Abstract
Renin is a key enzyme for cardiovascular and renal homeostasis and is produced by highly specialized endocrine cells in the kidney, known as juxtaglomerular (JG) cells. The nature and origin of these cells remain as mysteries. Previously, we have shown that the nuclear hormone receptor liver X receptor-α (LXRα) is a major transcriptional regulator of the expression of renin, c-myc, and other genes involved with growth/differentiation. In this study we test the hypothesis that LXRα plays an important role not only in renin expression but also in renin-containing cell differentiation, specifically from the mesenchymal stem cell (MSC), which may be the origin of the JG cell. Indeed, our data demonstrated that LXRα activation by its ligands or cAMP stimulated renin gene expression in both murine and human MSCs. Furthermore, sustained cAMP stimulation of murine MSCs overexpressing LXRα led to their differentiation into JG-like cells expressing renin and α-smooth muscle actin. These MSC-derived JG-like cells contained renin in secretory granules and released active renin in response to cAMP. In conclusion, the activation of LXRα stimulates renin expression and induces MSCs differentiation into renin-secreting, JG-like cells. Our results suggest that the MSC may be the origin of the juxtaglomerular cell and provide insight into novel understanding of pathophysiology of the renin-angiotensin system.
Keywords: Differentiation, Gene Expression, Gene Regulation, Nuclear Receptors, Stem Cell, Juxtaglomerular Apparatus, LXR, Mesenchymal Stem Cell, Renin, Renin-Angiotensin system
Introduction
Renin, which is an aspartyl protease, is the first and rate-limiting step of the renin-angiotensin cascade that ultimately integrates cardiovascular and renal homeostasis. Circulating renin is produced from highly specialized endocrine cells, accounting for less than 0.1% of all kidney cells, which are located in the afferent arterioles at the entrances to the kidney glomeruli; hence, their name juxtaglomerular (JG)3 cells. JG cell releases renin from storage granules in its cytoplasm (1, 2). Until recently, JG cells were thought to be derived from smooth muscle cells through metaplastic transformation (3). However, Sequeira Lopez et al. (4, 5) have suggested that renin-containing cells can give rise to smooth muscle cells rather than originating from them. They reported the possible existence of renin precursor cells in the prevascular metanephric kidney. Because mesenchymal stem cells play an important role in developmental biology contributing to the genesis of various tissues, we hypothesize that MSC is the origin of JG cells. Previously, we have demonstrated that adult human mesenchymal stem cells express at modest levels all the components of the renin-angiotensin system (6). In this study we examined for direct evidence that mesenchymal stem cells can differentiate into JG-like cells as characterized by co-expression of high levels of renin and smooth muscle actin and their capability of releasing renin from secretory granules in response to cAMP.
Cyclic AMP is suggested to be the central stimulator of renin gene expression (7). Our previous studies demonstrated that the upstream region of the Ren-1d promoter (−619 to −588) is involved in tissue-specific gene expression (8–10). We termed this unique element CNRE because it contains a cAMP-responsive cis element and a negative regulatory element that share an overlapping sequence (11). The CNRE element is conserved in the human and rodent genomes (11). We further identified and cloned a CNRE-binding protein using the yeast one-hybrid approach by screening a mouse kidney cDNA library (12). The cloned CNRE binding protein is liver X receptor-α (LXRα, also known as NR1H3), a transcriptional factor of the nuclear hormone receptor family (12).
Previously, we have demonstrated that LXRα induces renin expression in As4.1-transformed renin-containing cells (12, 13). We have also validated using LXRα knock-out mice that this transcription factor regulates renin expression in vivo (14). In addition, we have reported the LXRα also up-regulates in As4.1 cells the expression of c-myc and other genes involved in cell differentiation (13). Accordingly, we have hypothesized that LXRα is involved in JG differentiation through the expression of renin, c-myc, and other related cell differentiation genes (15).
In this study we test our hypothesis that LXRα plays an important role not only in renin expression but also in renin-containing cell differentiation. Our data demonstrate that the activation of LXRα up-regulates renin expression in both murine and human MSCs. Furthermore, the activation of LXRα induces murine MSCs differentiation into renin-producing and -secreting JG-like cells. Like JG cells, these cells also express α-smooth muscle actin. Taken together, our results support the hypothesis that the MSC is the origin of the juxtaglomerular cell and that LXRα may play an important role in JG cell differentiation.
EXPERIMENTAL PROCEDURES
Mesenchymal Stem Cell Isolation and Culture
All animal procedures were approved by the Duke University Institutional Animal Care and Use Committees. Murine mesenchymal stem cells (mMSCs) were isolated by their adherence to plastic as previously described (16–21). Bone marrow was collected from 12-week-old C57Bl/6 mice by flushing femurs and tibias with the mMSC growth medium constituted of minimum essential medium α with GlutaMAX (Invitrogen), 20% fetal bovine serum, and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively). The bone marrow cells were then filtered through a 40-μm nylon mesh filter. Mononuclear cells were separated by gradient density using Ficoll-Paque Plus (Amersham Biosciences). Cells were then washed twice with phosphate-buffered saline (PBS) and plated in plastic dishes. After 3 days, nonadherent cells were removed by two washes with PBS, and adherent cells were further cultured in the mMSC growth medium. Cells were then propagated in culture. Medium was changed every 3 days.
Human mesenchymal stem cells (hMSCs) were obtained from Cambrex (Walkersville, MD). Cells were positive for CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45. Human MSCs were grown in the hMSC growth medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 4 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin). Medium was changed every 3 days. Representative human renin-expressing cell line Calu-6 was obtained from the American Type Culture Collection, Manassas, VA. Human Calu-6 cells were grown in the Calu-6 growth medium constituted of Eagle's minimum essential medium (ATCC) with 10% fetal bovine serum and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively). Medium was changed every 3 days.
Fluorescence-activated Cell Sorting (FACS) Analysis
For surface protein expression, murine MSCs were trypsinized, washed, resuspended in PBS, and filtered through a 40-μm nylon mesh filter. Cells were then incubated with the following primary antibodies for 60 min (min) at room temperature: monoclonal phycoerythrin-conjugated anti-CD44 (Santa Cruz Biotechnology, INC., Santa Cruz, CA), goat polyclonal anti-CD105 (Santa Cruz Biotechnology, INC.), rabbit polyclonal anti-CD34 (Santa Cruz Biotechnology, INC.), and monoclonal phycoerythrin-conjugated anti-CD45 (Santa Cruz Biotechnology, INC.). Then, if the first antibody was unlabeled, cells were washed and incubated with the second antibody (Texas Red-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology, INC.) for CD105, or Alexa Fluor 350-conjugated goat anti-rabbit IgG (Invitrogen) for CD34) for 60 min at room temperature. After washing, cells were fixed with 1% paraformaldehyde for 10 min at 4 °C. Negative controls for antibody type were performed by using isotype control immunoglobulin G. FACS analysis was performed on a FACS Vantage SE (BD Biosciences) or a FACSCalibur (BD Biosciences) flow cytometer.
Pharmacologic Treatment
8-Bromo-cAMP and 22-hydroxycholesterol were purchased from Sigma. LXRα synthetic ligand T0901317 was purchased from Cayman Chemical (Ann Arbor, MI). For gene expression analysis, cells were subjected to serum depletion for 16 h before the treatment. For the prolonged treatment, cAMP or 22-hydroxycholesterol was added to culture media daily during the treatment period.
Quantitative RT-PCR
Total RNA was isolated by the Tri Reagent (Sigma) and further purified using RNeasy columns (Qiagen, Valencia, CA). The concentration of RNA was determined using spectrophotometry. First-strand cDNA was synthesized from total RNA using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) following the manufacturer's protocol. The TaqMan probe primer system (Applied Biosystems) was used for quantitative RT-PCR. The primers and probes for human renin, murine Ren1c, and human housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were adopted from literature (6, 14, 22, 23). The primer and probe sets for murine Abca1, murine LXRα, murine LXRβ, murine GAPDH, human LXRα, and human LXRβ were purchased from Applied Biosystems. TaqMan PCR was performed using ABI Prism 7700 Sequence Detection System as instructed by manufacturer (Applied Biosystems). Target gene mRNA expression was normalized to GAPDH mRNA expression, and the relative amounts of all mRNAs were calculated using the comparative cycle threshold method (24).
RNA Interference
Knockdown experiments were performed using Pre-designed siRNA. Silencer Pre-designed and Validated LXRα siRNA (ID# s19568) and Silencer Select Negative Control siRNA were obtained from Applied Biosystems. The sequence for LXRα siRNA is sense (5′-GGAUGCUAAUGAAACUGGUtt-3′) and antisense (5′-ACCAGUUUCAUUAGCAUCCgt-3′). hMSCs were transfected with siRNAs using siPORT NeoFX Transfection Agent (Applied Biosystems) according to the manufacturer's protocol. Briefly, siRNA was diluted in Opti-MEM I (Invitrogen) and siPORT NeoFX Transfection Agent before mixing with 2 × 105 cells. The final concentration of siRNA was 20 nm. After 24 h, media were replaced with hMSC growth medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 4 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin), and cells were incubated overnight for recovery. The cells were then subjected to serum depletion for 16 h for gene expression analysis. After serum depletion, cAMP (1 mmol/liter) or 22-hydroxycholesterol (0.1 μmol/liter) was added to the media. After 6 h of pharmacological treatment, we harvested the cells and investigated mRNA expression of LXRα and renin by using real-time RT-PCR.
Cell Lines Stably Overexpressing GFP or GFP-LXRα
For the generation of murine mesenchymal stem cell lines stably expressing GFP or GFP-LXRα, LXRα cDNA was cloned into a MSCV-IRES-GFP plasmid backbone. Retroviral particles were then obtained by tripartite transfection in HEK 293 T cells and concentrated by ultracentrifugation. Murine MSCs were infected with retroviral particles in the presence of Polybrene (Sigma). Each infection was repeated twice. Pools of infected cells were then subcultured.
Immunocytochemistry
For surface protein expression, the same antibodies were used as in the FACS analysis. For renin staining, rabbit polyclonal anti-rat renin antibody was generously provided by Dr. Tadashi Inagami (Vanderbilt University, Nashville, TN). For α-smooth muscle actin staining, monoclonal Cy3-conjugated anti-α-smooth muscle actin antibody was purchased from Sigma. Cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. After washing with PBS three times, cells were incubated with blocking solution (2% goat serum or 2% donkey serum) for 15 min at room temperature. Solution was removed and replaced by the primary antibody. Slides were incubated for 2 h at room temperature for renin staining. For other staining, slides were incubated for 1 h at room temperature. After washing with PBS three times, if the first antibody was unlabeled, cells were incubated with the second antibody. For renin staining, slides were incubated with goat anti-rabbit IgG (Sigma) for 1 h at room temperature, washed, incubated with rabbit peroxidase-anti-peroxidase (Sigma) for 1 h at room temperature, washed, and incubated with diaminobenzidine solution (Sigma). Staining was viewed in the Axiovert 200 inverted microscope (Carl Zeiss Inc., Thornwood, NY), and images were captured with the AxioCam MRc camera (Carl Zeiss, Inc.).
Immunoblotting
Cells were lysed at 4 °C with radioimmune precipitation assay lysis buffer (Upstate Biotechnology, Inc., Lake Placid, NY). Equal amounts of proteins (20 μg/lane) were separated by NuPAGE 4–12% Bis-Tris gel (Invitrogen) electrophoresis. Human renin recombinant protein (Cayman Chemical) was also applied as a positive control. Protein fractions were then electrophoretically transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with Blocker/Diluent solution (Western blot kit, Invitrogen). Then the membrane was incubated with rabbit polyclonal antibody to rat renin (a kind gift from Dr. Tadashi Inagami, Vanderbilt University) for 2 h at room temperature. After washing in wash buffer (Western blot kit, Invitrogen), the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Western blot kit, Invitrogen) for 1 h at room temperature. The antigen antibody-peroxidase complex was visualized using the ECL chemiluminescence solution (Western blot kit, Invitrogen). The blot was subsequently stripped with Re-Blot Plus Western blot recycling kit (Chemicon International, Inc., Temecula, CA) and rehybridized with an anti-GAPDH antibody (Santa Cruz Biotechnology, Inc.) as a control for protein loading.
Renin Activity in Conditioned Media
Active renin in conditioned media was measured according to the reported methods (25–28) with modifications. Conditioned media from cAMP-LXRα-activated mMSCs were prepared by treating mMSC overexpressing GFP-LXRα with cAMP treatment (1 mmol/liter) daily for 8 weeks. Conditioned media from 22-hydroxycholesterol-LXRα-activated mMSCs were prepared by treating mMSC-overexpressing GFP-LXRα with 22-hydroxycholesterol treatment (0.1 μmol/liter) daily for 8 weeks. After 8 weeks of treatment, conditioned media from the above cAMP-LXRα-activated mMSCs, 22-hydroxycholesterol-LXRα-activated mMSCs, or control mMSCs (mMSC overexpressing GFP alone without treatment) were collected. Fifteen microliters of conditioned media were incubated for 60 min at 37 °C with excess porcine angiotensinogen (4 μmol/liter, Sigma) in a 150-μl reaction containing sodium acetate (50 mmol/liter, pH 6.5, Sigma), 4-(2-aminomethyl)benzenesulfonyl fluoride hydrochloride (AEBSF; 2.5 mmol/liter, Sigma), 8-hydroxyquinoline (1 mmol/liter, Sigma), and EDTA (5 mmol/liter, Sigma). The reaction was stopped by boiling, and the generated angiotensin I was measured by using an enzyme immunoassay kit (Phoenix Pharmaceuticals, Belmont, CA) following the manufacturer's protocol. Results are expressed as angiotensin I generated/ml/60 min.
Statistical Analysis
All values are expressed as the mean ± S.E. Student's t test and one-way analysis of variance followed by post hoc Fisher's least significant difference test were performed. Differences were considered to be significant when p < 0.05. All statistical procedures were done using the Statgraphics Plus Version 5.0 software (StatPoint, Inc., Herndon, VA).
RESULTS
Characteristics of Murine Mesenchymal Stem Cells
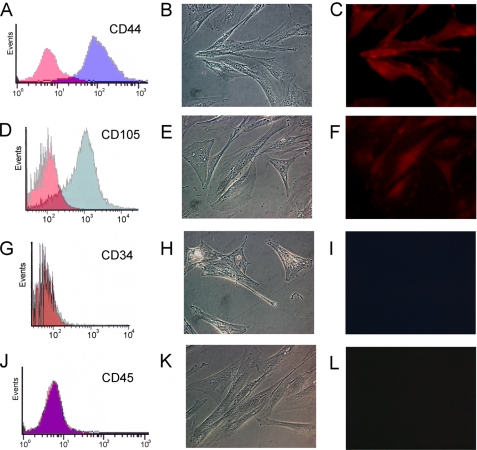
Murine MSCs were isolated from mouse bone marrow and propagated in culture as described (18, 19, 29). To characterize these cells, FACS and immunofluorescent staining were performed using antibodies against mesenchymal stem cell surface markers CD44 and CD105. As shown in Fig. 1, A–F, cells were positive for both markers. We further excluded the presence of hematopoietic stem cells by FACS and immunofluorescent staining analysis using anti-CD34- and -CD45-specific antibodies. The results showed that there was no contaminating hematopoietic population (Fig. 1, G–L). Negative control staining was also performed by using isotype control immunoglobulin G (for CD 44 and CD45) or by omitting the primary antibody (for CD 105 and CD 34). Immunofluorescent staining in negative controls showed no positive signal (data not shown).
FIGURE 1.
Characteristics of murine MSCs. Murine MSCs were analyzed for the expression of the cell surface markers CD44 (A–C), CD105 (D–F), CD34 (G–I), and CD 45 (J–L) by FACS (A, D, G, and J) and immunofluorescent staining (C, F, I, and L). Negative controls for antibody type were performed (red area in A, D, G, and J) by using isotype control immunoglobulin G (for CD 44 and CD45) or by omitting the primary antibody (for CD 105 and CD 34). B, C, E, F, H, I, K, and L, original magnification, ×400.
LXRα Ligands Stimulate Renin Gene Expression
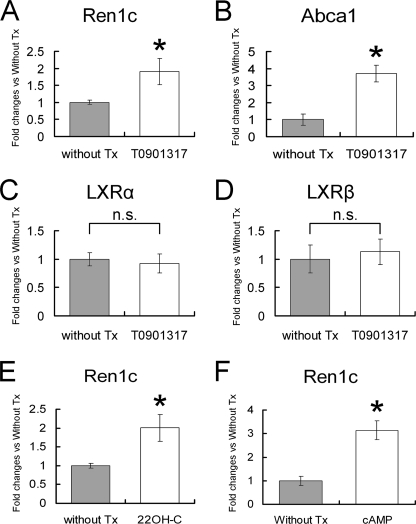
For gene expression analysis, murine MSCs were subjected to serum depletion for 16 h. We then added the LXRα synthetic ligand T0901317 (1 μmol/liter), LXRα natural ligand 22-hydroxycholesterol (0.1 μmol/liter), or 8-bromo-cyclic AMP (1 mmol/liter) and investigated mRNA expression of renin after 6 h of pharmacological treatment by using real-time RT-PCR. As shown in Fig. 2A, mMSCs treated with LXRα synthetic ligand T0901317 exhibited a significant increase in expression of renin (1.9 ± 0.4-fold changes for mMSCs without treatment, p < 0.05). In response to T0901317, the expression of Abca1, the ATP binding cassette transporter A1 gene known to be up-regulated by LXR activation (30), was also significantly increased (3.7 ± 0.5-fold changes for mMSCs without treatment, p < 0.05), but no significant change in LXRα or LXRβ expression was observed, suggesting that T0901317 acted only at the post-transcriptional level in mMSCs (Fig. 2, B–D). 22-hydroxycholesterol, one of the oxysterols known as the natural ligands for LXR, has been suggested to play a role in the regulation of cholesterol homeostasis through LXR activation (31–34). As shown in Fig. 2, E and F, 6 h of 22-hydroxycholesterol or cAMP treatment also stimulated renin mRNA expression in murine MSCs (2.0 ± 0.4-fold and 3.1 ± 0.4-fold changes for mMSCs without treatment, respectively; p < 0.05).
FIGURE 2.
Effects of LXRα ligands on the expression of Ren1c- and LXRα-related genes in murine MSCs. One μmol/liter LXRα synthetic ligand T0901317, 0.1 μmol/liter LXRα natural ligand 22-hydroxycholesterol (22OH-C), or 1 mmol/liter 8-bromocyclic AMP (cAMP) was added to media after 16 h of serum depletion. Murine MSCs were harvested after 6 h of pharmacological treatment, and total RNA was analyzed by quantitative RT-PCR for mRNA expression of Ren1c (A, E, and F), Abca1 (B), LXRα (C), and LXRβ (D). -Fold changes versus without treatment group (Without Tx). Data are the mean ± S.E.; n = 4; *, p < 0.05 versus without treatment group. n.s., statistically non-significant.
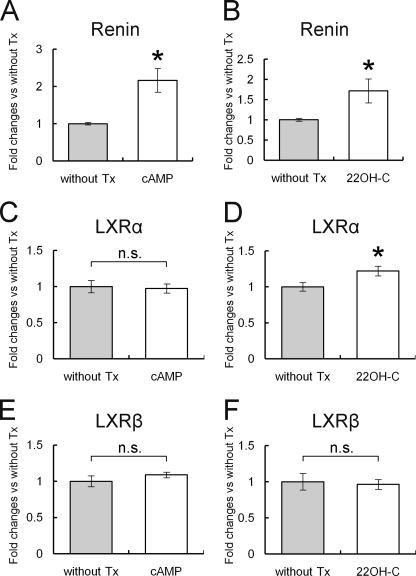
We examined the effects of cAMP (1 mmol/liter) or 22-hydroxycholesterol (0.1 μmol/liter) on human MSCs as well. hMSCs were subjected to serum depletion for 16 h before the pharmacological treatments. As shown in Fig. 3, A and B, 6 h of cAMP or 22-hydroxycholesterol treatment significantly stimulated renin mRNA expression in human MSCs (2.2 ± 0.3- and 1.7 ± 0.3-fold changes for hMSCs without treatment, respectively; p < 0.05). LXRα mRNA expression was slightly up-regulated by 22-hydroxycholesterol treatment but not changed by cAMP treatment. There was no significant change in LXRβ expression by 22-hydroxycholesterol or cAMP treatment. Whitney et al. (35) reported that LXR agonists did not induce the expression of the LXRα gene in murine macrophages, human adipocytes, or human hepatocytes but induced it in human macrophages. Indeed we did not detect increased LXR mRNA expression in response to its agonist in mMSCs and observed only a mild increase in hMSCs. The difference of LXRα up-regulation by LXR agonists between mMSCs and hMSCs may reflect differences in species. We also compared the relative level of LXRα expression between hMSCs and the representative human renin-expressing cell line Calu-6 (36, 37). The relative expression level of LXRα in hMSCs was 8.8 ± 1.3% that in Calu-6 cells.
FIGURE 3.
Effect of cAMP or 22-hydroxycholesterol on the expression of Renin, LXRα, and LXRβ in human MSCs. One mmol/liter 8-bromo-cyclic AMP (cAMP) or 0.1 μmol/liter LXRα natural ligand 22-hydroxycholesterol (22OH-C) was added to media after 16 h of serum depletion. Human MSCs were harvested after 6 h of pharmacological treatment, and total RNA was analyzed by quantitative RT-PCR for mRNA expression of renin (A and B), LXRα (C and D), and LXRβ (E and F). Data are -fold changes versus without the treatment group (Without Tx). Data are the mean ± S.E.; n = 4; *, p < 0.05 versus without treatment group. n.s., statistically non significant.
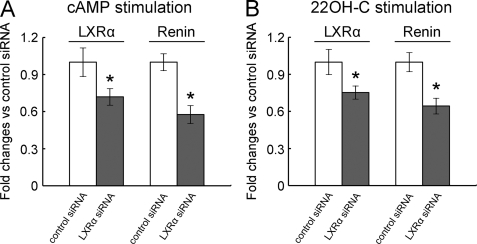
Knockdown of LXRα Inhibited cAMP- or 22-Hydroxycholesterol-induced Up-regulation of Renin Expression
To further confirm the role of LXRα in regulation of renin expression, we knocked down LXRα using LXRα siRNA. Nonspecific siRNA was used as a negative control. We then added cAMP (1 mmol/liter) or 22-hydroxycholesterol (0.1 μmol/liter) and investigated mRNA expression of renin after 6 h of pharmacological treatment by using real-time RT-PCR. As shown in Fig. 4A, transfection with LXRα-specific siRNA, in comparison with nonspecific negative control siRNA, resulted in a significant decrease in expression of LXRα (28.1 ± 6.6% decrease, p < 0.05). Knockdown of LXRα inhibited cAMP-induced up-regulation of renin expression by 42.3 ± 7.1% (p < 0.05, Fig. 4A). Similar to cAMP stimulation, hMSCs with decreased LXRα expression (24.7 ± 5.3% decrease, p < 0.05) attenuated the up-regulation of renin expression (35.6 ± 6.3% decrease, p < 0.05) by 22-hydroxycholesterol stimulation (Fig. 4B).
FIGURE 4.
Effect of LXRα siRNA in cAMP- or 22-hydroxycholesterol-induced up-regulation of renin expression in human MSCs. Human MSCs were transfected with LXRα siRNA or control siRNA. 1 mmol/liter 8-bromo-cyclic AMP (cAMP) (A) or 0.1 μmol/liter LXRα (B) natural ligand 22-hydroxycholesterol (22OH-C) was added to media after 16 h of serum depletion. Cells were harvested after 6 h of pharmacological treatment, and total RNA was analyzed by quantitative RT-PCR for mRNA expression of LXRα and Renin. Data are -fold changes versus the control siRNA group. Data are the mean ± S.E.; n = 5 in each group; *, p < 0.05 versus control siRNA group.
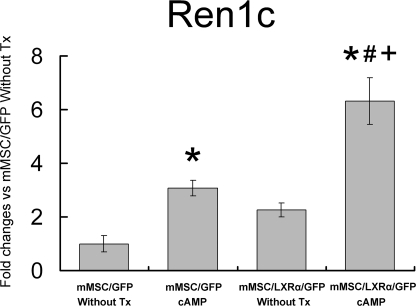
Murine MSCs Overexpressing LXRα with cAMP Treatment Further Stimulate Ren1c Gene Expression
We next generated murine MSC cell lines stably expressing GFP-LXRα by retroviral infection. Cell lines expressing GFP alone were also generated as a control. As shown in Table 1, murine MSCs overexpressing GFP-LXRα (mMSC/LXRα/GFP) exhibited a 178-fold increase in LXRα expression compared with murine MSCs overexpressing GFP alone (mMSC/GFP) (p < 0.05). There was no significant difference in LXRβ expression between these cell lines (Table 1). Murine MSC/LXRα/GFP and mMSC/GFP were treated with or without cAMP for 6 h after serum depletion for 16 h. Quantitative RT-PCR showed that there were significant differences (p < 0.05 by analysis of variance) in the expression of Ren1c among the mMSC/GFP without treatment (Tx), mMSC/GFP with cAMP Tx, mMSC/LXRα/GFP without Tx, and mMSC/LXRα/GFP with cAMP Tx groups (Fig. 5). As shown in Fig. 5, post hoc Fisher's least significant difference test revealed that mMSC/LXRα/GFP with cAMP treatment exhibited significantly higher renin mRNA levels (6.3 ± 0.9-fold changes for mMSC/GFP without Tx; p < 0.05) than mMSC/GFP with or without cAMP Tx (3.1 ± 0.3- and 1.0 ± 0.3-fold changes for mMSC/GFP without Tx, respectively; p < 0.05) and mMSC/LXRα/GFP without Tx (2.3 ± 0.3-fold changes for mMSC/GFP without Tx; p < 0.05).
TABLE 1.
Expression of LXRα and LXRβ in murine MSC cell lines stably overexpressing GFP (mMSC/GFP) or GFP-LXRα (mMSC/LXRα/GFP)
Murine MSC cell lines stably overexpressing GFP (mMSC/GFP) or GFP-LXRα (mMSC/LXRα/GFP) were generated by retroviral infection. Cells were harvested, and total RNA was analyzed by quantitative RT-PCR for mRNA expression of LXRα or LXRβ. Data are -fold changes versus mMSC/GFP. Data are the mean ± S.E.; n = 3; NS = statistically non-significant.
| mMSC/GFP | mMSC/LXRα/GFP | p value | |
|---|---|---|---|
| LXRα | 1.00 ± 0.12 | 177.59 ± 32.72 | p < 0.05 |
| LXRβ | 1.00 ± 0.20 | 1.16 ± 0.25 | NS |
FIGURE 5.
Effect of cAMP on the expression of Ren1c gene in mMSC/GFP and mMSC/LXRα/GFP. One mmol/liter 8-bromo-cyclic AMP (cAMP) was added to media after 16 h of serum depletion. Murine MSCs overexpressing GFP (mMSC/GFP) or GFP-LXRα (mMSC/LXRα/GFP) with or without cAMP treatment were harvested after 6 h of pharmacological treatment, and total RNA was analyzed by quantitative RT-PCR for mRNA expression of Ren1c. Without Tx, without the treatment group. Data are -fold changes versus mMSC/GFP without Tx. Data are the mean ± S.E.; n = 3. *, p < 0.05 versus mMSC/GFP without Tx. #, p < 0.05 versus mMSC/GFP, cAMP. +, p < 0.05 versus mMSC/LXRα/GFP without Tx.
Cyclic AMP or 22-Hydroxycholesterol Induces mMSC/LXRα/GFP into Renin-synthesizing Granulated Cells
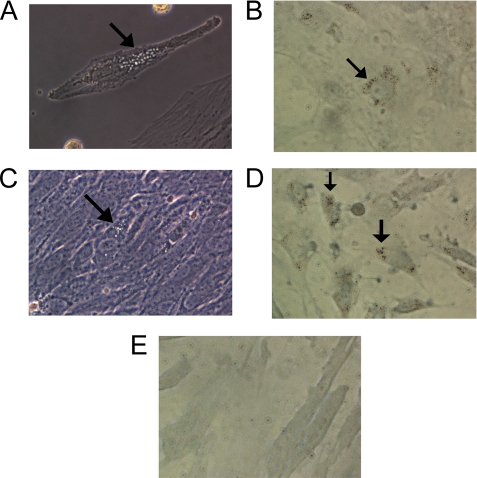
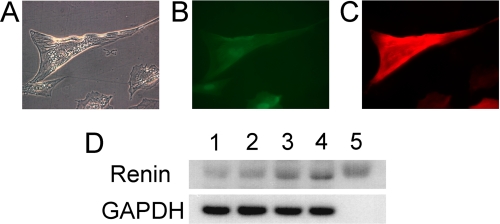
We treated mMSCs overexpressing GFP-LXRα (mMSC/LXRα/GFP) with cAMP (1 mmol/liter) or 22-hydroxycholesterol (0.1 μmol/liter) daily for 2–8 weeks to examine the phenotypic changes. Morphologically, about 10–20% of mMSC/LXRα/GFP became granulated cells after a couple of weeks of treatment of cAMP or 22-hydroxycholesterol (Fig. 6, A and C, respectively). We further performed immunocytochemical analysis using anti-renin antibody. The results showed that granules in cytoplasm exhibited positive staining for renin (Fig. 6, B and D). A negative control using isotype control immunoglobulin G showed no positive signal (Fig. 6E). Interestingly, we observed that many of the granulated cells were positive for α-smooth muscle actin as well. As shown in Fig. 7, A and B, a granulated cell induced from mMSC/LXRα/GFP with cAMP treatment for 5 weeks exhibited green fluorescence. Fig. 7C showed that the same cell was positive for α-smooth muscle actin.
FIGURE 6.
Phenotypic changes of mMSC/LXRα/GFP induced by the prolonged treatment with cAMP or 22-hydroxycholesterol. Prolonged treatment of mMSC/LXRα/GFP with cAMP (A, B, and E) or 22-hydroxycholesterol (C and D) was performed. The arrows show granules in cytoplasm (A, cAMP treatment for 3 weeks; C, 22-hydroxycholesterol treatment for 6 weeks). Immunocytochemistry for renin antibody exhibited that granules were positive for renin (B, cAMP treatment for 2 weeks; D, 22-hydroxycholesterol treatment for 6 weeks). E, negative control for renin staining using isotype control immunoglobulin G. Original magnification, ×400.
FIGURE 7.
Immunofluorescent staining for α-smooth muscle actin and immunoblotting for renin. A–C, murine MSCs overexpressing GFP-LXRα (mMSC/LXRα/GFP) were treated with cAMP for 5 weeks. A, microscopic findings showed granules in cytoplasm. B, cells induced from mMSC/LXRα/GFP exhibited green fluorescence. C, granulated cells were positive for α-smooth muscle actin. Original magnification, ×400. D, murine MSCs overexpressing GFP alone (mMSC/GFP) and murine MSCs overexpressing GFP-LXRα (mMSC/LXRα/GFP) were treated with or without cAMP daily for 8 weeks. Cell lysates were isolated and subjected to immunoblotting analysis using antibodies for renin and GAPDH. Lane 1, mMSC/GFP without treatment (Tx); lane 2, mMSC/GFP with cAMP Tx; lane 3, mMSC/LXRα/GFP without Tx; lane 4, mMSC/LXRα/GFP with cAMP Tx; lane 5, positive control (human renin recombinant protein).
Murine MSC/LXRα/GFP with cAMP Treatment Stimulates Renin Protein Accumulation
We also examined renin protein expression by immunoblotting as well. Previously, we have reported that MSCs have, at modest levels, renin gene expression (6). Consistent with it, control murine MSCs (mMSC/GFP without any treatment) showed renin protein expression at very low levels (Fig. 7D, lane 1). Murine MSC/LXRα/GFP and mMSC/GFP were treated with or without cAMP daily for 8 weeks. Murine MSC/LXRα/GFP with cAMP treatment showed higher renin protein levels than cells mMSC/GFP with or without cAMP treatment and mMSC/LXRα/GFP without treatment (Fig. 7D).
Renin-expressing Cells Release Active Renin
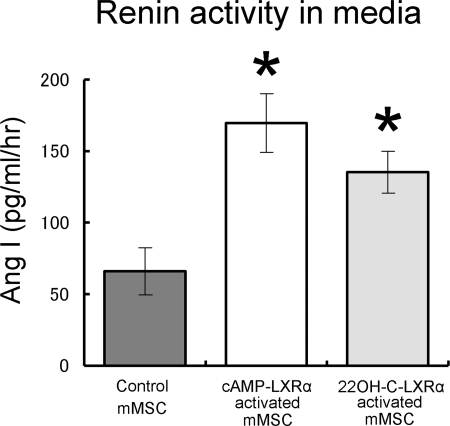
We measured active renin in conditioned media. Cyclic AMP-LXRα-activated mMSCs (mMSC/LXRα/GFP with cAMP treatment), 22-hydroxycholesterol-LXRα-activated mMSCs (mMSC/LXRα/GFP with 22-hydroxycholesterol treatment), and control MSCs (mMSC/GFP without treatment) were cultured for 8 weeks. Fig. 8 shows the cumulated renin activities in the culture media as measured by angiotensin I generation upon incubation of the samples (15 μl of conditioned media) with excess amounts of angiotensinogen for 60 min. To test for nonspecific degradation of the substrate, minimum essential medium α media were also incubated with the same amount of angiotensinogen for 60 min. No significant renin activity was detected from minimum essential medium α media (data not shown). As shown in Fig. 8, renin activity in conditioned media from cAMP-LXRα-activated mMSCs or 22-hydroxycholesterol-LXRα-activated mMSCs was significantly higher than that from the control mMSCs (169.6 ± 20.5 pg/ml/h, 135.2 ± 14.5 pg/ml/h, and 66.0 ± 16.5 pg/ml/h, respectively. p < 0.05).
FIGURE 8.
Renin activity in conditioned media. Cyclic AMP-LXRα-activated mMSCs (mMSC/LXRα/GFP with cAMP treatment), 22-hydroxycholesterol-LXRα-activated mMSCs (mMSC/LXRα/GFP with 22-hydroxycholesterol treatment), and control MSCs (mMSC/GFP without treatment) were cultured for 8 weeks. Conditioned media from cells were collected, and active free renin activity in conditioned media was measured by angiotensin I (Ang I) generation. Data are the mean ± S.E.; n = 6; *, p < 0.05 versus control mMSC.
DISCUSSION
JG cells are highly specialized endocrine cells that express renin in a cell-specific manner. JG cells account for less than 0.1% of all kidney cells. Renin is stored in granules and is secreted in response to specific stimuli. The biology and origin of JG cells remain as mysteries as they are endocrine cells that express α-smooth muscle actin and are coupled to vascular smooth muscle cells in the renal afferent arterioles. Are they endocrine cells that have a unique contractile phenotype, or are they derived from vascular smooth muscle cells that have acquired endocrine characteristics? Not much is known of the biology of JG cells because they are difficult to maintain in culture. In vitro they rapidly lose the secretory phenotype and, thus, are impossible to study. Sigmund et al. (38) have successfully cloned renin-producing cells by transforming them with SV40 T antigen. These transformed cells have been useful for studies of renin gene transcription but provide little insight into the biology of JG cells.
Recently, Sequeira Lopez et al. reported that renin progenitor cells can be detected in the prevascular metanephric kidney (4, 5). These renin progenitor cells migrate to and become incorporated into the developing afferent arterioles. These cells become JG renin-producing cells and only later acquire smooth muscle markers. Furthermore, da Silva Meirelles et al. (39) reported that adult kidney stem cells exist within the glomerulus, and these stem cells are similar to bone marrow-derived MSCs. Because mesenchymal stem cells can differentiate into various tissues and play an important role in developmental biology, we hypothesize that MSC is the origin of JG cells. In this regard, we have recently demonstrated that adult human mesenchymal stem cells express at modest levels all the components of the renin-angiotensin system, including renin (6). In this study we examined for direct evidence that MSCs can be induced to differentiate into JG-like cells expressing high levels of renin and demonstrated that MSCs with sustained LXRα activation can differentiate into renin-secreting cells with α-smooth muscle expression.
The transcription control of renin gene expression has been studied by many investigators (8–10, 40–44), and cyclic AMP is assumed to be the main activator of renin gene expression (7). We have previously demonstrated the role of LXRα in renin gene expression using a clonal renin-expressing cell line, As4.1 cells, that is derived from a kidney JG tumor of a mouse renin promoter/SV40 T antigen transgenic mice (38). As4.1 cells overexpressing GFP-LXRα (As4.1/LXRα) showed higher renin gene expression than control As4.1 cells overexpressing GFP alone (As4.1/GFP) at basal levels (12, 13). Cyclic AMP treatment of As4.1/LXRα also exhibited higher renin gene up-regulation than the same treatment of As4.1/GFP with a maximum peak at 6 h post-stimulation (12, 13). We further demonstrated that the mechanism of renin gene up-regulation by cAMP-LXRα activation is through a unique conserved sequence in the renin promoter region termed the CNRE element, which contains a cAMP-responsive cis element and a negative regulatory element that share an overlapping sequence (8–12). We have examined the effect of LXRα on renin gene expression in vivo. β-Adrenergic stimulation, which is known to increase intracellular cAMP (7), induced renin gene up-regulation in mouse kidney (14). The administration of the synthetic LXRα agonist also significantly up-regulated renin gene expression accompanied by the up-regulation of Abca1 gene in mouse kidney (14). Importantly, the up-regulation of renin triggered by β-adrenergic stimulation was abolished in LXRα null mice (14). Moreover, we showed that JG cells were enriched in LXRα gene expression, displaying the highest levels of LXRα gene expression in the mouse kidney cortex (14). These results demonstrated that LXRα plays an important role in the regulation of renin expression in vivo, although other factors may cooperate with it. In this study we examined the effect of sustained LXRα activation on MSC renin expression. Upon prolonged stimulation of LXRα-overexpressing MSCs with cAMP or LXRα natural ligand 22-hydroxycholesterol, we observed the MSCs differentiated into renin-containing cells with secretory granules and α-smooth muscle actin staining, which is characteristic of JG cells. Taken together, our data would suggest that LXRα plays an important role not only in renin expression but also in renin-containing cell differentiation. It is conceivable that during specific stages of embryonic development, the milieu in the kidney favors significant local LXRα activation that induces MSC renin expression and renin-containing cell differentiation. Further experiments are necessary to address this interesting biological question.
In the adult kidney, in response to chronic stimuli (such as chronic ischemia, prolonged adrenergic activation, and sodium depletion), JG cells exhibit the enigmatic property of undergoing “hyperplasia” while maintaining the ability to express high levels of renin. Although the exact cellular and genetic mechanisms of JG cell hyperplasia remain as mysteries, we (45) and Owen et al. (3) have reported that bromodeoxyuridine incorporation into JG cells was increased during JG cell hyperplasia. Furthermore, we demonstrated colocalization of renin and bromodeoxyuridine staining in JG hyperplasia. These data would suggest that the increased renin gene expression in JG cell hyperplasia involves not only an increase in renin expression per cell but also an increase in the number of cells that express renin. In other words, JG cell hyperplasia possibly involves both differentiation and proliferation. In this regard, recent studies suggest that adult renal stem cells exist in the kidney and are a potential source of cells when the body needs them (39, 46–49). In addition, Yokoo et al. (50) reported that human MSCs, which were injected into rodent embryos, differentiated and contributed to the structures of the developing kidney. Morigi et al. (51) also demonstrated that intravenously injected bone marrow MSCs were engrafted to tubular epithelial cells and protected the kidney from tubular injury. Furthermore, da Silva Meirelles et al. (39) reported that adult renal stem cells exist within the glomerulus, and these stem cells are similar to bone marrow-derived MSCs. Taken together, we speculate MSCs of either renal or extrarenal origin might play a role in JG cell hyperplasia in pathological conditions.
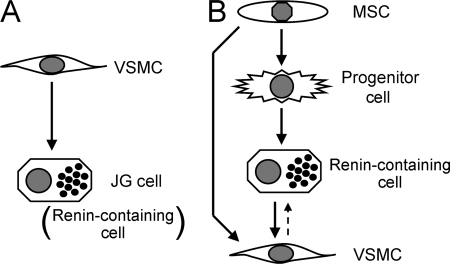
In conclusion, we have shown that LXRα agonists up-regulate renin mRNA expression in MSCs and that murine MSCs overexpressing LXRα (mMSC/LXRα/GFP) with cAMP treatment exhibit renin granule formation and stimulate renin expression and release. Our data demonstrate that the activation of LXRα induces murine MSCs differentiation into renin-producing cells with α-smooth muscle actin expression and suggest that these cells may be the origin of juxtaglomerular renin-producing cells in the kidney (Fig. 9).
FIGURE 9.
Hypotheses of the lineage of renin-containing cell. A, shown is a traditional hypothesis. The JG cell (Renin-containing cell) is hypothesized to be derived from the vascular smooth muscle cell (VSMC) through metaplastic transformation. B, shown is a new hypothesis. Renin progenitor cell may exist, and the renin-containing cell can give rise to vascular smooth muscle cells. The MSC may be the origin of renin-containing cell.
Acknowledgments
We thank Hui Mu and Tomoko Masuda for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL35610, HL58516, HL72010, and HL73219 from NHLBI (to V. J. D.). This work was also supported by the Edna Mandel Foundation and the Leducq Foundation (to V. J. D.) and in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI 21790745, to K. M.).
- JG
- juxtaglomerular
- MSC
- mesenchymal stem cell
- mMSC
- murine MSC
- hMSC
- human MSC
- LXRα
- liver X receptor-α
- PBS
- phosphate-buffered saline
- FACS
- fluorescence-activated Cell Sorting
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Tx
- treatment
- RT
- reverse transcription
- siRNA
- small interfering RNA
- GFP
- green fluorescent protein
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1.Ice K. S., Geary K. M., Gomez R. A., Johns D. W., Peach M. J., Carey R. M. (1988) Clin. Exp. Hypertens. A 10, 1169–1187 [DOI] [PubMed] [Google Scholar]
- 2.Kon Y., Hashimoto Y., Murakami K., Sugimura M. (1992) Acta Anat. 144, 354–362 [DOI] [PubMed] [Google Scholar]
- 3.Owen R. A., Molon-Noblot S., Hubert M. F., Kindt M. V., Keenan K. P., Eydelloth R. S. (1995) Toxicol. Pathol. 23, 606–619 [DOI] [PubMed] [Google Scholar]
- 4.Sequeira López M. L., Pentz E. S., Nomasa T., Smithies O., Gomez R. A. (2004) Dev. Cell 6, 719–728 [DOI] [PubMed] [Google Scholar]
- 5.Sequeira Lopez M. L., Pentz E. S., Robert B., Abrahamson D. R., Gomez R. A. (2001) Am. J. Physiol. Renal. Physiol. 281, F345–F356 [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K., Wu Y., Okamoto Y., Pratt R. E., Dzau V. J. (2006) Hypertension 48, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 7.Bader M., Ganten D. (2000) J. Mol. Med. 78, 130–139 [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi M., Nakamura N., Tang S. S., Barrett G., Dzau V. J. (1991) J. Biol. Chem. 266, 16247–16254 [PubMed] [Google Scholar]
- 9.Horiuchi M., Pratt R. E., Nakamura N., Dzau V. J. (1993) J. Clin. Invest. 92, 1805–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura N., Burt D. W., Paul M., Dzau V. J. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt D. W., Nakamura N., Kelley P., Dzau V. J. (1989) J. Biol. Chem. 264, 7357–7362 [PubMed] [Google Scholar]
- 12.Tamura K., Chen Y. E., Horiuchi M., Chen Q., Daviet L., Yang Z., Lopez-Ilasaca M., Mu H., Pratt R. E., Dzau V. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8513–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson L. M., Choe S. E., Yukhananov R. Y., Hopfner R. L., Church G. M., Pratt R. E., Dzau V. J. (2003) J. Biol. Chem. 278, 15252–15260 [DOI] [PubMed] [Google Scholar]
- 14.Morello F., de Boer R. A., Steffensen K. R., Gnecchi M., Chisholm J. W., Boomsma F., Anderson L. M., Lawn R. M., Gustafsson J. A., Lopez-Ilasaca M., Pratt R. E., Dzau V. J. (2005) J. Clin. Invest. 115, 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K., Zhang Z., Pratt R. E., Dzau V. J. (2007) J. Am. Soc. Hypertens. 1, 164–168 [DOI] [PubMed] [Google Scholar]
- 16.Bruder S. P., Jaiswal N., Haynesworth S. E. (1997) J. Cell. Biochem. 64, 278–294 [DOI] [PubMed] [Google Scholar]
- 17.Colter D. C., Class R., DiGirolamo C. M., Prockop D. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 3213–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangi A. A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J. S., Dzau V. J. (2003) Nat. Med. 9, 1195–1201 [DOI] [PubMed] [Google Scholar]
- 19.Mirotsou M., Zhang Z., Deb A., Zhang L., Gnecchi M., Noiseux N., Mu H., Pachori A., Dzau V. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira R. F., Halford K. W., O'Hara M. D., Leeper D. B., Sokolov B. P., Pollard M. D., Bagasra O., Prockop D. J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4857–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prockop D. J. (1997) Science 276, 71–74 [DOI] [PubMed] [Google Scholar]
- 22.Janke J., Engeli S., Gorzelniak K., Luft F. C., Sharma A. M. (2002) Diabetes 51, 1699–1707 [DOI] [PubMed] [Google Scholar]
- 23.Schoof E., Girstl M., Frobenius W., Kirschbaum M., Dörr H. G., Rascher W., Dötsch J. (2001) J. Clin. Endocrinol. Metab. 86, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 24.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 25.Ahn D., Ge Y., Stricklett P. K., Gill P., Taylor D., Hughes A. K., Yanagisawa M., Miller L., Nelson R. D., Kohan D. E. (2004) J. Clin. Invest. 114, 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujino T., Nakagawa N., Yuhki K., Hara A., Yamada T., Takayama K., Kuriyama S., Hosoki Y., Takahata O., Taniguchi T., Fukuzawa J., Hasebe N., Kikuchi K., Narumiya S., Ushikubi F. (2004) J. Clin. Invest. 114, 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantelme P., Rohrwasser A., Gociman B., Hillas E., Cheng T., Petty G., Thomas J., Xiao S., Ishigami T., Herrmann T., Terreros D. A., Ward K., Lalouel J. M. (2002) Hypertension 39, 1007–1014 [DOI] [PubMed] [Google Scholar]
- 28.Mackins C. J., Kano S., Seyedi N., Schäfer U., Reid A. C., Machida T., Silver R. B., Levi R. (2006) J. Clin. Invest. 116, 1063–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ip J. E., Wu Y., Huang J., Zhang L., Pratt R. E., Dzau V. J. (2007) Mol. Biol. Cell 18, 2873–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz G., Langmann T. (2005) Biochim. Biophys. Acta 1735, 1–19 [DOI] [PubMed] [Google Scholar]
- 31.Björkhem I., Diczfalusy U. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 734–742 [DOI] [PubMed] [Google Scholar]
- 32.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. (1996) Nature 383, 728–731 [DOI] [PubMed] [Google Scholar]
- 33.Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. (1997) J. Biol. Chem. 272, 3137–3140 [DOI] [PubMed] [Google Scholar]
- 34.Repa J. J., Mangelsdorf D. J. (2000) Annu. Rev. Cell Dev. Biol. 16, 459–481 [DOI] [PubMed] [Google Scholar]
- 35.Whitney K. D., Watson M. A., Goodwin B., Galardi C. M., Maglich J. M., Wilson J. G., Willson T. M., Collins J. L., Kliewer S. A. (2001) J. Biol. Chem. 276, 43509–43515 [DOI] [PubMed] [Google Scholar]
- 36.Lang J. A., Yang G., Kern J. A., Sigmund C. D. (1995) Hypertension 25, 704–710 [DOI] [PubMed] [Google Scholar]
- 37.Tamura K., Chen Y. E., Tanaka Y., Sakai M., Tsurumi Y., Koide Y., Kihara M., Pratt R. E., Horiuchi M., Umemura S., Dzau V. J. (2004) Mol. Cell. Endocrinol. 224, 11–20 [DOI] [PubMed] [Google Scholar]
- 38.Sigmund C. D., Okuyama K., Ingelfinger J., Jones C. A., Mullins J. J., Kane C., Kim U., Wu C. Z., Kenny L., Rustum Y., Dzau V. J., Gross K. W. (1990) J. Biol. Chem. 265, 19916–19922 [PubMed] [Google Scholar]
- 39.da Silva Meirelles L., Chagastelles P. C., Nardi N. B. (2006) J. Cell Sci. 119, 2204–2213 [DOI] [PubMed] [Google Scholar]
- 40.Catanzaro D. F., Sun J., Gilbert M. T., Yan Y., Black T., Sigmund C., Gross K. W. (1994) Kidney Int. 46, 1513–1515 [DOI] [PubMed] [Google Scholar]
- 41.Germain S., Konoshita T., Philippe J., Corvol P., Pinet F. (1996) Biochem. J. 316, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan L., Black T. A., Shi Q., Jones C. A., Petrovic N., Loudon J., Kane C., Sigmund C. D., Gross K. W. (2001) J. Biol. Chem. 276, 45530–45538 [DOI] [PubMed] [Google Scholar]
- 43.Pan L., Gross K. W. (2005) Hypertension 45, 3–8 [DOI] [PubMed] [Google Scholar]
- 44.Tamura K., Umemura S., Yamaguchi S., Iwamoto T., Kobayashi S., Fukamizu A., Murakami K., Ishii M. (1994) J. Clin. Invest. 94, 1959–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Wang H., Matsushita K., Pratt R. E., Dzau V. J. (2007) Hypertension 50, e100 [Google Scholar]
- 46.Al-Awqati Q., Oliver J. A. (2002) Kidney Int. 61, 387–395 [DOI] [PubMed] [Google Scholar]
- 47.Anglani F., Forino M., Del Prete D., Tosetto E., Torregrossa R., D'Angelo A. (2004) J. Cell Mol. Med. 8, 474–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver J. A., Maarouf O., Cheema F. H., Martens T. P., Al-Awqati Q. (2004) J. Clin. Invest. 114, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watorek E., Klinger M. (2006) Arch. Immunol. Ther. Exp. 54, 45–50 [DOI] [PubMed] [Google Scholar]
- 50.Yokoo T., Ohashi T., Shen J. S., Sakurai K., Miyazaki Y., Utsunomiya Y., Takahashi M., Terada Y., Eto Y., Kawamura T., Osumi N., Hosoya T. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3296–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morigi M., Imberti B., Zoja C., Corna D., Tomasoni S., Abbate M., Rottoli D., Angioletti S., Benigni A., Perico N., Alison M., Remuzzi G. (2004) J. Am. Soc. Nephrol. 15, 1794–1804 [DOI] [PubMed] [Google Scholar]