cx43), Cx43 lacking the carboxyl terminus (M257: truncated at position 257), and the Cx43 carboxyl terminus itself (CT: amino acids 255–382). Two days after plating, transfected and mock-transfected cultures were immunostained with β-III-tubulin. As shown in Fig. 6, both Cx43 and CT constructs but not M257 abrogated the onset of neuronal differentiation seen in Cx43-null cells. The percentage of mock-treated Cx43-null neurospheres with differentiated neurons decreased from 65.8 ± 3.9% (n = 50 neurospheres from 7 animals from 6 litters) to 6.3 ± 6.3% (n = 44 neurospheres from 5 animals derived from 4 litters) and to 9.0 ± 9.0% (n = 33 neurospheres from 3 animals derived from 3 litters; p < 0.05 ANOVA followed by Newman-Keuls test) in neurospheres transfected with full-length Cx43 and with Cx43CT, respectively. The number of neurospheres with neurons following transfection with Cx43 lacking CT, i.e. transfected with the M257 construct (59.67 ± 0.9%, n = 74 neurospheres from 5 animals derived from 3 litters) was similar to that recorded from mock-transfected Cx43-null (65.8 ± 3.9%, n = 50 neurospheres from 6 litters; p > 0.05 ANOVA followed by the Newman-Keuls test) (Fig. 6). Examples of Western blots showing transfection efficiency and immunofluorescence for cellular distribution of Cx43 and its mutants are shown in supplemental Figs. 6S and 7S, respectively. Thus, these results support the hypothesis that expression of Cx43 itself, and specifically its carboxyl terminus, modulates neuronal differentiation during early stages of brain development.
FIGURE 6.
Cx43 carboxyl terminus modulates neuronal differentiation. A–D, examples of epifluorescence images showing β-III-tubulin-positive cells in Cx43-null neurospheres mock transfected (A), transfected with full-length Cx43 (B), transfected with Cx43 truncated at position 257 (Cx43-M257) (C), and transfected with the Cx43 carboxyl terminus itself (Cx43-CT) (D). Scale bar, 50 μm. E, bar histograms showing the mean ± S.E. (error bars) values obtained for the percent Cx43-null neurospheres with differentiated neurons (β-III-tubulin-positive cells with neurite projections) following 48 h treatment with transfection reagents alone (Mock) and after transfection with full-length Cx43, Cx43 lacking the carboxyl terminus (M257), and with Cx43 carboxyl terminus itself (CT). A significant decrease in the percent neurospheres with differentiated neurons was obtained after expression of full-length Cx43 and the CT itself, but not with Cx43 constructs lacking the CT. These two effective constructs significantly reduced the number of neurospheres displaying neuronal neurite projections to levels seen in WT neurospheres. Transfection efficiency and cellular distribution of Cx43 and its mutants are shown in supplemental Figs. 6S and 7S.
DISCUSSION
About one-third of the 20 mammalian connexins are expressed in the adult central nervous system, which are developmentally regulated, with different neural cell types expressing specific subsets of gap junction proteins at particular stages of development. Cx43 is prominent in early embryonic days (E12–E18) of central nervous system development, being found in all neural precursor types located in the VZ and in radial glia and neuroblasts extending to the CP (10, 12, 25). During postnatal stages, however, Cx43 becomes restricted to the astrocyte population and is replaced by Cx36 and Cx30.2 in postmitotic neuroblasts (13, 16, 26–28).
Because of the wide distribution of Cx43 in early stages of brain development, it has been proposed that Cx43 is involved in important central nervous system functions. Indeed, previous studies have indicated that Cx43 promotes cell division (29, 30) and neural cell migration (6, 8, 23, 24) and that it delays neuronal differentiation (15, 31).
Embryonic brain derived from Cx43-null mice, labeled with bromodeoxyuridine, indicated an accumulation of bromodeoxyuridine-positive cells within the innermost zones and reduction of cell number in the cortical plate at particular embryonic stages compared with similarly treated brains of WT animals (6). Similar to results obtained with mice totally lacking Cx43, delayed neuronal migration was also reported in embryonic brains of the nestin-Cre:Cx43fl/fl (24) and following in utero electroporation of Cx43 shRNA or Cx43 siRNA in WT embryonic brain ventricles (23, 24).
Our results showing a reduction of CP size in Cx43-null brains compared with those of WT siblings are in accordance with previous studies showing delayed neuronal migration in brains in which Cx43 is deleted or down-regulated (6, 23, 24). However, unlike previous reports showing accumulation of cells in the IZ (23, 24), we found an enlargement of the VZ/SVZ layers in brains of mice lacking Cx43. Such a difference may be related to the distinct stages of brain development analyzed (E16 versus E18 and E21) and/or to the different approaches used (total deletion of Cx43 versus shRNA knockdown and Cx43 conditional knock-out). Moreover, our results showing, for the first time, increased β-III-tubulin immunoreactivity in the germinal zones of brains of mouse embryos lacking Cx43 suggest that the slowly migrating neuroblasts assume a more mature neuronal phenotype when Cx43 is absent. This early onset of neuronal maturation was also observed in Cx43-null neurospheres, as indicated by the increased number of neurospheres producing β-III-tubulin-positive cells, longer and more numerous neurite extensions, and increased number of cells with neuronal-like inward currents in cells derived from Cx43-null neurospheres than those from WT littermates (Figs. 3 and 4). We did not detect alteration in the ratio of nestin to glial fibrillary acidic protein expression levels (supplemental Fig. 3S) or number of O4-positive cells (oligodendrocyte progenitors; data not shown) between the two genotypes (which after 2–3 days of plating of WT neurospheres represent no more than 2–3% of total cell population) (20). Thus, we favor the hypothesis that increased neuronal differentiation in Cx43-null brains is more likely related to faster progression of neuronal progenitors to a more mature phenotype rather than to increased neuronal commitment at the expense of other lineages. Nevertheless, further studies are necessary to provide evidence in favor or against this hypothesis.
Although the reports cited above strongly support a role for Cx43 during neurogenesis, the mechanism(s) by which gap junctions, specifically Cx43, regulate brain development is still debated. Early studies had emphasized gap junctional coupling as a mechanism by which a cohort of coupled cells migrating along the radial glial fibers would maintain their identity and therefore contribute to the specification of the neocortical columnar cytoarchitecture by sharing gap junctional diffusable signaling molecules and/or by displaying synchronous activity (14, 32–40). This hypothesis was supported by evidence indicating an inverse relationship between gap junctional communication and neuronal differentiation. For instance, newborn neurons derived from cultured immortalized hippocampal progenitor cells were shown to be dye and electrically coupled and to express high levels of Cx43, whereas upon differentiation, Cx43 levels as well as cell coupling were shown to be dramatically reduced as the cells became electrically excitable (15).
However, more recent studies have emphasized a channel-independent effect of Cx43 on neural cell development, and at least three mechanisms were proposed, including (i) a ripple effect of Gja1 gene ablation on other genes such as the purinergic receptors involved in cell migration (8); (ii) an adhesiveness property provided by the interaction between the extracellular loops of Cx43 from radial glia with those of Cx43 expressed in migrating neuroblasts (23); and (iii) a scaffolding property of Cx43CT due to its interaction with cytoskeletal elements (24), mediated in part by linker proteins such as ZO-1 and N-cadherins.
Results described here are in contrast with the early hypothesis described above that functional gap junction-mediated coupling modulates neuronal differentiation. Our results showing that blockade of gap junctional communication with CBX did not recapitulate the effects seen when Cx43 was deleted (knock-out mice) or knocked down (siRNA) provide strong evidence that expression of Cx43 itself but not direct cell-cell communication is involved in neuronal differentiation. Furthermore, because we found that expression of the Cx43CT fragment alone, but not of Cx43 lacking this domain, was sufficient to restore the WT phenotype, as determined by percent neurospheres with β-III-tubulin-positive cells, we favor the interpretation that the carboxyl-terminal domain of Cx43 is an integral part of the signal transduction pathways that control neuronal differentiation and neurite outgrowth, such as the Wnt and Src-tyrosine kinase pathways (41, 42). In this regard, studies have shown that the association of Cx43 with β-catenin at the cell membrane is disrupted following deletion of Cx43, leading to β-catenin translocation into the nucleus where the protein complexes with transcription factors and modulates the expression of specific target genes (43). Moreover, the association of Cx43 with Src-tyrosine kinases is well known (44, 45), and a recent study indicated that the lack of a Src binding site (SH3 domain) located within amino acids 264–287 of Cx43CT (46) impairs purinergic receptor-mediated calcium rises (47), an important signaling mechanism involved in neural cell migration (8, 48).
Besides interacting with key proteins involved in neural development, the Gja1 gene encoding Cx43 has been proposed to modulate a large number of gene networks (49). Microarray analyses of gene expression levels in WT and Cx43-null E19 brains revealed that 5% of the spotted genes had their expression levels altered in the null genotype (49). Of these regulated genes, we found that calbindin, a calcium-binding protein expressed in some pyramidal neurons and in GABAergic interneurons originating from the ganglionic eminence, was up-regulated (1.6-fold) in brains of Cx43-null E18 embryos. This is in accordance with our observation of intense β-III-tubulin in the IZ of Cx43-null brains, where inhibitory interneurons tangentially migrate from the ganglionic eminence to the forebrain. Similarly up-regulated (1.9-fold) in the null genotype were δ-catenin (plakophillin), a protein complexing with the cadherin cell adhesion molecule which promotes neurite outgrowth, Gli2 (1.5-fold), a transcription factor promoting premature neuronal differentiation, and drebrin (2.3-fold), a neuronal specific actin-binding protein involved in neuronal morphogenesis.
Certainly, further studies are necessary to disclose completely the intricate mechanisms by which Cx43 influences neurodevelopment. However, the findings described here indicating that the cytoplasmic carboxyl-terminal domain of Cx43 is a negative modulator of neuronal differentiation may provide a new target molecule to study brain malformation and diseases.
Supplementary Material
Acknowledgments
We thank Drs. Dominick P. Purpura and Michael V. L. Bennett for comments and suggestions on an earlier version of this manuscript; Dr. Steve Taffet (SUNY Upstate Medical University, Syracuse, NY) for the Cx43-M254-FLAG construct; Melissa Aleksey and Aisha Cordero for technical assistance with animal husbandry and genotyping, neurosphere cultures, and initial brain cryosections; and Kevin Fisher for assistance with image analyses.
This work was supported, in whole or in part, by NINDS/National Institutes of Health Grants NS052245 (to E. S.) and NS041282 (to D. C. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1S–7S.
- Cx
- connexin
- WT
- wild-type
- E
- embryonic day
- DMEM
- Dulbecco's modified Eagle's medium
- CT
- carboxyl-terminal
- siRNA
- small interfering RNA
- CBX
- carbenoxolone
- ANOVA
- analysis of variance
- CP
- cortical plate
- IZ
- intermediate zone
- VZ
- ventricular zone
- SVZ
- subventricular zone
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Ayala R., Shu T., Tsai L. H. (2007) Cell 128, 29–43 [DOI] [PubMed] [Google Scholar]
- 2.Chao D. L., Shen K. (2008) Mol. Cell. Neurosci. 39, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dehay C., Kennedy H. (2007) Nat. Rev. Neurosci. 8, 438–450 [DOI] [PubMed] [Google Scholar]
- 4.Molyneaux B. J., Arlotta P., Menezes J. R., Macklis J. D. (2007) Nat. Rev. Neurosci. 8, 427–437 [DOI] [PubMed] [Google Scholar]
- 5.Elias L. A., Kriegstein A. R. (2008) Trends Neurosci. 31, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fushiki S., Perez-Velazquez J. L., Zhang L., Bechberger J. F., Carlen P. L., Naus C. C. G. (2003) J. Neuropathol. Exp. Neurol. 62, 304–314 [DOI] [PubMed] [Google Scholar]
- 7.Kunze A., Congreso M. R., Hartmann C., Wallraff-Beck A., Hüttmann K., Bedner P., Requardt R., Seifert G., Redecker C., Willecke K., Hofmann A., Pfeifer A., Theis M., Steinhäuser C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11336–11341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scemes E., Duval N., Meda P. (2003) J. Neurosci. 23, 11444–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiencken-Barger A. E., Djukic B., Casper K. B., McCarthy K. D. (2007) Glia 55, 675–686 [DOI] [PubMed] [Google Scholar]
- 10.Cina C., Bechberger J. F., Ozog M. A., Naus C. C. (2007) J. Comp. Neurol. 504, 298–313 [DOI] [PubMed] [Google Scholar]
- 11.Duval N., Gomès D., Calaora V., Calabrese A., Meda P., Bruzzone R. (2002) J. Cell Sci. 115, 3241–3251 [DOI] [PubMed] [Google Scholar]
- 12.Nadarajah B., Jones A. M., Evans W. H., Parnavelas J. G. (1997) J. Neurosci. 17, 3096–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scemes E., Spray D. C. (2009) in Astrocytes in (Patho)Physiology of the Nervous System (Parpura V., Haydon P. G. eds) pp. 107–150, Springer, New York [Google Scholar]
- 14.Rozental R., Mehler M. F., Morales M., Andrade-Rozental A. F., Kessler J. A., Spray D. C. (1995) Dev. Biol. 167, 350–362 [DOI] [PubMed] [Google Scholar]
- 15.Rozental R., Morales M., Mehler M. F., Urban M., Kremer M., Dermietzel R., Kessler J. A., Spray D. C. (1998) J. Neurosci. 18, 1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozental R., Srinivas M., Gökhan S., Urban M., Dermietzel R., Kessler J. A., Spray D. C., Mehler M. F. (2000) Brain Res. Brain Res. Rev. 32, 57–71 [DOI] [PubMed] [Google Scholar]
- 17.Bani-Yaghoub M., Bechberger J. F., Underhill T. M., Naus C. C. (1999) Exp. Neurol. 156, 16–32 [DOI] [PubMed] [Google Scholar]
- 18.Bani-Yaghoub M., Underhill T. M., Naus C. C. (1999) Dev. Genet. 24, 69–81 [DOI] [PubMed] [Google Scholar]
- 19.Shamekh R., Cameron D. F., Willing A. E., Saporta S. (2006) Exp. Brain Res. 170, 277–284 [DOI] [PubMed] [Google Scholar]
- 20.Striedinger K., Meda P., Scemes E. (2007) Glia 55, 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Striedinger K., Scemes E. (2008) J. Neuroimmunol. 196, 116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacobas D. A., Iacobas S., Urban-Maldonado M., Scemes E., Spray D. C. (2008) Cell Commun. Adhes. 15, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias L. A., Wang D. D., Kriegstein A. R. (2007) Nature 448, 901–907 [DOI] [PubMed] [Google Scholar]
- 24.Cina C., Maass K., Theis M., Willecke K., Bechberger J. F., Naus C. C. (2009) J. Neurosci. 29, 2009–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittman K. S., LoTurco J. J. (1999) Cereb. Cortex 9, 188–195 [DOI] [PubMed] [Google Scholar]
- 26.Belluardo N., Mudò G., Trovato-Salinaro A., Le Gurun S., Charollais A., Serre-Beinier V., Amato G., Haefliger J. A., Meda P., Condorelli D. F. (2000) Brain Res. 865, 121–138 [DOI] [PubMed] [Google Scholar]
- 27.Gulisano M., Parenti R., Spinella F., Cicirata F. (2000) Neuroreport 11, 3823–3838 [DOI] [PubMed] [Google Scholar]
- 28.Kreuzberg M. M., Deuchars J., Weiss E., Schober A., Sonntag S., Wellershaus K., Draguhn A., Willecke K. (2008) Mol. Cell. Neurosci. 37, 119–134 [DOI] [PubMed] [Google Scholar]
- 29.Bittman K., Owens D. F., Kriegstein A. R., LoTurco J. J. (1997) J. Neurosci. 17, 7037–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissman T. A., Riquelme P. A., Ivic L., Flint A. C., Kriegstein A..R. (2004) Neuron 43, 647–661 [DOI] [PubMed] [Google Scholar]
- 31.Belliveau D. J., Bani-Yaghoub M., McGirr B., Naus C. C., Rushlow W. J. (2006) J. Biol. Chem. 281, 20920–20931 [DOI] [PubMed] [Google Scholar]
- 32.Bani-Yaghoub M., Bechberger J. F., Naus C. C. (1997) J. Neurosci. Res. 49, 19–31 [DOI] [PubMed] [Google Scholar]
- 33.Connors B. W., Benardo L. S., Prince D. A. (1983) J. Neurosci. 3, 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman C. S., Spitzer N. C. (1979) Nature 280, 208–214 [DOI] [PubMed] [Google Scholar]
- 35.Kandler K., Katz L. C. (1995) Curr. Opin. Neurobiol. 5, 98–105 [DOI] [PubMed] [Google Scholar]
- 36.LoTurco J. J., Kriegstein A. R. (1991) Science 252, 563–566 [DOI] [PubMed] [Google Scholar]
- 37.Mehler M. F., Rozental R., Dougherty M., Spray D. C., Kessler J. A. (1993) Nature 362, 62–65 [DOI] [PubMed] [Google Scholar]
- 38.Mienville J. M., Lange G. D., Barker J. L. (1994) Brain Res. Dev. Brain Res. 77, 89–95 [DOI] [PubMed] [Google Scholar]
- 39.Peinado A., Yuste R., Katz L. C. (1993) Cereb. Cortex 3, 488–498 [DOI] [PubMed] [Google Scholar]
- 40.Yuste R., Peinado A., Katz L. C. (1992) Science 257, 665–669 [DOI] [PubMed] [Google Scholar]
- 41.Ingraham C. A., Cox M. E., Ward D. C., Fults D. W., Maness P. F. (1989) Mol. Chem. Neuropathol. 10, 1–14 [DOI] [PubMed] [Google Scholar]
- 42.Patapoutian A., Reichardt L. F. (2000) Curr. Opin. Neurobiol. 10, 392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ai Z., Fischer A., Spray D. C., Brown A. M., Fishman G. I. (2000) J. Clin. Invest. 105, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W., Hertzberg E. L., Spray D. C. (2005) J. Biol. Chem. 280, 7941–7948 [DOI] [PubMed] [Google Scholar]
- 45.Loo L. W., Kanemitsu M. Y., Lau A. F. (1999) Mol. Carcinog. 25, 187–195 [PubMed] [Google Scholar]
- 46.Sorgen P. L., Duffy H. S., Sahoo P., Coombs W., Delmar M., Spray D. C. (2004) J. Biol. Chem. 279, 54695–54701 [DOI] [PubMed] [Google Scholar]
- 47.Scemes E. (2008) Glia 56, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agresti C., Meomartini M. E., Amadio S., Ambrosini E., Serafini B., Franchini L., Volonté C., Aloisi F., Visentin S. (2005) Glia 50, 132–144 [DOI] [PubMed] [Google Scholar]
- 49.Iacobas D. A., Iacobas S., Spray D. C. (2007) Genomics 89, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.