Abstract
The OR class insect odorant receptors are ligand-gated ion channels comprised of at least one common subunit (OR83b in Drosophila) and at least one putative odorant-binding subunit. However, little else is known about the molecular details of insect OR architecture. For example, nothing is known about how these receptors bind odorants, greatly limiting efforts to develop insect OR-targeted compounds for the control of insects involved in disease propagation and agricultural damage. Here we identify a portion of a Drosophila OR that is involved in odorant activation of the receptor. Using the substituted cysteine accessibility method, we identified residues 146–150 of OR85b, located at the predicted interface between transmembrane segment 3 (TMS3) and extracellular loop 2 (ECL2), as playing a role in odorant (2-heptanone) activation. We found that occupation of the receptor by the competitive antagonist 2-nonanone protected the receptor from methanethiosulfonate action at position 148, placing this region close to the odorant-binding site. In addition, mutations at positions 142 and 143 within TMS3 altered odorant sensitivity. Our results identify the involvement of the extracellular half of TMS3 in Drosophila OR85b in odorant activation of the receptor. This finding can serve as a starting point for future detailed analysis of the molecular basis for odorant recognition by insect ORs, a novel class of ligand-gated channel.
Keywords: Channels/Ion, Membrane/Proteins, Protein/Structure, Receptors/7-Helix Ligand-gated Channels, Receptors/Membrane, Receptors/Structure-Function
Introduction
The accurate assessment of volatile chemicals in an environment requires an olfactory system to be both sensitive and broadly tuned. An organism can then quickly process new information about its surroundings and make behavioral decisions necessary for survival. To this end, the insect olfactory system employs a large array of chemosensory receptors for the detection of odorants and pheromones. These receptors are appealing targets for the control of insects involved in disease propagation and agricultural damage. Realizing this goal requires development of a detailed understanding of insect chemosensory receptor structure and function.
Members of the OR3 class of insect odorant receptors are broadly expressed in olfactory sensory neurons and are formed by a novel, highly divergent family of subunits (1). Insect ORs exhibit significant differences from mammalian odorant receptors, a large family of G-protein-coupled receptors (2, 3). For example, although insect OR subunits are thought to have seven transmembrane domains, the topology is inverted, with respect to G-protein-coupled receptors, with an intracellular N terminus (4). Further, insect ORs are formed as multimeric complexes of subunits. Most notably, insect ORs function as ligand-gated ion channels, generating a non-selective cation current upon odorant binding (5, 6). At present, it is unknown whether this current is sufficient for action potential generation or whether other ion channel openings are necessary. In addition to ion channel activity, insect OR activation may also initiate or be modified by a second messenger pathway, although the details of how this might occur are controversial (2). Recently, a second class of insect olfactory receptor has been described (7), termed “ionotropic receptors.” The ionotropic receptors are expressed in a subset of olfactory sensory neurons and are homologous to ionotropic glutamate receptors (7). Interestingly, despite the differences in receptor structure, the mammalian and insect olfactory systems share a strategy for accurate odorant discrimination, which involves the use of a large repertoire of receptors with various degrees of specificity (8). Odorants activate multiple receptors and, therefore, are represented in the brain as a combinatorial code of detection events, dramatically increasing discriminatory power.
In the Drosophila melanogaster fly, the OR subunit gene family consists of 62 members (9). Each functional OR is a complex of unknown stoichiometry, comprised of at least one copy of OR83b and at least one copy of a non-OR83b subunit (4, 10–13). Changing the non-OR83b subunit in the complex alters the receptor specificity to odorants (14), suggesting that the non-OR83b specificity subunit is involved in odorant binding and recognition. The OR83b subunit has a role in receptor localization as its presence targets the OR complex to the olfactory sensory neuron dendrites (4). Whether this role includes communication with trafficking machinery and/or chaperone and protein-folding functions is unknown. A direct association between OR83b and the specificity subunits has been demonstrated (4, 12), but it is unclear how the highly conserved OR83b (15, 16) is able to associate with the highly divergent specificity subunits. Although OR83b has not been shown to respond to any odorants on its own (13, 14), it is essential for odor-induced ion flux (5, 6), suggesting that OR83b directly contributes to the structure of the channel pore.
Investigations of insect OR function have focused mainly on determining ligand specificities. In vivo experiments utilizing genetic manipulations in Drosophila have deciphered ligand specificities for a large collection of Drosophila OR complexes (14). This work suggests that these ORs span a range from broadly tuned receptors to those with highly restricted specificities. Odorant specificities of insect ORs can also be studied in heterologous expression systems, such as Xenopus oocytes (5, 11, 13, 17, 18). However, although substantial progress has been made in “deorphanizing” insect ORs, the molecular details of how these receptors distinguish among and bind odorants and how odorant binding results in receptor activation remain unknown. Here we identify a portion of an insect OR that contributes to ligand-receptor interactions: transmembrane domain 3 of Drosophila OR85b. This is a critical first step toward the elucidation of the molecular basis of odorant recognition by insect ORs.
EXPERIMENTAL PROCEDURES
Materials
Xenopus laevis frogs were purchased from Nasco. The care and use of X. laevis frogs in this study were approved by the University of Miami Animal Research Committee and meet the guidelines of the National Institutes of Health. Methanethiosulfonate (MTS) reagents were from Toronto Research Chemicals. Odorants and other chemicals were from Sigma-Aldrich.
Receptor Cloning and Mutagenesis
Wild-type (WT) Canton S Drosophila melanogaster flies were frozen in liquid nitrogen and shaken through a three-stage sieve. Antennae were collected and homogenized in a Dounce homogenizer. Total RNA was extracted with TRIzol (Invitrogen) and used as template for cDNA synthesis with gene-specific primers. Products were amplified by PCR, subcloned into pGEMHE (19), and verified by sequencing. ORs 35a and 83b were generously provided by J. Carlson and L. Vosshall, respectively. Mutations were introduced using QuikChange Lightning kits (Stratagene). Each mutant construct was verified by sequencing.
Expression of ORs in Xenopus Oocytes
Oocytes were surgically removed from mature X. laevis frogs. Follicle cells were removed by treatment with collagenase B (Roche Applied Science) for 2 h at room temperature. Capped cRNA encoding each OR subunit was generated using mMessage mMachine kits (Ambion). 25 ng of cRNA encoding each OR subunit was injected into stage V–VI Xenopus oocytes. Oocytes were incubated at 18 °C in Barth's saline (in mm: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.3 CaNO3, 0.41 CaCl2, 0.82 MgSO4, 15 HEPES, pH 7.6, and 100 μg/ml amikacin) for 2–5 days prior to electrophysiological recording.
Electrophysiology and Data Capture
Odorant-induced currents were recorded under two-electrode voltage clamp from X. laevis oocytes expressing ORs, using an automated parallel electrophysiology system (OpusXpress 6000A; Molecular Devices). Oocytes were perfused with ND96 (in mm: 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 5 HEPES, pH 7.5). Odorant stock solutions (usually 1 m) of each odorant were prepared in dimethyl sulfoxide (DMSO). Odorants were diluted in ND96 and, unless otherwise noted, applied for 20 s at a flow rate of 1.65 ml/min, with extensive washing in ND96 (10 min at 4.6 ml/min) between applications. Micropipettes were filled with 3 m KCl and had resistances of 0.2–2.0 megaohms. The holding potential was −70 mV. Current responses, filtered (4-pole, Bessel, low pass) at 20 Hz (−3 db) and sampled at 100 Hz, were captured and stored using the OpusXpress 1.1 software (Molecular Devices).
Experimental Protocols
For the MTS susceptibility screens presented in Fig. 1, a pre-MTS receptor response to odorant was measured by taking the average response from two odorant applications. After extensive washing, MTS reagent was then applied at 1 mm for 2 min followed by a 4-min wash period. Finally, two post-MTS receptor responses to odorant were recorded, with a 10-min (OR35a/83b, OR85b/83b) or a 12-min (OR85a/83b) wash between each odorant application. An additional post-MTS odorant response was obtained for OR85b/83b after a further 40-min wash. Post-MTS responses are presented as a percentage of the averaged pre-MTS odorant response for each post-treatment time point.
FIGURE 1.
Drosophila ORs are differentially susceptible to functional modulation by MTS reagents. Traces, odorant-induced current responses of OR-expressing oocytes before and after treatment with MTSES (1 mm, 2 min). OR35a/83b (A) was activated by 3 μm hexanol (HEX), OR85a/83b (B) was activated by 100 μm ethyl 3-hydroxybutyrate (E3HB), and OR85b/83b (C) was activated by 100 μm 2-heptanone (HEP). Odorant applications were for 20 s and are indicated by arrowheads. Graphs, quantification of odorant-induced current responses after MTS treatment (1 mm, 2 min). Remaining response values were assayed after wash periods of 4 min (4′), 14 min (14′), or 16 min (16′) and 54 min (54′) and are presented as means ± S.E. A, OR35a/83b-expressing oocyte responses to 3 μm hexanol (n values from left to right: 4, 3, 5, 5, 7, 7). B, OR85a/83b-expressing oocyte responses to 100 μm ethyl 3-hydroxybutyrate (n values from left to right: 5, 4, 5, 5, 3, 3). C, OR85b/83b-expressing oocyte responses to 100 μm HEP (n values from left to right: 4, 4, 4, 4, 4, 4, 4, 4, 5).
For the dithiothreitol (DTT) treatment experiment in Fig. 2, a pretreatment response was first determined by application of 100 μm 2-heptanone. Following a 10-min wash period, oocytes were presented with either ND96 (Sham or DTT alone) or 10 mm ethylsulfonate-MTS (MTSES) (MTSES alone or MTSES + DTT) for 2 min. After an additional 10-min wash period, oocytes were presented with either ND96 (Sham or MTS alone) or 20 mm DTT (DTT alone or MTSES + DTT) for 2 min followed by a 10-min wash. Finally, a post-treatment response to 100 μm 2-heptanone was measured. Data were analyzed by calculating the response remaining (post-treatment 2-heptanone/pretreatment 2-heptanone) and are presented by normalizing this value as a percentage of the response remaining after sham treatment.
FIGURE 2.
Inhibition of OR85b/83b by MTSES is partially reversed by treatment with the reducing agent DTT. Quantification of current responses from OR85b/83b-expressing oocytes to 100 μm 2-heptanone after the indicated treatment (see ”Experimental Procedures“) is shown. MTSES was applied at 10 mm for 2 min, and DTT was applied at 20 mm for 2 min. Data are presented as means ± S.E. Statistical significance was assessed by one-way analysis of variance and Dunnett's multiple comparisons post test: one asterisk, p < 0.05; two asterisks, p < 0.01; NS, not significant (n values from left to right: 5, 4, 12, 5).
For the MTS susceptibility screens presented in Figs. 3 and 4, 2-heptanone was applied for 240 s, and 2-heptanone plus 10 mm MTSES were then applied for 200 s followed by washout in ND96. MTSES effects were calculated by measuring current amplitudes 440 s after initiation of odorant application (response in the presence of 2-heptanone and MTSES) and dividing by the response amplitude 240 s after initiation of the odorant application (maximal response to 2-heptanone alone).
FIGURE 3.
MTSES inhibits OR85b/83b function by action at cysteine 146 of OR85b. A, current responses from OR-expressing oocytes when challenged with 300 μm HEP for 440 s, with MTSES (10 mm) co-applied during the last 200 s of the 2-heptanone application. Oocytes expressed OR83b and either the WT OR85b subunit or one of several OR85b mutants: C124S, C146S, C208S, C278S, or C311S. B, effect of MTSES treatment on the response of WT and mutant receptors at several 2-heptanone concentrations. Data are presented as means ± S.E. (n values from left to right: WT, 4, 5, 4; C124S, 4, 7, 5; C146S, 5, 9, 4; C208S, 5, 9, 4; C278S, 4, 5, 9, 4; C311S, 4, 7, 4).
FIGURE 4.
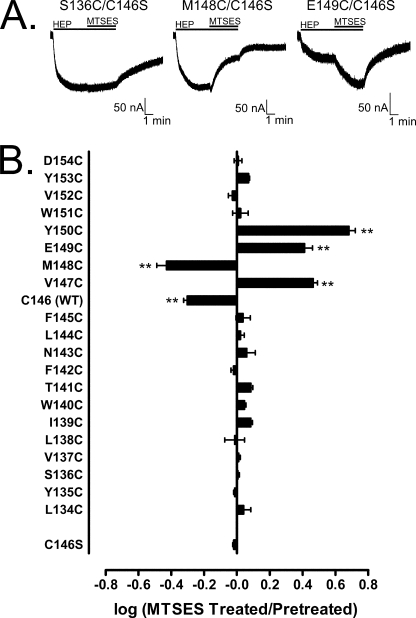
SCAM analysis identifies a functionally relevant region in OR85b. A, current responses from OR-expressing oocytes challenged with 100 μm HEP for 440 s. MTSES (10 mm) was co-applied during the last 200 s of the HEP application. Oocytes expressed OR83b and one of several Or85b mutants (each within the C146S pseudo-WT background): S136C (left), M148C (middle), and E149C (right). B, quantification of the effect of MTSES treatment on the response of WT OR85b/83b (Cys-146 (WT)) and mutant receptors to 100 μm 2-heptanone (data are means ± S.E.). Bars extending to the left indicate inhibition, whereas bars extending to the right indicate potentiation. Each mutant was constructed in the C146S pseudo-WT background. Statistical significance was assessed by one-way analysis of variance and Bonferroni's post test comparing each mutant response with the C146S pseudo-WT response: two asterisks, p < 0.001 (n values from top to bottom: 6, 6, 5, 6, 7, 5, 5, 6, 3, 7, 5, 4, 6, 4, 6, 5, 6, 7, 7, 7, 4, 7).
To generate the concentration-inhibition curve shown in Fig. 5C, OR85b/83b-expressing oocytes were challenged with 100 μm 2-heptanone for 420 s. 240 s after initiation of the 2-heptanone application, various concentrations of 2-nonanone were co-applied for 60 s. The response amplitude measured at the end of the 2-nonanone co-application was then compared with the response amplitude immediately preceding the 2-nonanone co-application. To generate the concentration-response curves shown in Fig. 5D and supplemental Figs. 3 and 9, various concentrations of 2-heptanone or 2-heptanol were applied to OR85b/83b-expressing oocytes for 20 s, with 10-min washes between applications. Response amplitudes were normalized to the response to application of 100 μm 2-heptanone.
FIGURE 5.
2-Nonanone is a competitive antagonist of OR85b/83b. A, quantification of current responses of OR85b/83b-expressing oocytes challenged with a panel of ketones (applied at 1 mm: 5, 2-pentanone; 6, 2-hexanone; 7, 2-heptanone; 8, 2-octanone; 9, 2-nonanone). Responses from each oocyte were normalized to the 2-heptanone response (data are means ± S.E., n = 9). B, current responses from OR85b/83b-expressing oocytes challenged with HEP (100 or 3000 μm for 420 s). 2-Nonanone (NONA) (1000 μm for 60 s) was applied starting 240 s after initiation of the HEP application. C, concentration-inhibition relationship of 2-nonanone block of OR85b/83b activity in the presence of 100 μm HEP (IC50 = 550 ± 270 μm, means ± S.E., n = 4). D, dose-response relationship comparison for the activation of OR85b/83b by HEP (untreated or in the presence of 1 mm 2-nonanone). Responses were normalized to the response elicited by 100 μm HEP for all data points (EC50s, untreated, 70 ± 20 μm, n = 10; 1 mm 2-nonanone-treated, 2070 ± 1380 μm, n = 5, means ± S.E.). Statistical significance was assessed by F-test of the EC50 values (p = 0.0007).
For protection from MTSES action by 2-nonanone in Fig. 6A, a pretreatment response was first determined by application of 100 μm 2-heptanone. Following a 10-min wash period, oocytes were presented with either ND96 (Sham or MTSES alone treatments) or 6 mm 2-nonanone (MTSES + 2-nonanone or 2-nonanone alone treatments) for 50 s, immediately followed by application of ND96 (Sham), 1 mm MTSES (MTSES alone), 1 mm MTSES and 6 mm 2-nonanone (MTSES + 2-nonanone), or 6 mm 2-nonanone (2-nonanone alone) for 1 min followed by a 10-min washout. Finally, a post-treatment response to 100 μm 2-heptanone was measured. Data were analyzed by calculating the response remaining (post-treatment 2-heptanone/pretreatment 2-heptanone) and are presented by normalizing this value as a percentage of the response remaining after sham treatment.
FIGURE 6.
Transmembrane domain 3 is near the odorant-binding site of OR85b and contributes to odorant sensitivity. A, quantification of current responses from OR85b C146S,M148C/OR83b-expressing oocytes to 100 μm 2-heptanone after the indicated treatment (see ”Experimental Procedures“). MTSES was applied at 1 mm for 60 s, and 2-nonanone was applied at 6 mm. Data are presented as means ± S.E. Statistical significance was assessed by one-way analysis of variance and Dunnett's multiple comparisons post test: asterisk, p < 0.05; two asterisks, p < 0.01; NS, not significant (n values from left to right: 12, 7, 6, 8). B, normalized odorant preference ratios (see ”Experimental Procedures“) of receptors formed by WT or mutant OR85b subunits for 2-heptanol when compared with 2-heptanone. Odorants were applied at 1 mm, and data are presented as means ± S.E. A ratio of <1 indicates a preference for 2-heptanone over 2-heptanol. Statistical significance was assessed by one-way analysis of variance and Bonferroni's post test comparing each mutant response with WT: two asterisks, p < 0.01; three asterisks, p < 0.001 (n values from top to bottom: 13, 6, 6, 5, 5, 18, 7, 7, 7).
For odorant preference ratio measurements in Fig. 6B, two sets of oocytes were utilized for each OR85b subunit tested. The first set of oocytes was challenged twice with 1 mm 2-heptanol, and an average desensitization factor (D) was calculated by: second 2-heptanol response/first 2-heptanol response. The second set of oocytes was challenged first with 1 mm 2-heptanol and then with 1 mm 2-heptanone, and a preference ratio (P) was calculated by: 2-heptanone response/2-heptanol response. The normalized preference ratio, corrected for desensitization, was calculated as: P/D. All odorant applications were followed by 10-min wash periods in ND96 before subsequent applications, and data collection for each subunit was performed on the same day.
Data Analysis
Initial analyses of electrophysiological data were done using the Clampfit 9.1 software (Molecular Devices). Statistical analyses, curve fitting, and EC50 and IC50 calculations were done using Prism 4 (GraphPad). Concentration-response data were fit to the equation: I = Imax/(1+(EC50/X)n), where I represents the current response at a given concentration of odorant, X; Imax is the maximal response; EC50 is the concentration of odorant yielding a half-maximal response; and n is the apparent Hill coefficient. For concentration-inhibition curves, data were fit to the equation: I = Imax/(1+ (X/IC50)n), where IC50 is the concentration of inhibitor present that still allows a half-maximal response from odorant. Statistical significance was assessed using a two-tailed unpaired t test, a one-tailed unpaired t test, a two-tailed paired t test, an F-test, or a one-way analysis of variance followed by Bonferroni's post test or Dunnett's multiple comparisons post test, as appropriate.
RESULTS
We expressed a series of ORs with known odorant specificities (14, 20) in Xenopus oocytes (11, 13) with the goal of identifying functionally important structural features of this novel receptor class. Odorant-induced current responses were only observed upon co-expression of a putative odorant-binding subunit (for example, OR85b) and the “common” OR83b subunit (supplemental Fig. 1A), in agreement with previous reports of heterologous expression of insect ORs and the functional requirement for OR83b (4, 10–13). The odorant specificities of ORs expressed in Xenopus oocytes (13) are comparable with what has been reported in vivo (14). For example, oocytes expressing OR85b/83b respond to 2-heptanone, amyl acetate, and butyl acetate but not to ethyl octanoate or methyl acetate, whereas oocytes expressing OR85a/83b respond to hexanol, ethyl butyrate, and ethyl-3-hydroxybutyrate but not to amyl acetate or benzaldehyde (supplemental Fig. 1B). The similarity of these results to what has been reported in vivo (14) indicates that the Xenopus oocyte system can serve as an accurate expression platform for insect OR characterization.
One approach to identifying regions of functional importance on receptors is the substituted cysteine accessibility method (SCAM) (21). SCAM is performed by inserting a cysteine residue at a series of positions within a region of interest. Structural and functional information can then be derived by measuring the function of each cysteine mutant before and after treatment with an MTS reagent. However, our almost complete lack of structural knowledge about this receptor class makes this method unwieldy as an initial approach. Noting that a conventional application of SCAM often requires construction of a “pseudo-wild-type” (pseudo-WT) receptor in which one or more cysteine residues are removed from a functionally important region (21), we reasoned that among the highly divergent insect ORs, some receptors might have a cysteine in just such a functionally important location. What we are seeking is a covalent interaction between an MTS reagent and an endogenous cysteine residue in an OR subunit that results in a detectable change in receptor function. By systematically mutating each cysteine residue in the affected OR subunit, we would then be able to identify the cysteine residue that confers MTS sensitivity. We pursued this strategy by screening several Drosophila ORs (35a/83b, 85a/83b, 85b/83b) for susceptibility to irreversible functional modification by MTS reagents, each applied at 1 mm for 2 min (Fig. 1). Odorant responses of OR35a/83b were slightly potentiated after treatment with trimethylammonium-MTS (MTSET) and were unaffected by treatment with MTSES or ethylammonium-MTS (MTSEA) (Fig. 1A, supplemental Fig. 2A). OR85a/83b was also unaffected by MTSES treatment but was transiently potentiated after MTSEA or MTSET treatment (Fig. 1B, supplemental Fig. 2B). Both MTSEA and MTSET appeared to have modest agonist activity when applied alone to OR85a/83b (supplemental Fig. 2B), which may be contributing to the subsequent potentiation of the odorant response. No agonist activity was observed during application of MTSEA, MTSET, or MTSES (in the absence of odorant) to oocytes expressing OR35a/83b or OR85b/83b (Fig. 1, supplemental Fig. 2). The transient nature of the MTSEA- and MTSET-induced potentiation of OR85a/83b indicates that the interaction is not covalent and thus is not of interest in our screen.
OR85b/83b was partially inhibited by each of the three MTS reagents (Fig. 1C, supplemental Fig. 2C). OR85b/83b inhibition by MTSEA and MTSET appeared to be slowly reversible, again indicating that the desired disulfide bond was not formed. However, inhibition of OR85b/83b by MTSES was maintained for at least 54 min after washout of the MTSES (Fig. 1C). Although the effect of MTSES on OR85b/83b was partial (50 ± 2% response remaining, mean ± S.E., n = 4), this effect was saturating; increasing the concentration of MTSES to 10 mm had no further inhibitory effect (Fig. 2, compare MTSES alone with Sham). The failure of OR85b/83b function to recover after a long wash period suggests that MTSES is reacting to form a disulfide bond with one or more cysteine residues in the receptor. If so, then application of a reducing agent, such as DTT, should be able to reverse the effect of MTSES. We found that a 2-min treatment with 20 mm DTT could significantly restore receptor function after MTSES treatment (Fig. 2). These results suggest MTSES inhibition of OR85b/83b as a probe for exploration of Drosophila OR structure.
To gain insight into the mechanism of MTSES action on OR85b/83b, we examined the effect of MTSES on odorant activation and channel permeability. MTSES treatment shifted the 2-heptanone concentration-response relationship to the right (supplemental Fig. 3A). Untreated WT receptor responded to 2-heptanone with an EC50 70 ± 20 μm. Treatment with MTSES (10 mm, 2 min) significantly reduced 2-heptanone sensitivity (EC50: 472 ± 213 μm, p = 0.0005, F-test). This shift in the concentration-response curve explains why MTSES only partially inhibits the response to 100 μm 2-heptanone. We also performed current-voltage analysis to determine whether MTSES treatment affected the general properties of the ion pore (supplemental Fig. 4). Although MTSES treatment reduced odorant-induced macroscopic current (supplemental Fig. 4A), the reversal potential and the shape of the curve were unchanged (supplemental Fig. 4B), indicating that MTSES treatment did not alter the general properties of the ion pore. We conclude that MTSES treatment alters the odorant activation properties of OR85b/83b.
Our observation that MTSES affected only one of the three ORs tested suggested that the site of MTSES action was on the putative odorant-binding subunit OR85b and not the OR83b subunit (which is common to all three ORs). Thus, we constructed a series of mutant receptors in which each cysteine in OR85b (supplemental Fig. 5) was replaced with a serine and screened for susceptibility to block by MTSES (Fig. 3). The C124S, C208S, C278S, and C311S mutant receptors each remained sensitive to inhibition by MTSES, although this was only apparent for the C278S mutant at low 2-heptanone concentrations (see below). The C146S mutant receptor was insensitive to MTSES treatment. In these experiments, odorant is applied for 440 s, with MTSES applied for the last 200 s (starting at 240 s after initiation of the odorant application). This relatively long odorant application time raises the concern that receptor desensitization might affect our results. We examined this issue by applying 2-heptanone alone for 440 s to WT receptor, as well as each of the cysteine to serine mutant receptors. Comparison of the response amplitude at 440 s with the response amplitude at 240 s showed no evidence for desensitization (supplemental Fig. 6, A and B).
The C278S mutant receptor appears to be insensitive to MTSES treatment when 100 or 300 μm 2-heptanone is used to activate the receptor. This is due to the C278S mutation causing a significant shift in odorant sensitivity (supplemental Fig. 3E), with the C278S receptor being 23-fold more sensitive to 2-heptanone (EC50 = 3 ± 0.4 μm, p < 0.0001, F-test) than the WT receptor. Because the effect of MTSES is to decrease odorant sensitivity, such an effect would be masked by the increased sensitivity of the C278S mutant when screening with a high concentration of 2-heptanone. We did not generate full concentration-response curves for each mutant after MTSES treatment but tested each mutant for MTSES sensitivity at several 2-heptanone concentrations. When the 2-heptanone concentration was lowered to 10 or 30 μm, an inhibitory effect of MTSES became apparent for the C278S mutant (Fig. 3B). In contrast, the C146S mutant receptor, with a 2-heptanone sensitivity (EC50 = 67 ± 16 μm, supplemental Fig. 3C) very similar to that of WT receptor, was insensitive to MTSES treatment at all tested concentrations of 2-heptanone. Importantly, MTSES fails to inhibit responses to 30 μm 2-heptanone, a concentration below the EC50 for activation of this receptor (supplemental Fig. 3C). We conclude that Cys-146 of the OR85b subunit is the sole site of action for MTSES at this receptor.
Next we examined the region surrounding position 146 of OR85b using the SCAM approach (21). Because MTSES treatment exerts a functional effect at Cys-146 (Fig. 3), we constructed a series of cysteine substitutions from positions 134 to 154 within a C146S pseudo-WT background and screened for restoration of susceptibility to 10 mm MTSES in the presence of 100 μm 2-heptanone (Fig. 4). We found that MTSES could alter receptor function when a cysteine was placed at positions 147, 148, 149, or 150, providing further confirmation that the region around Cys-146 of OR85b is functionally relevant. MTSES treatment inhibited the M148C mutant, similar to what was observed for WT receptor (Fig. 4, A and B). Surprisingly, MTSES treatment resulted in potentiation of function for the V147C, E149C, and Y150C mutants (Fig. 4, A and B). Similar to WT receptor, MTSES effects on the V147C, M148C, E149C, and Y150C mutants persisted for at least 20 min (data not shown), demonstrating long term perturbation of the receptors. We did not generate full concentration-response curves for each MTSES sensitive mutant, but the effect of MTSES on the V147C, M148C, E149C, and Y150C mutants was greatly diminished at a higher odorant concentration (supplemental Fig. 7, A and B), suggesting that similar to the effect on WT receptor, MTSES acts by shifting the 2-heptanone concentration-response relationship of the mutant receptors.
The experimental protocol used in Fig. 4 is the same as what was used in Fig. 3. Thus, similar concerns arise regarding the long odorant application (440 s) and whether receptor desensitization might affect our results. We examined this issue by applying 2-heptanone alone for 440 s to each of the cysteine insertion mutants that displayed sensitivity to MTSES (C146S,V147C; C146S,M148C; C146S,E149C; C146S,Y150C), as well as two representative cysteine insertion mutants that did not display sensitivity to MTSES (C146S,S136C; C146S,D154C). Comparison of the response amplitude at 440 s with the response amplitude at 240 s showed no evidence for desensitization (supplemental Fig. 6C). Our previous observation that MTSEA and MTSET could exert modest agonist activity at the OR85a receptor (supplemental Fig. 2B) raises a concern about the ability of MTSES to potentiate the odorant responses of several cysteine insertion mutants of OR85b (C146S,V147C; C146S,E149C; C146S,Y150C). We examined this issue by applying 10 mm MTSES alone for 200 s to oocytes expressing WT OR85b or a selection of mutant receptors (supplemental Fig. 8). No effect was seen with oocytes expressing WT receptor or mutant receptors: C146S; C146S,S136C; C146S,M148C; C146S,Y150C; C146S,D154C. Very slight deflections from base line were seen for oocytes expressing the C146S,V147C or C146S,E149C mutant receptors, suggesting that some caution should be used when interpreting results with these two particular mutants.
Our data indicate that residues 146–150 of OR85b are involved in agonist activation of the receptor. However, these residues are unlikely to be directly involved in odorant binding. Deposition of the large MTSES moiety onto the side chain of a residue that directly interacts with odorant would most likely eliminate odorant binding. Although the WT receptor is less sensitive to odorant after MTSES treatment, the receptor remains responsive to odorant (supplemental Figs. 3A and 7A). This is also the case for the M148C mutant receptor (supplemental Fig. 7A). Also, MTSES treatment actually makes the V147C, E149C, and Y150C mutant receptors more responsive to odorant. Instead of participating in direct binding of odorant, these residues, predicted to be located at the interface of TMS3 and ECL2 (supplemental Fig. 5), may serve as part of the access pathway through which odorants reach the binding site. The effect of MTSES could be to hinder (WT and M148C receptors) or improve (V147C, E149C, and Y150C receptors) binding site access. Alternatively, alterations within this region could be exerting an effect on receptor activation through an allosteric mechanism.
To determine whether residues 146–150 are located near the odorant-binding site, we sought to protect the receptor from MTSES action by occupation of the odorant-binding site. Use of an agonist such as 2-heptanone is problematic due to the high concentration and long application that would be necessary to protect the receptor from MTSES action. Instead, we screened for a competitive antagonist by examining a series of compounds closely related to 2-heptanone (Fig. 5A). We found that although 2-nonanone failed to activate OR85b/83b (Fig. 5A), it was able to block 2-heptanone activation of the receptor and that this blockade could be overcome by increasing the concentration of 2-heptanone (Fig. 5B). We examined the ability of 2-nonanone to antagonize the receptor in more detail by generating a concentration-inhibition curve (Fig. 5C), finding that 2-nonanone inhibited receptor activation by 100 μm 2-heptanone with an IC50 of 550 ± 270 μm. We also found that the presence of 1 mm 2-nonanone shifted the 2-heptanone concentration-response curve to the right, without reducing the maximal response amplitude (Fig. 5D). These results show 2-nonanone to be a competitive antagonist of the OR85b receptor and an ideal compound for use in a protection assay. Indeed, the presence of 2-nonanone (6 mm) protected the C146S,M148C mutant receptor from functional inhibition by 1 mm MTSES (Fig. 6A). These results indicate that to act at residue 148, MTSES must compete for physical space with 2-nonanone. This result indicates that residue 148 is physically near the odorant-binding site of the OR85b subunit.
To further explore the portion of TMS3 near residues 146–150, we scanned a subset of mutant receptors, comparing the relative sensitivity to two odorants (2-heptanol and 2-heptanone). Receptors formed by WT OR85b, pseudo-WT (C146S), and most of the mutant receptors showed no preference between these two odorants when applied at a concentration of 1 mm (Fig. 6B). However, two mutants (F142C and N143C, each within the pseudo-WT C146S context) exhibited a significantly altered odorant preference (2-heptanone > 2-heptanol, Fig. 6B). The preference of the F142C mutant for 2-heptanone over 2-heptanol was diminished at a higher odorant concentration (preference ratio at 1 mm = 0.50 ± 0.04, n = 18; at 10 mm = 0.81 ± 0.06, n = 5, p = 0.0025, unpaired t test), suggesting that this altered odorant preference is due to a shift in the concentration-response relationships for the two odorants. We examined this in more detail by generating concentration-response curves for 2-heptanone and 2-heptanol activation of WT receptor and the F142C mutant. The WT receptor was significantly less sensitive to 2-heptanol when compared with 2-heptanone (supplemental Fig. 9A). However, the 1 mm test concentration used in Fig. 6B is near saturation for both odorants, resulting in similar response amplitudes. The F142C mutant was also significantly less sensitive to 2-heptanol than to 2-heptanone, but both concentration-response curves were shifted to the right (supplemental Fig. 9B). That is, the F142C mutant receptor is less sensitive to both odorants than is the WT receptor. Thus, the 1 mm test concentration used in Fig. 6B is no longer near saturation for 2-heptanol, and the response to 2-heptanol is less than the response to 2-heptanone. This shift in odorant responsiveness provides further evidence of the involvement of TMS3 in odorant activation of OR85b.
DISCUSSION
Our results indicate that in the OR85b/83b receptor complex, a portion of the OR85b subunit is involved in odorant activation of the receptor. Within OR85b, we identified an MTSES-susceptible region comprising residues 146–150 and located at the predicted transition from TMS3 to ECL2 of OR85b (supplemental Fig. 5). The ability of a competitive antagonist (2-nonanone) to protect the receptor from the MTSES reagent suggests that this region is near the odorant-binding site of this receptor. Further, mutations within the extracellular end of TMS3 alter the sensitivity of the receptor to odorants. Our results demonstrate an involvement of TMS3 in odorant activation of OR85b/83b and suggest this region as a component of the odorant-binding site.
It is clear that non-OR83b subunits make a major contribution to the odorant specificity of insect ORs (14, 20), and this has led to these subunits being referred to as odorant-binding subunits. Our results with the OR85b subunit support this designation. Could the OR83b subunit also contribute to the structure of the odorant-binding site? This seems unlikely as it is difficult to imagine how a subunit shared by all the ORs of an insect species could participate in forming binding sites with sufficient diversity to account for the wide range of odorant specificities among insect ORs (14, 20). Thus, the odorant-binding sites of insect ORs are mostly likely formed entirely by contributions from the non-OR83b subunits.
The extracellular half of TMS3, which we have shown to participate in odorant activation, may form part of the odorant-binding site of OR85b. However, the complete odorant-binding site would likely be formed by contributions from several discontinuous protein segments in addition to TMS3. This idea is supported by our finding that mutation of Cys-208 or Cys-278 to serine increases odorant sensitivity (supplemental Fig. 3). Cys-208 is located near the middle of the predicted TMS4, whereas Cys-278 is located toward the extracellular end of the predicted TMS5 of OR85b (supplemental Fig. 5). Both are plausible locations for participation in odorant binding and, together with the extracellular end of TMS3, could form part of the odorant-binding site in this OR subunit.
It is important to note that the locations of residues within OR85b that we are discussing are based on a topology prediction and not on direct experimental results. Therefore, it is important to consider the accuracy of such topology predictions. The topology diagram that we present in supplemental Fig. 5 was generated using the TMRPres2D program (22), which obtains topology predictions from the UniProt data base. UniProt generates topology predictions using a combination of predictive tools including TMHMM (23), Memsat (24), and Phobius (25). These predictions are in general agreement with experimentally obtained topology results for several insect OR subunits. The cytoplasmic location of the N terminus has been convincingly demonstrated for the OR83b subunit and several odorant-binding subunits (ORs 43a, 22a, and 9a) using a variety of techniques, including β-galactosidase fusion, yellow fluorescent protein reconstitution, epitope tagging, and engineered glycosylation site mapping (4, 26, 27). This result has also been generated using bioinformatics approaches (4, 28). The C terminus has been demonstrated to be extracellular in OR83b by epitope tagging (27) and in OR22a by engineered glycosylation site mapping (26). The locations (extracellular or cytoplasmic) of loop sequences between the seven proposed transmembrane domains have also been determined in OR83b and OR22a using a variety of methods (4, 26, 27). All of these results agree quite well with the topology predictions generated in the UniProt data base, supporting our use of such predictions, at least as a ”low resolution“ guide, when interpreting our experimental results.
Many insect species exert a negative impact on humans through serving as disease propagation vectors or through agricultural damage. The novel structure of insect ORs and lack of similar receptors in humans and other mammals (4) promise control of deleterious insect species through the development of compounds with higher selectivity and lower environmental toxicity than currently available insecticides. Our demonstration of the involvement of TMS3 of Drosophila OR85b in odorant activation of the receptor will now allow a more detailed analysis of the molecular basis for odorant recognition by this important receptor class.
Supplementary Material
Acknowledgments
We thank Dr. Grace Zhai for help with Drosophila care and antennae isolation; Drs. Steve Roper and Grace Zhai for critical reading of an early version of the manuscript; Ana Castro for help with oocyte preparation; Dr. John Carlson for the OR35a clone; and Dr. Leslie Vosshall for the OR83b clone.
This work was supported, in whole or in part, by National Institutes of Health Grant DC008119 (to C. W. L.). This work was also supported by United States Department of Agriculture Grant 2008-35302-18815.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–9.
- OR
- odorant receptor
- MTS
- methanethiosulfonate
- MTSEA
- ethylammonium-MTS
- MTSES
- ethylsulfonate-MTS
- MTSET
- trimethylammonium-MTS
- SCAM
- substituted cysteine accessibility method
- WT
- wild-type
- DTT
- dithiothreitol
- HEP
- 2-heptanone.
REFERENCES
- 1.Vosshall L. B., Amrein H., Morozov P. S., Rzhetsky A., Axel R. (1999) Cell 96, 725–736 [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa T., Vosshall L. B. (2009) Curr. Opin. Neurobiol. 19, 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touhara K., Vosshall L. B. (2009) Annu. Rev. Physiol. 71, 307–332 [DOI] [PubMed] [Google Scholar]
- 4.Benton R., Sachse S., Michnick S. W., Vosshall L. B. (2006) PLoS Biol. 4, e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato K., Pellegrino M., Nakagawa T., Nakagawa T., Vosshall L. B., Touhara K. (2008) Nature 452, 1002–1006 [DOI] [PubMed] [Google Scholar]
- 6.Wicher D., Schäfer R., Bauernfeind R., Stensmyr M. C., Heller R., Heinemann S. H., Hansson B. S. (2008) Nature 452, 1007–1011 [DOI] [PubMed] [Google Scholar]
- 7.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B. (2009) Cell 136, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallem E. A., Dahanukar A., Carlson J. R. (2006) Annu. Rev. Entomol. 51, 113–135 [DOI] [PubMed] [Google Scholar]
- 9.Hallem E. A., Carlson J. R. (2004) Trends Genet 20, 453–459 [DOI] [PubMed] [Google Scholar]
- 10.Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., Vosshall L. B. (2004) Neuron 43, 703–714 [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T., Sakurai T., Nishioka T., Touhara K. (2005) Science 307, 1638–1642 [DOI] [PubMed] [Google Scholar]
- 12.Neuhaus E. M., Gisselmann G., Zhang W., Dooley R., Störtkuhl K., Hatt H. (2005) Nat. Neurosci. 8, 15–17 [DOI] [PubMed] [Google Scholar]
- 13.Wanner K. W., Nichols A. S., Walden K. K., Brockmann A., Luetje C. W., Robertson H. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14383–14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallem E. A., Carlson J. R. (2006) Cell 125, 143–160 [DOI] [PubMed] [Google Scholar]
- 15.Pitts R. J., Fox A. N., Zwiebel L. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5058–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones W. D., Nguyen T. A., Kloss B., Lee K. J., Vosshall L. B. (2005) Curr. Biol. 15, R119–R121 [DOI] [PubMed] [Google Scholar]
- 17.Wetzel C. H., Behrendt H. J., Gisselmann G., Störtkuhl K. F., Hovemann B., Hatt H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9377–9380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu T., Qiu Y. T., Wang G., Kwon J. Y., Rutzler M., Kwon H. W., Pitts R. J., van Loon J. J., Takken W., Carlson J. R., Zwiebel L. J. (2007) Curr. Biol. 17, 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liman E. R., Tytgat J., Hess P. (1992) Neuron 9, 861–871 [DOI] [PubMed] [Google Scholar]
- 20.Hallem E. A., Ho M. G., Carlson J. R. (2004) Cell 117, 965–979 [DOI] [PubMed] [Google Scholar]
- 21.Karlin A., Akabas M. H. (1998) Methods Enzymol. 293, 123–145 [DOI] [PubMed] [Google Scholar]
- 22.Spyropoulos I. C., Liakopoulos T. D., Bagos P. G., Hamodrakas S. J. (2004) Bioinformatics 20, 3258–3260 [DOI] [PubMed] [Google Scholar]
- 23.Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 24.Jones D. T., Taylor W. R., Thornton J. M. (1994) Biochemistry 33, 3038–3049 [DOI] [PubMed] [Google Scholar]
- 25.Käll L., Krogh A., Sonnhammer E. L. (2005) Bioinformatics 21, Suppl. 1, i251–i257 [DOI] [PubMed] [Google Scholar]
- 26.Lundin C., Käll L., Kreher S. A., Kapp K., Sonnhammer E. L., Carlson J. R., Heijne G., Nilsson I. (2007) FEBS Lett. 581, 5601–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart R., Kiely A., Beale M., Vargas E., Carraher C., Kralicek A. V., Christie D. L., Chen C., Newcomb R. D., Warr C. G. (2008) Insect Biochem. Mol. Biol. 38, 770–780 [DOI] [PubMed] [Google Scholar]
- 28.Wistrand M., Käll L., Sonnhammer E. L. (2006) Protein Sci. 15, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.