Abstract
Conformational differences in abnormal prion proteins (PrPSc) have been postulated to produce different prion phenotypes. During the interspecies transmission of prions, the conformation of PrPSc may change with passage; however, little is known about the mechanism of PrPSc transition. In this study, novel PrPSc-specific monoclonal antibodies (mAbs) were developed that could detect the PrPSc of mouse but not that of sheep. By using these mAbs, we attempted to examine PrPSc accumulated in mice inoculated with sheep scrapie serially up to five passages. The presence of PrPSc in the mice was confirmed at all passages; however, mAb-bound PrPSc conformer was detected only from the third passage onward. The generated mAb enabled tracing of a particular conformer during adaptation in sheep-to-mice transmission of prion, suggesting that the conformational transition of PrPSc was caused by propagation of this conformer. Such mAbs capable of discriminating conformational differences may allow us to address questions concerning PrPSc conformation and strain diversity.
Keywords: Diseased/Prions, Methods/Immunochemistry, Protein/Conformation, Protein/Posttranslational Modification, Antibodies, Conformational Differentiation, Conformational Transition, Interspecies Transmission
Introduction
Transmissible spongiform encephalopathies (also called prion diseases) are neurodegenerative diseases of humans and other animals. Affected animals accumulate an abnormal prion protein isoform (PrPSc), which is generated by posttranslational modification of cellular prion protein (PrPC).2 Unlike PrPC, PrPSc has a large number of β-sheets (1). This structure is thought to cause aggregation of PrPSc, which exhibits relative resistance to digestion by proteinase K (PK) (2). The moiety remaining after digestion by PK is detected as PrPcore. According to the “protein-only” hypothesis, PrPSc is believed to be the major, or only, component of the infectious agent, the prion (3, 4). Because the conformation of PrPSc seems to determine disease phenotype, conformational discrimination of PrPSc is considered to be a critical issue.
Many studies have demonstrated that transmissible spongiform encephalopathies can be transmitted across species. During adaptation, several passages are required for stabilization of the incubation period and attack rate. These phenomena are part of the “species barrier” (5). Under the protein-only hypothesis, the conformation of PrPSc is postulated to change as part of the adaptation process. Recently, a model of PrPSc conformation at the molecular level in interspecies transmission was proposed (6, 7). In this model, the PrPSc of one strain was believed to represent a cluster of several conformers. Among these conformers, one or more PrPSc conformers that were most readily adaptable in the host were thought to be selected; these conformers then propagated dominantly to become host-adapted PrPSc. Another possibility is that mutation of PrPSc of uniform conformation causes the emergence of new host-adapted PrPSc. However, in field sheep scrapie, the high diversity of strains present seems to favor the multiple conformer concept (8–10). During transmission of transmissible mink encephalopathy (TME) to hamster, previous studies have revealed the emergence of two PrPSc conformers during adaptation (11). In the case of TME, different conformers could be distinguished on the basis of their clearly different disease phenotypes; however, in other prion transmissions without such clear criteria, direct evidence of the adaptive progress of a particular conformer has not yet been presented. Thus, without such clear phenotypic criteria, there is no means by which to discriminate specific conformers from among others. Therefore, it is imperative to be able to discriminate various PrPSc conformers for biochemical investigation of the conformational transition of PrPSc.
The conformational differences in PrPSc can be estimated by the biochemical properties of PrPcore or PrPSc, and the glycoform profile and molecular weight of PrPcore exhibited by immunoblot has been used as the primary tool for such investigations (3, 12–14). Recent results have shown that a mixed banding pattern of PrPSc from TME (11) or a particular case of Creutzfeldt-Jakob disease (15) could exhibit the presence of different conformers in the same brain. However, in a study of in vitro mixed-brain homogenate containing different PrPSc conformers derived from scrapie and bovine spongiform encephalopathy-affected mice analyzed by immunoblot, PrPSc conformation was interpreted as a single property (15). This indicated that estimation of PrPSc conformation by immunoblot banding pattern could not distinguish the different conformers contained in one sample. Therefore, to differentiate PrPSc conformers, it is necessary to find a new strategy, for example, using probes to bind to PrPSc conformers.
We developed PrPSc-specific monoclonal antibodies (mAbs) by immunizing mice against PrPSc with the intention of producing a direct probe for PrPSc. The resulting PrPSc-specific mAbs showed unique binding specificity; they could detect mouse PrPSc but not sheep PrPSc. Taking advantage of this specificity, we traced the conformational transition of PrPSc during adaptation in sheep-to-mice transmission. The results of the immunoprecipitation assay revealed that the PrPSc conformer bound to mAb 3B7 was detected from the third passage despite observations of PrPSc accumulation from the first passage. Consistent with these data, the onset of stabilization of the incubation period and the change in conformational stability of PrPSc was observed from the third passage. These findings suggested that the increase in the particular PrPSc conformer detected by this mAb contributed largely to conformational transition. The unique conformational specificity of this mAb should be widely valuable in the molecular approach to conformational analysis of PrPSc.
EXPERIMENTAL PROCEDURES
Prions and Animals
The following strains of scrapie prion were prepared as 10% (w/v) homogenates of brains in phosphate-buffered saline (PBS). Mouse prion strains Obihiro (16), Chandler, and 79A were intracerebrally inoculated into 3-week-old ICR (SLC) mice, as described previously (17, 18). Prions of Sc237, which had been passaged through Syrian golden hamsters >10 times, were intracerebrally inoculated into 3-week-old Syrian hamsters (SLC) (17). The animals were euthanized at the terminal stage of illness, and the brains were collected from the infected animals for use in this study.
The brains of sheep with scrapie (provided by Dr. M. J. Schmerr, Iowa State University) (19), white-tailed deer with chronic wasting disease (provided by Dr. A. J. Davis, Animal and Plant Health Inspection Service) (20), cattle with bovine spongiform encephalopathy (21), and bovine spongiform encephalopathy-passaged mice (22) were also used. Unaffected brains of ICR mice, PrP−/− mice (23), hamsters, deer, and cattle served as controls.
Purification of Intact PrPSc
Intact PrPSc was purified from the brains of Obihiro strain-affected ICR mice in accordance with a protocol reported previously (24, 25). The purified PrPSc was suspended in PBS. The purity of intact PrPSc was confirmed by SDS-PAGE followed by silver staining. The concentration of intact PrPSc was estimated by the relative intensity of the immunoblot (as described below) compared with that of recombinant mouse PrP quantified in advance.
Generation of Monoclonal Antibodies
For immunization, ∼18 μg of purified intact PrPSc was given twice subcutaneously at the tail base to PrP−/− mice at 2–3-week intervals as an emulsion with TiterMax Gold (CytRx Corp.). The spleen cells of the immunized mice were fused to mouse myeloma cells (Sp2/0-Ag14, DS Pharma Biomedical) and cultured in accordance with the standard protocol (23). To screen the hybridomas, enzyme-linked immunosorbent assay (ELISA) was used with or without antigen treatment with guanidine thiocyanate for denaturation (see details below). mAbs were purified using a mAb trap kit (GE Healthcare), and the subclasses of the mAbs were determined by using an IsoStrip mouse monoclonal antibody isotyping kit (Roche Applied Science).
ELISA
PrPSc-specific mAbs were screened by ELISA using Seprion ligand (Microsense Biotechnologies), which is a ligand specific to PrPSc (26), with minor modifications. Briefly, the ELISA plate was coated with Seprion ligand. After the plate had been blocked, 100 μl of 1% (w/v) brain homogenate of scrapie-affected mice in detergent buffer was added to allow the native PrPSc to couple to the plate. The plate wells were then treated with 4 m guanidine thiocyanate or left untreated to prepare denatured PrPSc antigen or native PrPSc antigen, respectively, for screening of conformation-specific mAbs bound to native antigen dominantly. The supernatant of each hybridoma was added to a set of two wells (denatured PrPSc and native PrPSc antigen) and incubated for 1 h at room temperature. After the plates had been washed, the bound antibody was visualized by the addition of horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories) and SureBlue 3,3′,5,5′-tetramethylbenzidine 1-component microwell peroxidase substrate (KPL). To determine the amounts of PrPSc recognized by the mAbs obtained or HRP-conjugated anti-PrP mAb T2 (HRP-T2) (22), the same ELISA procedure was used, but only on the native PrPSc antigen.
Immunoprecipitation
Brain homogenates (from 0.16 to 1.25% (v/w)) were prepared with PBS containing 2% Triton X-100 (PBST) and Pefablock (final concentration, 1 mm; Merck). In some experiments, brain homogenate was digested with 50 μg/ml PK (Roche Applied Science) for 30 min at 37 °C before immunoprecipitation. After centrifugation of the samples at 400 × g for 1 min at 4 °C, the supernatant was incubated for 1 h at room temperature with 1–2 μg of purified mAb obtained in this experiment or control IgG2a (generated against plant-derived protein, not related to PrP). After an additional incubation with 25 μl of Dynabeads M-280 Sheep anti-mouse IgG (Invitrogen) for 1 h at room temperature, the beads were washed four times with PBST. For PK digestion of bound proteins, beads were divided into two aliquots and then treated with 20 μg/ml PK for 30 min at 37 °C or left untreated under the same conditions. After addition of 1 mm (final concentration) Pefablock, the beads were mixed with SDS sample buffer and heated for 10 min at 100 °C. The eluted protein was then subjected to immunoblot.
Immunoblot
The sample was electrophoresed on NuPAGE Novex 12% Bis-Tris gels and NuPAGE MOPS-SDS running buffer in accordance with the manufacturer's instructions (Invitrogen). After SDS-PAGE, the proteins were transferred onto Immobilon-P transfer membrane (Millipore) by using a TE 22 mini tank transfer unit (GE Healthcare) and NuPAGE transfer buffer (Invitrogen) at 90 V for 1 h at 4 °C. After being blocked with Block Ace (Dainippon Pharma) for 30 min at room temperature, the membrane was incubated with HRP-T2 for 1 h at room temperature. PrPs were detected using SuperSignal West Dura extended duration substrate (Pierce) in accordance with the supplier's instructions and then processed with a FluorChem gel imaging system (Alpha Innotech). For quantitative analysis, the intensity of the bands was analyzed with Spot Denso software (Alpha Innotech).
Transmission of Sheep Scrapie to Mice
Brain homogenate of sheep scrapie (D216) was intracerebrally inoculated into 3-week-old ICR mice as described previously (17, 18). The incubation period was calculated as the interval between injection and a standard clinical endpoint at which the mice were showing clear signs of disease (22). Scrapie-affected mice were euthanized, and their brains were collected. Part of each individual mouse brain homogenate derived from each passage level was passaged into another mouse in subsequent transmission experiments, and the rest was used for the experiments described below.
Long Term PK Digestion
The relative degree of PK resistance was measured by a procedure described previously (27), with modification. Brain homogenate (10% w/v) of scrapie-affected mice was prepared in 5 mm Tris-HCl (pH 7.6), containing 150 mm NaCl, 5 mm EDTA, 0.5% Triton X-100, and 0.5% sodium deoxycholate. The homogenate was digested with 100 μg/ml PK for 1, 3, or 6 h at 37 °C. The reaction was stopped by adding Pefablock to give a final concentration of 1 mm. The samples were subjected to ELISA by HRP-T2 as mentioned above, and the optical density at a wavelength of 450 nm (A450) was measured. The PK resistance of PrPcore at each passage level was represented by the signal ratio; the A450 of PrPcore after 1 h of digestion was defined as 1.0, and the signal ratios of PrPcore after 3 and 6 h of digestion were calculated.
RESULTS
Generation of mAbs Specific to Mouse PrPSc
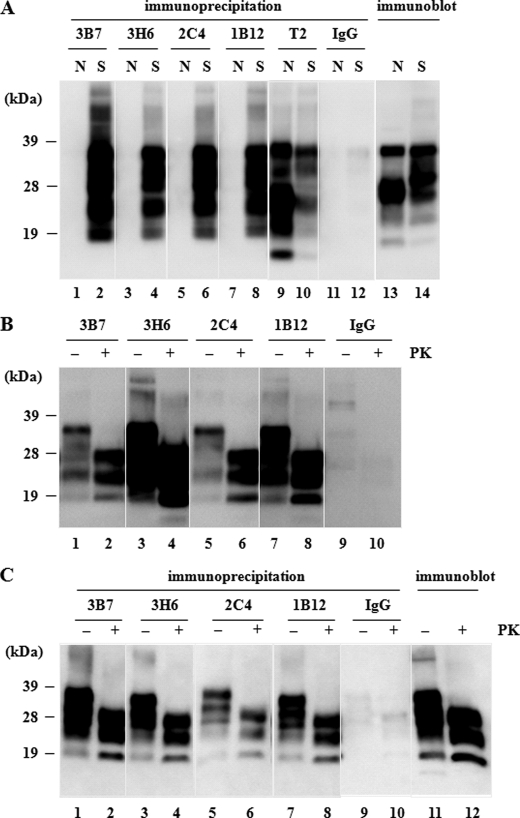
Hybridomas derived from splenocytes of PrP-deficient mice immunized with intact mouse PrPSc were screened according to their reactivity to scrapie brain homogenate incubated with or without guanidine thiocyanate. Candidate clones that produced mAbs were then selected to act as conformation-specific mAbs. These mAbs exhibited stronger immunoreactivity toward the native scrapie brain homogenate than toward the denatured homogenate; this suggested that the mAbs recognized conformational, rather than linear, epitopes. By using this approach, four clones (mAbs 3B7, 3H6, 2C4, and 1B12) were established (Table 1) from >800 screened hybridoma cells (supplemental Fig. 1). In comparison with these mAbs, the pan-PrP antibody mAb T2 showed the inverse result (Table 1). We then performed immunoprecipitation assays to confirm the specificity of the mAbs. The four mAbs captured abundant PrP from scrapie-affected mouse brain homogenates, whereas no PrP was detected in brain homogenates of normal mice (Fig. 1A) or PrP−/− mice (data not shown). In contrast, mAb T2 precipitated PrP from brain homogenates of both scrapie-affected and normal mice (Fig. 1A). Upon PK treatment of the precipitated PrP, the 27- to 38-kDa form of PrP was shortened to 19 to 28 kDa, which was identical to the PK-digested PrPSc (PrPcore) (Fig. 1B). When brain homogenate was treated with PK before the precipitation, the four mAbs were able to precipitate PrPcore (Fig. 1C). In the immunoblot, mAb T2 recognized denatured PrPSc (Fig. 1C), whereas the four mAbs did not react with any form of PrP (supplemental Fig. 2). Furthermore, the four mAbs did not react with any synthetic peptides derived from the whole PrP sequence, including the Tyr-Tyr-Arg motif (29) in ELISA and the peptide arrays on the cellulose support used for epitope mapping in a previous report (23) (supplemental Fig. 3). These results suggested that mAbs 3B7, 3H6, 2C4, and 1B12 were PrPSc conformation-specific antibodies.
TABLE 1.
Immunoreactivity of mAbs to native or denatured scrapie-affected mouse brain homogenates
Immunoreactivities of mAbs to native homogenate (−Gdn, guanidine-untreated) and to denatured homogenate (+Gdn, guanidine-treated were compared (n = 3). The mAbs obtained showed stronger immunoreactivity to −Gdn antigen than to +Gdn antigen, whereas mAb T2 yielded a contrasting result. Values between −Gdn and +Gdn are significantly different.
| mAb |
A450 (means ± S.E.) |
|
|---|---|---|
| −Gdn | +Gdn | |
| 3B7 | 0.686 ± 0.061 | 0.421 ± 0.056a |
| 3H6 | 1.017 ± 0.017 | 0.104 ± 0.011b |
| 2C4 | 1.362 ± 0.077 | 0.404 ± 0.092b |
| 1B12 | 0.643 ± 0.202 | 0.127 ± 0.003c |
| T2 | 0.963 ± 0.040 | 1.939 ± 0.004b |
ap < 0.01.
b p < 0.001.
cp < 0.05.
FIGURE 1.
Detection of PrPSc by immunoprecipitation with mAbs. Before immunoprecipitation, the concentration of each homogenate was adjusted to ensure that the total PrP would give the same signal intensity by immunoblot. All precipitated protein was analyzed by immunoblot and detected with HRP-T2. We confirmed that control IgG2a (IgG) bound no PrP in any of the assays. A, immunoprecipitation of PrP. The four mAbs (3B7, 3H6, 2C4, and 1B12) detected PrP specifically in scrapie-affected mouse brain homogenate (S), whereas no signal was observed in the normal mouse brain homogenate (N; lanes 1, 3, 5, and 7). In contrast, mAb T2 (Pan-PrP mAb) detected PrP in both types of homogenate (lanes 9 and 10). B, PK digestion of immunoprecipitant. Precipitated protein was divided into two aliquots and treated with 20 μg/ml PK (+) or left untreated (−). All the protein precipitated by these mAbs contained PrPcore (lanes 1–8). C, immunoprecipitation of PrPSc from PK-treated brain homogenates of scrapie-affected mice. The four mAbs captured PrPSc in the presence or absence of PK treatment before reaction (lanes 1–8). Lanes 11 and 12 show total PrP in the homogenate used in this experiment.
Species Specificity of the PrPSc-specific Generated mAbs
We further used prion-affected animal brain homogenates to analyze the species specificity of the generated mAbs. MAbs 3B7, 2C4, and 1B12 reacted with PrPSc from scrapie-affected mice and hamsters and from chronic wasting disease-affected deer brains. mAb 3H6 reacted only with PrPSc from scrapie-affected mouse brains (Table 2 and supplemental Fig. 4). mAbs 3B7 and 3H6 could detect PrPSc of other mouse-adapted scrapie strains (Chandler and 79A) besides the Obihiro strain (data not shown). In contrast, none of the four mAbs reacted with PrPSc from sheep with scrapie and bovine spongiform encephalopathy cattle (Table 2 and supplemental Fig. 4). According to these results, the PrPSc conformer precipitated by mAb 3B7 was considered to be at least part of mouse PrPSc. By using this mAb, we attempted to detect this conformer during adaptation of sheep scrapie to mice.
TABLE 2.
Species specificity of PrPSc-specific mAbs
Reactivity was graded as (−, +, or ++) in accordance with the results of at least three immunoprecipitation assays. In the case of mouse-adapted bovine spongiform encephalopathy (BSE), PrPSc strongly bound to control IgG, and its reactivity was determined by comparison with the intensity of the control IgG (same as control, ±; stronger than control, +). CWD, chronic wasting disease.
| mAb | Scrapie |
CWD, deer | BSE |
Subclass | |||
|---|---|---|---|---|---|---|---|
| Sheep | Mouse | Hamster | Cattle | Mouse | |||
| 3B7 | − | ++ | ++ | ++ | − | + | IgG2a |
| 2C4 | − | ++ | ++ | + | − | ± | IgG2b |
| 1B12 | − | ++ | ++ | + | − | ± | IgG2b |
| 3H6 | − | ++ | − | − | − | ± | IgG2b |
Conformational Transition of PrPSc in Interspecies Transmission
Transmission Experiment
Natural sheep scrapie prion was intracerebrally inoculated into ICR mice; the prion was subsequently passaged through mouse brains up to five times. All the inoculated mice showed neurological clinical signs (weight loss, depression, and lack of coordination). The length of the incubation period gradually declined with each passage until it became constant at the fourth (160.7 days) and fifth passages (160.9 days) (Table 3). The results of the incubation period assay indicated that the sheep scrapie prion required at least four passages for adaptation to the new host. Brain homogenate at each passage level was subjected to the following experiments.
TABLE 3.
Disease incubation periods in mice inoculated with sheep scrapie
| Passagea | n/n0b | Incubation periodc |
|---|---|---|
| Days | ||
| 1st | 8/8 | 403.6 ± 11.2 |
| 2nd | 6/6 | 308.5 ± 11.7 |
| 3rd | 6/6 | 287.0 ± 9.4 |
| 4th | 8/8 | 160.7 ± 5.4 |
| 5th | 8/8 | 160.9 ± 3.2 |
a The passage history of sheep scrapie in ICR mice is shown.
b The number of mice that developed signs of scrapie/number of mice inoculated is shown.
c The incubation period to signs of disease (mean ± S.D.) is shown.
Characterization of PrPSc in Affected Mice
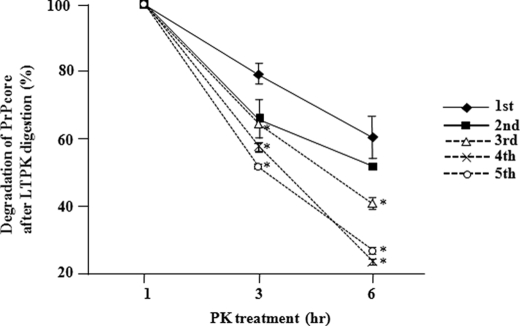
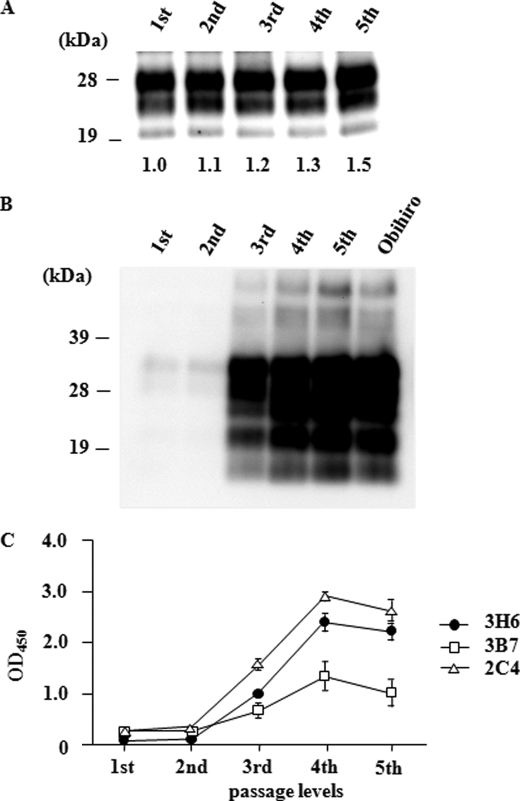
Brain homogenate prepared from affected mice by serial passage of sheep scrapie was initially examined by immunoblot. Three obvious bands of PrPcore were detected after PK treatment, and all the examined mice harbored PrPSc with similar banding patterns (supplemental Fig. 5A). The biochemical characteristics of PrPSc in the mice subjected to prion passage were analyzed by a long term PK resistance assay. PrPcore after the first and second passages showed significantly stronger resistance (p < 0.01) to long term PK digestion than PrPcore after the third, fourth, and fifth passages (Fig. 2 and supplemental Fig. 5B). Thus, the PK resistance of PrPcore changed between the second and third passages, indicating conformational transition of PrPSc. By using generated mAbs, we attempted to trace mouse PrPSc in the process of conformational transition.
FIGURE 2.
Transition of PrPSc in scrapie-passaged mice during the long term PK (LTPK) resistance assay. Brain homogenates prepared from the first to fifth passages of scrapie in mice were digested with 100 μg/ml PK for 1, 3, or 6 h. The transition of PK resistance of PrPcore was monitored by ELISA (see “Experimental Procedures”). The A450 nm of PrPcore (1 h) was defined as 1.0 and used as a basis for calculating the degree of PK resistance of PrPcore after 3 and 6 h of digestion. All values except that after the second passage were significantly lower (*, p < 0.01) than those after the first passage (n = 3 for each analysis).
Tracing of mAb-bound PrPSc Conformer in Sheep-to-Mouse Prion Transmission
Before this experiment, the concentrations of brain homogenate used were adjusted to contain approximately the same amount of PrPcore according to the intensity of the bands obtained by immunoblot (Fig. 3A). As a result of immunoprecipitation with mAb 3B7, PrPSc bound to this mAb was detected after the third to fifth passages but not after the first and second passages (Fig. 3B). Similar results were obtained by using mAb 3H6 (data not shown). Furthermore, application of ELISA to the brain homogenates of scrapie-passaged mice demonstrated that the amount of mouse PrPSc bound to mAb 3B7 increased in the fourth and fifth passages. This result was confirmed with mAbs 2C4 and 3H6 (Fig. 3C).
FIGURE 3.
Monitoring of the conformational transition of PrPSc in interspecies transmission. A, PrPSc in the homogenate used for the immunoprecipitation assay. Each brain homogenate derived from passage of sheep scrapie in mice (first to fifth passages) was confirmed by immunoblot to contain PrPcore. The average relative intensity of each band, as calculated from the results of two separate experiments, is shown under each lane. B, immunoprecipitation assay with mAb 3B7. No signal was detected in first and second prion-passaged mouse brains. However, mAb 3B7 detected PrPSc in the mouse brains from the third passage onward. Obihiro, mouse-adapted scrapie strain. C, estimation of amounts of PrPSc recognized by the mAbs that we obtained. ELISA using Seprion ligand was performed without denaturing treatment. mAbs 3H6, 3B7, and 2C4 exhibited specific reactivity to PrPSc in mice from the third passage onward; the result was therefore similar to that in the immunoprecipitation assay. The amounts of PrPSc detected by these mAbs approximately doubled between the third and fourth passages; it then plateaued or declined slightly at the fifth passage.
DISCUSSION
The novel PrPSc-specific mAbs generated in this study can be considered the first probe to detect a particular PrPSc conformer during prion adaptation. The mAbs generated in the present study, in all likelihood, directly detected the PrPSc conformer by conformation-specific recognition. Thus far, only a few mAbs have been reported to show conformational specificity for PrPSc (28–31); they showed the cross-reactivity against PrPSc from different animal species. In contrast, generated mAbs showed the species specificity, indicating that they could discriminate fine structural difference of PrPSc. This unique function of our mAbs allowed us to trace the conformer during adaptation and revealed that the proportional transition of it was consistent with the stabilization of incubation period and the change in biochemical characteristics of PrPSc. This indicated that the proportional change of this PrPSc conformer was essential to prion adaptation.
There is little biochemical evidence supporting the concept that the conformational property of PrPSc may change due to proportional differences of PrPSc conformers. However, a case using HY (hyper strain of TME) and DY (drowsy strain of TME) strains of TME showed that the disease phenotype, which is directed by PrPSc conformation, changed in correspondence to the HY/DY proportion in inoculums (11). However, coexisting PrPSc conformers in one prion-affected animal could not be discriminated by banding patterns of PrPcore or disease phenotype, as opposed to the case of TME strains. To accumulate evidence that the types and proportions of multiple conformers determine the properties of PrPSc, mAbs that can discriminate conformers will be of clear value.
The species/conformer specificity of our mAbs allowed us to eliminate the possibility of binding between those mAbs and PrPSc aggregates through nonspecific interactions, as demonstrated previously (32). It remains to be determined how our mAbs recognized the conformational properties of the PrPSc conformer, which could not be discriminated by other PrPSc-specific mAbs reported previously. Furthermore, the mechanism underlying PrPSc recognition of these four mAbs may have been different because they showed different reactivity to PrPSc from various species, although all of the mAbs detected mouse PrPSc (Table 2). We expected that the epitopes recognized by our mAbs would include mouse-specific sequences. This was probable if the conformational epitope, which was exposed on the surface of the PrPSc aggregate, was composed solely of peptide or assembled from several discontinuous locations, as was the case with the epitopes detected by previous PrPSc-specific mAbs (28–30). However, epitope mapping by a peptide array with immobilized 13-residue synthetic peptides failed to determine the peptide associated with the conformational epitope (supplemental Fig. 3). This might have been because the synthetic peptides corresponding to the epitopes detected by our mAbs formed particular conformations under our experimental conditions of epitope mapping as even such small peptides are thought to form secondary structures such as β-strand structures (33). These conformations may have differed significantly from those of native PrPSc or may have hidden the sequence required for mAb binding. Another possibility is that the epitopes detected by our mAbs consisted of peptides too short to be recognized when they were divided into independent segments. More definitive experiments are needed to determine the epitopes essential for conformation- and species-specific recognition.
It is also important to determine which conditions in our strategy were advantageous for the generation of conformation-specific mAbs. One of the key factors might have been that we used native brain homogenate as the antigen for mAb screening; various forms of PrPSc aggregate could have been contained in the homogenate. Recently, it was reported that small, nonfibrillar particles showed greater infectivity and conformation-converting activity against PrPC than did large fibrils (34). Similar small aggregates have been found in suspensions of amyloid formed by recombinant PrP in vitro (35). Interestingly, this small aggregate was detected only by PrPSc-specific mAb (31) but not by other general, non-PrPSc-specific mAbs. These results suggest that the small aggregate represents the characteristic conformation of PrPSc, providing support for our hypothesis that the reason why our previous study using purified PrPSc for screening failed to establish conformation-specific mAbs was that centrifugation of detergent-insoluble PrPSc eliminated the small aggregates. Another factor might have been that, in the present study, we used denatured brain homogenate together with the native homogenate as antigens for mAb screening, and we selected clones showing higher reactivity against the latter. As a result, we could discriminate conformation-specific mAbs from general mAbs by recognizing the linear epitopes exposed by denaturation. This approach might be valid for screening other conformation-specific mAbs against proteins other than PrP.
Our strategy can generate repertoires of conformation-specific mAbs, and these mAbs will be useful for isolating the different PrPSc conformers that emerge in various interspecies transmissions. Biochemical or biophysical characterization of these PrPSc conformers may enable us to discover the factors that determine the conformational properties behind the variations in susceptibility observed in interspecies transmission. The discovery of these factors would provide new insights into mechanism of prion adaptation in interspecies transmission.
Supplementary Material
Acknowledgments
We thank Dr. M. J. Schmerr, Iowa State University, and Dr. A. J. Davis, Animal and Plant Health Inspection Service, for providing brain samples and Dr. M. Shinagawa, National Institute of Animal Health, Japan, Dr. Y. Kaku, National Institute of Infectious Diseases, Japan, and Dr. K. Ogawa-Goto, Nippi Reserch Institute of Biomatirx, for providing comments and suggestions on this article. We also thank N. Tabeta and other laboratory staff of the National Institute of Animal Health, Japan, for technical support and for maintaining the mouse colony.
This work was supported by grants from the Ministry of Agriculture, Forestry, and Fisheries of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6 and additional references.
- PrP
- prion protein
- PrPC
- cellular PrP
- PK
- proteinase K
- TME
- transmissible mink encephalopathy
- ELISA
- enzyme-linked immunosorbent assay
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- mAb
- monoclonal antibody
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E., Prusiner S. B. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10962–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner S. B. (1982) Science 216, 136–144 [DOI] [PubMed] [Google Scholar]
- 3.Prusiner S. B. (1991) in Current Topics in Microbiology and Immunology, pp. 233–257, Springer-Verlag, Berlin-Heidelberg: [DOI] [PubMed] [Google Scholar]
- 4.Prusiner S. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimberlin R. H. (1991) Sub-acute Spongiform Encephalopathies (Bradley R., Savey M., Marchant B. eds) pp. 137–147, Kluwer Academic Publishers, Dordrecht [Google Scholar]
- 6.Collinge J., Clarke A. R. (2007) Science 318, 930–936 [DOI] [PubMed] [Google Scholar]
- 7.Béringue V., Vilotte J. L., Laude H. (2008) Vet. Res. 39, 47. [DOI] [PubMed] [Google Scholar]
- 8.Bruce M. E., Boyle A., Cousens S., McConnell I., Foster J., Goldmann W., Fraser H. (2002) J. Gen. Virol. 83, 695–704 [DOI] [PubMed] [Google Scholar]
- 9.Bruce M. E., Dickinson A. G. (1987) J. Gen. Virol. 68, 79–89 [DOI] [PubMed] [Google Scholar]
- 10.Bruce M. E. (1993) Br. Med. Bull. 49, 822–838 [DOI] [PubMed] [Google Scholar]
- 11.Bartz J. C., Bessen R. A., McKenzie D., Marsh R. F., Aiken J. M. (2000) J. Virol. 74, 5542–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinge J., Sidle K. C., Meads J., Ironside J., Hill A. F. (1996) Nature 383, 685–690 [DOI] [PubMed] [Google Scholar]
- 13.Telling G. C., Parchi P., DeArmond S. J., Cortelli P., Montagna P., Gabizon R., Mastrianni J., Lugaresi E., Gambetti P., Prusiner S. B. (1996) Science 274, 2079–2082 [DOI] [PubMed] [Google Scholar]
- 14.Bessen R. A., Marsh R. F. (1994) J. Virol. 68, 7859–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron T. G., Biacabe A. G. (2001) J. Virol. 75, 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinagawa M., Takahashi K., Sasaki S., Doi S., Goto H., Sato G. (1985) Microbiol. Immunol. 29, 543–551 [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama T., Kimura K., Tagawa Y., Yuasa N. (1995) Clin. Diagn. Lab. Immunol. 2, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamaru Y., Takenouchi T., Ogihara K., Hoshino M., Takata M., Imamura M., Tagawa Y., Hayashi-Kato H., Ushiki-Kaku Y., Shimizu Y., Okada H., Shinagawa M., Kitani H., Yokoyama T. (2007) J. Virol. 81, 1524–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada K., Hayashi H. K., Ookubo Y., Iwamaru Y., Imamura M., Takata M., Schmerr M. J., Shinagawa M., Yokoyama T. (2005) Microbiol. Immunol. 49, 801–804 [DOI] [PubMed] [Google Scholar]
- 20.Masujin K., Shimada K., Kimura K. M., Imamura M., Yoshida A., Iwamaru Y., Mohri S., Yokoyama T. (2007) Microbiol. Immunol. 51, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi H., Takata M., Iwamaru Y., Ushiki Y., Kimura K. M., Tagawa Y., Shinagawa M., Yokoyama T. (2004) J. Vet. Med. Sci. 66, 515–520 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi H. K., Yokoyama T., Takata M., Iwamaru Y., Imamura M., Ushiki Y. K., Shinagawa M. (2005) Biochem. Biophys. Res. Commun. 328, 1024–1027 [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama T., Kimura K. M., Ushiki Y., Yamada S., Morooka A., Nakashiba T., Sassa T., Itohara S. (2001) J. Biol. Chem. 276, 11265–11271 [DOI] [PubMed] [Google Scholar]
- 24.Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. (1987) Arch. Biochem. Biophys. 258, 579–590 [DOI] [PubMed] [Google Scholar]
- 25.Caughey B., Raymond G. J., Priola S. A., Kocisko D. A., Race R. E., Bessen R. A., Lansbury P. T., Jr., Chesebro B. (1999) Mol. Biotechnol. 13, 45–55 [DOI] [PubMed] [Google Scholar]
- 26.Lane A., Stanley C. J., Dealler S., Wilson S. M. (2003) Clin. Chem. 49, 1774–1775 [Google Scholar]
- 27.Kuczius T., Groschup M. H. (1999) Mol. Med. 5, 406–418 [PMC free article] [PubMed] [Google Scholar]
- 28.Korth C., Stierli B., Streit P., Moser M., Schaller O., Fischer R., Schulz-Schaeffer W., Kretzschmar H., Raeber A., Braun U., Ehrensperger F., Hornemann S., Glockshuber R., Riek R., Billeter M., Wüthrich K., Oesch B. (1997) Nature 390, 74–77 [DOI] [PubMed] [Google Scholar]
- 29.Paramithiotis E., Pinard M., Lawton T., LaBoissiere S., Leathers V. L., Zou W. Q., Estey L. A., Lamontagne J., Lehto M. T., Kondejewski L. H., Francoeur G. P., Papadopoulos M., Haghighat A., Spatz S. J., Head M., Will R., Ironside J., O'Rourke K., Tonelli Q., Ledebur H. C., Chakrabartty A., Cashman N. R. (2003) Nat. Med. 9, 893–899 [DOI] [PubMed] [Google Scholar]
- 30.Curin Serbec V., Bresjanac M., Popovic M., Pretnar Hartman K., Galvani V., Rupreht R., Cernilec M., Vranac T., Hafner I., Jerala R. (2004) J. Biol. Chem. 279, 3694–3698 [DOI] [PubMed] [Google Scholar]
- 31.Moroncini G., Kanu N., Solforosi L., Abalos G., Telling G. C., Head M., Ironside J., Brockes J. P., Burton D. R., Williamson R. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10404–10409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morel N., Simon S., Frobert Y., Volland H., Mourton-Gilles C., Negro A., Sorgato M. C., Créminon C., Grassi J. (2004) J. Biol. Chem. 279, 30143–30149 [DOI] [PubMed] [Google Scholar]
- 33.Osterman D. G., Kaiser E. T. (1985) J. Cell. Biochem. 29, 57–72 [DOI] [PubMed] [Google Scholar]
- 34.Silveira J. R., Raymond G. J., Hughson A. G., Race R. E., Sim V. L., Hayes S. F., Caughey B. (2005) Nature 437, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novitskaya V., Makarava N., Bellon A., Bocharova O. V., Bronstein I. B., Williamson R. A., Baskakov I. V. (2006) J. Biol. Chem. 281, 15536–15545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.