Abstract
Rapamycin is a macrolide antibiotic that inhibits vascular smooth muscle cell proliferation and migration and that is used clinically on drug-eluting stents to inhibit in-stent restenosis. Although inhibition of cell migration is an asset in preventing restenosis, it also leads to impaired stent endothelialization, a significant limitation of current drug-eluting stent technology that necessitates prolonged antiplatelet therapy. We measured the ability of rapamycin to inhibit the migration of human umbilical vein endothelial cells (HUVECs) and human coronary artery endothelial cells (HCAEC) toward the chemoattractant vascular endothelial cell growth factor. Although acute administration of rapamycin had no effect, exposure for 24 h inhibited HUVEC and HCAEC migration. Disruption of the mTORC2 via small interfering RNA was also effective in inhibiting HCAEC migration. Treatment of HCAECs for this period with rapamycin produced an increase in the cyclin-dependent kinase inhibitor p27Kip, through a decrease in the targeting of the protein for degradation by phosphorylation at Thr187. ECs isolated from a knock-in mouse expressing p27Kip1 with a mutation of this residue to an alanine, blocking this phosphorylation, exhibited reduced migration compared with wild-type controls. Silencing of p27Kip1 with small interfering RNA blocked the effects of rapamycin on migration and tube formation as well as RhoA activation and cytoskeletal reorganization. We conclude that prolonged exposure of ECs to rapamycin increases p27Kip1 and in turn inhibits RhoA activation, blocking cell migration and differentiation. These data elucidate the molecular mechanism underlying regulation of p27Kip1 protein and cell migration by rapamycin.
Keywords: Cell/Chemotaxis, Cell/Migration, Chemotaxis, Growth Factors, Phosphorylation/Kinases/Tyrosine, Signal Transduction, Tissue/Organ Systems/Endothelium
Introduction
Re-endothelialization is a key step in the healing of an artery following vascular injury, including that of percutaneous coronary interventions. Stents eluting rapamycin and its analogs have proven effective at preventing neointimal growth following angioplasty (1), but the antimigratory properties of these agents prevent stent endothelialization, resulting in late stent thrombosis (2). Late stent thrombosis has emerged as a major factor diminishing the benefits of drug-eluting stents, highlighting the need for a better understanding of the antimigratory mechanism of rapamycin and its analogs (3–6).
Rapamycin, when bound to its intercellular receptor, FKBP12 (FK506-binding protein-12), inhibits the activity of the two mammalian target of rapamycin (mTOR)3 complexes. mTORC1 consists of mTOR, the rapamycin-sensitive adapter protein of mTOR (raptor), and mLST8 (also known as GβL) (7). This complex regulates ribosomal biogenesis and protein synthesis through activation of p70S6K to initiate ribosomal S6 kinase and inhibition of the ability of 4E-BP1 (4E-binding protein-1) to restrict mRNA translation (8, 9). mTORC2 is defined by the presence of the rapamycin-insensitive companion of mTOR (rictor), SIN1 (stress-activated protein kinase-interacting protein-1), and mLST8. mTORC2 is linked to actin cytoskeleton regulation through phosphorylation of the protein kinase Akt (protein kinase B) (10–14). Inhibition of mTORC1 occurs immediately following binding of rapamycin to FKBP12, whereas prolonged exposure to rapamycin is required to block formation of nascent mTORC2 (11).
Inhibition of mTOR has been shown to block the actions of a critical regulator of angiogenesis and endothelial cell (EC) migration, vascular endothelial cell growth factor A (VEGF), through both inhibition of VEGF synthesis and signal transduction (15, 16). Treatment with rapamycin also blocks mitogen-induced down-regulation of the cyclin-dependent kinase inhibitor p27Kip1 through an unknown mechanism (17, 18). In G1 phase, a decrease in p27Kip1 protein levels releases cyclin E/cyclin-dependent kinase 2 complexes to hyperphosphorylate pRb (retinoblastoma protein), resulting in the transcription of genes required for the G1/S transition (19). Beyond cell cycle regulation, p27Kip1 has also been shown to regulate migration directly through its ability to bind and block activation of the small GTPase, RhoA (20). Vascular smooth muscle cells (VSMCs) lacking p27Kip1 exhibit a relative rapamycin resistance (21).
Here we report that inhibition of mTORC2 through prolonged exposure of ECs to rapamycin increases p27Kip1, resulting in inhibition of VEGF-directed endothelial cell chemotaxis and differentiation. The elevation in p27Kip1 protein occurs through an inhibition in the phosphorylation of p27Kip1 at a specific residue, Thr187, that targets the protein for degradation and results in an inhibition of activation of the small Rho GTPase RhoA and cytoskeletal reorganization. These data extend our understanding of the molecular mechanisms behind the regulation of p27Kip1 and cell migration.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant human vascular endothelial cell growth factor A-165 was purchased from Upstate Biotechnology (Waltham, MA). Paclitaxel and rapamycin were purchased from LC Laboratories (Woburn, MA).
Cell Culture
Human endothelial cells, growth medium (EGM-2 and EGM-2MV), and basal endothelial cell medium (EBM-2) were purchased from Lonza (Basel, Switzerland). Human umbilical vein endothelial cells (HUVECs) and human coronary artery endothelial cells (HCAECs) were subcultured in EGM-2 or EGM-2MV, respectively, at 37 °C in a humidified 5% CO2 atmosphere with the medium replaced every ∼48 h. Murine ECs were isolated from wild-type and knock-in mice (n = 3, p27Kip1T187A) homozygous for a mutation of Thr187 of p27Kip1 to alanine (22) according to the method of Kobayashi et al. (23) with minor modification and subcultured in a similar manner in basal medium, Dulbecco's modified Eagle's medium, supplemented with 20% fetal bovine serum (Invitrogen), 80 μg/ml streptomycin, 80 units/ml penicillin, 100 μg/ml heparin, and 100 μg/ml endothelial cell growth supplement (Sigma-Aldrich). Greater than 90% purity of the murine EC isolations was confirmed by positive staining for CD31. Only cells less than passage 8 were used. For starvation, ECs were incubated in basal medium supplemented with 0.5% fetal bovine serum, and for serum stimulation, ECs were incubated in basal medium supplemented with 20% fetal bovine serum. For experiments including proteasome inhibition, MG-132 (Sigma-Aldrich) was added to the medium 8 h prior to cell harvest at a concentration of 20 μmol/liter.
Small Interfering RNA (siRNA) Protocol
Oligonucleotides and transfection reagent were purchased from Dharmacon, Inc. (Chicago, IL). The siRNAs targeting p27Kip1, rictor, and raptor were the predesigned ON-TARGETplus SMARTpool set of four oligonucleotides (Qiagen, Inc., Valencia, CA). HCAECs were incubated for 24 h prior to rapamycin treatment in EBM-2 supplemented with 20% fetal bovine serum, 20 nmol/liter siRNA, and 500 ng/liter DharmaFECT. Transfection efficiency was measured in parallel cultures transfected as described above with siGLO fluorescent siRNA and compared with mock transfection. Efficiency was maintained at >95%.
Migration Assay
Transwell cell inserts (BD Biosciences) with 8-μm pores were coated with 250 μg/ml Matrigel (BD Biosciences). ECs at less than 50% confluence were pretreated with rapamycin in normal growth medium for up to 48 h prior to seeding onto the cell inserts. For studies on the acute effects of paclitaxel (10 nmol/liter) and rapamycin (100 nmol/liter), drug was added to an aliquot of the untreated cells immediately prior to seeding. ECs (2 × 105) were seeded into the cell inserts and incubated for 4 h at 37 °C and 5% CO2 over medium containing chemoattractant (VEGF, 10 ng/ml) and 0.1% bovine serum albumin. Unmigrated ECs were scraped from the upper side of the inserts, and the migrated ECs were fixed in 10% zinc formalin and visualized with a hematoxylin stain (Fisher). Data are expressed as the average of four high-power fields of triplicate samples after subtraction of the average of cells migrating toward medium without chemoattractant.
Tube Formation Assay
HCAECs were treated with siRNA as described above and then treated with either vehicle or 100 nmol/liter rapamycin for 24 h. Following the pretreatment, HCAECs were seeded in 96-well tissue culture plates coated with 50 μl of Matrigel at 25,000 cells/well and incubated in EBM-2 for 24 h. Four high-power random fields were obtained, and tube formation was quantified by determining the total tube length in each field using ImageJ analysis software (24).
RhoA Activity Assay
For measurement of RhoA activity, HCAECs were seeded in 150-mm tissue culture dishes and treated with siRNA and/or rapamycin as indicated. RhoA activity was measured in cells harvested from the 150-mm culture dishes with the G-LISA™ RhoA Activation Assay Biochem Kit (Cytoskeleton, Inc., Denver, CO) according to the manufacturer's instructions.
Western Blots
ECs were grown under identical conditions as in the migration assays. Western blots were prepared as described previously (21) and probed with antibodies for p27Kip1 (BD Biosciences), p27Kip1 phosphorylated at Thr187 (Santa Cruz Biotechnology, Santa Cruz, CA), pRb, rictor, raptor, mTOR, and β-actin (Cell Signaling Technology, Danvers, MA).
Protein Half-life Measurements
HCAECs were incubated in EGM-2MV medium for 24 h with either vehicle or 100 nmol/liter rapamycin. The cells were then treated with cycloheximide (20 μmol/liter; EMD Chemicals, Inc., San Diego, CA) for the indicated times, and lysates were prepared as described above. Protein content was determined by the Bradford assay, and p27Kip1 content was measured using an enzyme-linked immunoassay (R&D Systems, Minneapolis, MN). Protein half-life was calculated as t½ = ln(2)/k, where k is the first-order rate of decay constant. Results were confirmed by Western blotting.
Actin Stress Fiber Visualization
HCAECs were seeded on tissue culture chamber slides and pretreated as described with siRNA and rapamycin. The medium was then replaced with EBM-2 supplemented with 10 ng/ml VEGF or vehicle and incubated for 2 h. Cells were permeabilized with 0.5% Triton X-100 and stained with 1 unit of Alexa Fluor 647-phalloidin (Invitrogen) in phosphate-buffered saline containing 1% bovine serum albumin. Cells were mounted with Prolong Gold with 4′,6-diamidino-2-phenylindole (Invitrogen) to visualize the nuclei. Images were taken using an Axiovert 200M deconvoluting microscope (Carl Zeiss, Inc., Thornwood, NY). Four high-power fields were obtained from each treatment group to calculate the percentage of HCAECs exhibiting actin stress fiber formation.
Statistics
All data are expressed as the means ± S.E. Statistical analysis between groups was performed using one-way analysis of variance, and Tukey's honestly significant difference test was used to compare the individual mean values. A p value <0.05 was considered significant.
RESULTS
Inhibition of mTORC2, but Not mTORC1, Inhibits EC Migration
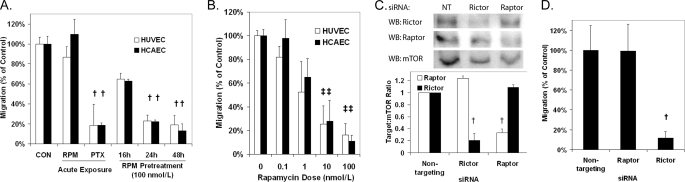
The ability of rapamycin to inhibit the migration of HUVECs and HCAECs toward VEGF was measured using Transwell migration assays. In contrast to a positive control, paclitaxel, acute addition of rapamycin to the migration chamber at the time of seeding was ineffective in inhibiting migration (Fig. 1A). Rapamycin was effective in inhibiting migration when ECs were incubated with the drug for 24–48 h prior to seeding into the migration chamber. The antimigratory effects of pretreatment with rapamycin were dose-dependent with an IC50 of ∼1–2 nmol/liter (Fig. 1B) and were not associated with a change in cell attachment (supplemental Fig. S1). Because the pretreatment period was required, we hypothesized that the effects of mTOR inhibition on EC migration were mediated through mTORC2. Using siRNAs targeting the mTOR complex proteins, raptor (mTORC1) and rictor (mTORC2), we depleted HCAECs of one of the two mTORCs (Fig. 1C) and measured migration toward VEGF. Knockdown of rictor, but not raptor, was effective in inhibiting HCAEC migration toward VEGF compared with HCAECs treated with nontargeting siRNA (Fig. 1D). These data suggest that mTOR regulates EC chemotaxis toward VEGF through mTORC2 and not mTORC1.
FIGURE 1.
Inhibition of mTORC2 with rapamycin pretreatment or siRNA blocks EC migration toward VEGF (10 ng/ml). A, migration of HUVECs and HCAECs following treatment with rapamycin (RPM; 100 nmol/liter) or paclitaxel (PTX; 10 nmol/liter) for the indicated time. B, migration of HUVECs and HCAECs following 48 h of treatment with the indicated doses of rapamycin. C, transfection with siRNA targeting rictor and raptor significantly reduces the target protein in HCAECs compared with nontargeting (NT) siRNA. D, migration of HCAECs following pretreatment with raptor, rictor, or nontargeting siRNA. † and ‡, p < 0.01 and p < 0.05, respectively, compared with the control. Error bars represent the mean ± S.E. (n = 3). WB, Western blot.
Rapamycin Treatment Increases p27Kip1
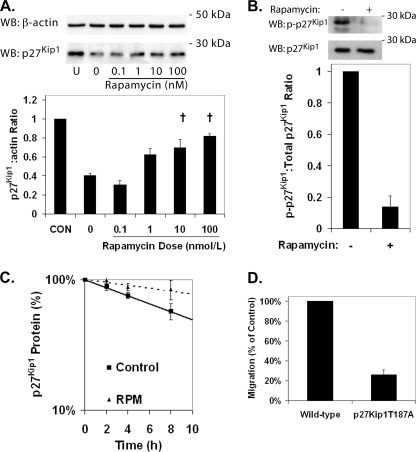
Pretreatment of HCAECs with rapamycin for 24 h increased p27Kip1 protein levels in a dose-dependent manner (Fig. 2A). Phosphorylation of Thr187 targets p27Kip1 for ubiquitinylation and proteasome-dependent degradation. In HCAECs treated with a proteasome inhibitor (MG-132) to allow assessment of p27Kip1 phosphorylation in the absence of degradation, phosphorylation of Thr187 of p27Kip1 was reduced following treatment with rapamycin (Fig. 2B). Measurement of p27Kip1 protein in HCAECs treated with cycloheximide to block nascent p27Kip1 protein translation demonstrated that pretreatment with rapamycin significantly increased the half-life of p27Kip1 (Fig. 2C) from 9.8 ± 0.3 h to 26.7 ± 7.4 h. Degradation of p27Kip1 protein appears to be the dominant mechanism for regulation of p27Kip1 by mTOR because mRNA encoding p27Kip1 remained unchanged by rapamycin treatment (supplemental Fig. S2). We confirmed the importance of phosphorylation of Thr187 to EC migration using ECs isolated from a knock-in mouse expressing p27Kip1 where the Thr187 has been mutated to an alanine (22). The p27Kip1T187A ECs exhibited significantly reduced migration compared with wild-type controls (Fig. 2D). These data suggest that pretreatment of ECs with rapamycin increases p27Kip1 levels via inhibition of phosphorylation targeting the protein for proteasome-dependent degradation.
FIGURE 2.
Rapamycin treatment increases the level of p27Kip1 protein in HCAECs through inhibition of its degradation. HCAECs were starved for 72 h and then serum-stimulated in the presence of rapamycin for 24 h. A, representative Western blots (WB) and densitometric analysis for p27Kip1 and β-actin in HCAECs treated with increasing doses of rapamycin. Error bars represent the mean ± S.E. (n = 3) of the ratio of p27Kip1 to β-actin normalized to the unstimulated control (CON). †, p < 0.05 compared with samples treated with vehicle alone (0). B, representative Western blot and densitometric analysis demonstrating the inhibition of phosphorylation of p27Kip1 at Thr187 in HCAECs treated with rapamycin (100 nmol/liter) and MG-132. Error bars represent mean ± S.E. (n = 3) relative to controls. †, p < 0.01. C, comparison of p27Kip1 protein half-life in HCAECs pretreated with either vehicle or 100 nmol/liter rapamycin (RPM) for 24 h. Data points represent mean ± S.E. (n = 3). D, shown is migration of wild-type and p27Kip1T187A ECs toward VEGF (10 ng/ml). Error bars represent mean ± S.E. (n = 3).
Down-regulation of p27Kip1 Reduces Rapamycin Inhibition of EC Migration
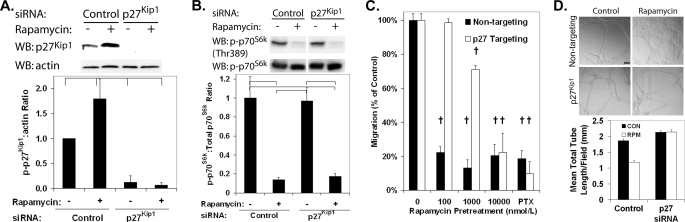
The dependence of the rapamycin antimigratory effects on p27Kip1 was confirmed using HCAECs depleted of p27Kip1 through transfection with siRNA (Fig. 3A). Down-regulation of p27Kip1 protein did not alter the ability of rapamycin to inhibit mTOR function because phosphorylation of p70S6K was reduced in HCAECs treated with both p27Kip1-targeting siRNA and rapamycin (Fig. 3B). Treatment of HCAECs with p27Kip1 siRNA induced the relative resistance to the antimigratory effect of rapamycin with the IC50 shifting to ∼2000 nmol/liter (Fig. 3C). No significant difference was observed in the total number of migrating cells between the HCAECs treated with nontargeting and p27Kip1-targeting siRNA. In a similar manner, rapamycin was effective in inhibiting HCAEC differentiation stimulated by growth on Matrigel in a p27Kip1-dependent manner. The formation of capillary-like tubes, as opposed to a cell monolayer, following growth on Matrigel was inhibited by rapamycin treatment in control HCAECs (Fig. 3D). In HCAECs treated with siRNA reducing p27Kip1 levels, rapamycin was ineffective at reducing tube formation. These data demonstrate that rapamycin inhibits HCAEC migration and differentiation, in part through an increase in p27Kip1 protein levels.
FIGURE 3.
Pretreatment with siRNA targeting p27Kip1 abolishes the effect of rapamycin on EC migration and angiogenesis. HCAECs were serum-stimulated for 24 h with nontargeting siRNA or siRNA targeting p27Kip1, followed by incubation for 24 h in medium supplemented with either rapamycin (100 nmol/liter) or vehicle. A, representative Western blot (WB) and densitometric analysis demonstrating the down-regulation of p27Kip1 protein by siRNA. Error bars represent mean ± S.E. (n = 3) of p27Kip1 to β-actin levels relative to HCAECs treated with vehicle and control siRNA. Brackets indicate p < 0.05. B, representative Western blot and densitometric analysis demonstrating that down-regulation of p27Kip1 protein by siRNA does not alter the effect of rapamycin on p70S6K phosphorylation. Error bars represent mean ± S.E. (n = 3) of phospho-p70S6K to total p70S6K protein levels relative to treatment with vehicle and control siRNA. Brackets indicate p < 0.05. C, migration of HCAECs treated with either p27Kip1-targeting or control siRNA toward VEGF (10 ng/ml) in the presence of increasing doses of rapamycin or a positive control (paclitaxel (PTX)). Error bars represent mean ± S.E. (n = 3) normalized to the vehicle sample. †, p < 0.01. D, representative micrographs (10×) and quantification of tube formation induced by growth on Matrigel of HCAECs treated with either p27Kip1-targeting or control (CON) siRNA and either vehicle or 100 nmol/liter rapamycin (RPM). Error bars represent mean ± S.E. (n = 3). The scale bar indicates 100 μm. †, p < 0.01.
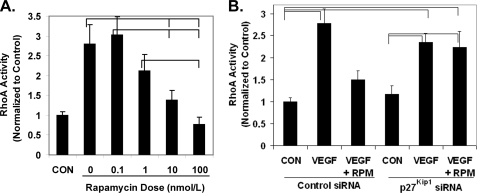
Rapamycin Inhibits RhoA Activation and Cytoskeletal Reorganization in a p27Kip1-dependent Manner
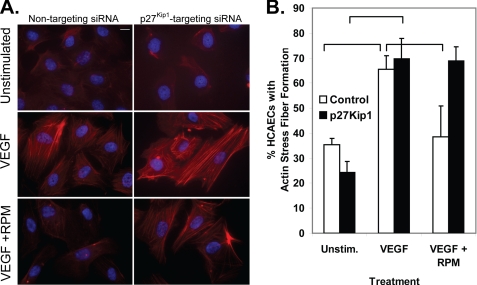
As early work on p27Kip1 focused on its ability to bind cyclin E/cyclin-dependent kinase 2 complexes and inhibit the phosphorylation of pRb (19), we verified that rapamycin treatment of HCAECs does inhibit pRb phosphorylation (supplemental Fig. S3). Of interest to this report is that cytoplasmic p27Kip1 protein is able to bind and inhibit the small GTPase RhoA, which is a critical mediator of cell migration (20). The ability of VEGF to stimulate RhoA activity decreased in a dose-dependent manner following pretreatment with rapamycin (Fig. 4A). Treatment of HCAECs with p27Kip1-targeting siRNA abolished the ability of rapamycin pretreatment to block VEGF stimulation of RhoA activity and the actin stress fiber formation required for cell migration (Figs. 4B and 5). These data suggest a model whereby rapamycin inhibits endothelial cell migration through an increase in p27Kip1 protein, which in turn inhibits RhoA activation, blocking the cytoskeletal reorganization required for cell migration.
FIGURE 4.
Rapamycin treatment inhibits RhoA activation by VEGF in a p27Kip1-dependent manner. A, HCAECs were starved for 72 h and then serum-stimulated in the presence of the indicated dose of rapamycin for 24 h. HCAECs were then stimulated with 10 ng/ml of VEGF for 5 min, and RhoA activation was measured. Error bars represent mean ± S.E. (n = 3) normalized to the unstimulated control (CON). B, HCAECs were serum-stimulated for 24 h in the presence of nontargeting siRNA or siRNA targeting p27Kip1. The medium was then supplemented with either rapamycin (VEGF + RPM; 100 nmol/liter) or vehicle (CON and VEGF), and the cells were incubated for an additional 24 h. RhoA activity was then stimulated for 5 min with VEGF (VEGF and VEGF + RPM; 10 ng/ml). Error bars represent mean ± S.E. (n = 3). Brackets indicate p < 0.05.
FIGURE 5.
Rapamycin pretreatment inhibits the formation of stress fibers in a p27Kip1-dependent manner. HCAECs were grown for 24 h in growth medium supplemented with nontargeting siRNA or siRNA targeting p27Kip1. The growth medium was then supplemented with either rapamycin (RPM; 100 nmol/liter) or vehicle, and the cells were incubated for 24 h. A, stress fiber formation was stimulated with VEGF (10 ng/ml) for 2 h and visualized with phalloidin staining coupled with 4′,6-diamidino-2-phenylindole counterstain. The scale bar indicates 10 μm. B, the percentage of HCAECs that were positive for stress fiber formation was calculated for each treatment group. Brackets indicate p < 0.05. Unstim., unstimulated.
DISCUSSION
The present data from ECs are similar to data previously obtained with VSMCs, suggesting a common p27Kip1-dependent mechanism for the regulation of cell migration by the mTOR pathway. In coronary artery VSMCs, an inhibition of growth factor-stimulated phosphorylation of p70S6K and 4E-BP1 is seen within 1 h of exposure to rapamycin (10 nmol/liter) with an increase in p27Kip1 occurring after a prolonged exposure to rapamycin (25). BC3H1 myogenic cells passaged repeatedly in the presence of rapamycin (100 or 1000 nmol/liter) exhibit a relative resistance to rapamycin-dependent inhibition of proliferation compared with the parental cell line. Characterization of these rapamycin-resistant cells showed a decrease in the phosphorylation of the p70S6K and 4E-BP1 proteins in response to rapamycin treatment as seen in the parental cell line but also constitutively low levels of p27Kip1 that did not increase with rapamycin treatment or growth in low serum medium (18).
The link between rapamycin regulation of p27Kip1 and its antimigratory effects was confirmed in VSMCs from p27Kip1−/− mice. The IC50 of the effects of rapamycin on cellular migration was ∼200 nmol/liter in p27Kip1−/− SMCs compared with ∼2 nmol/liter in wild-type VSMCs, demonstrating a p27Kip1-dependent pathway through which rapamycin inhibits migration (21). The ability of higher doses of rapamycin to inhibit SMC migration in the absence of p27Kip1 suggests there exists an additional p27Kip1-independent pathway through which rapamycin regulates migration (21). At these elevated doses of rapamycin, p70S6K and 4E-BP1 phosphorylation is inhibited, suggesting that this additional pathway is also p70S6K- and 4E-BP1-independent. In a separate study, rapamycin was equally effective in reducing intimal hyperplasia following femoral wire injury in p27Kip1−/− and wild-type mice, suggesting that rapamycin inhibits VSMC proliferation and migration in vivo in a p27Kip1-independent manner (26). However, neither VSMC migration nor re-endothelialization of the site of injury was examined in this study. Thus, it remains unclear whether the effects of rapamycin on VSMC or EC migration are altered in the p27Kip1−/− mice. In this study, HCAECs treated with siRNA targeting p27Kip1 exhibited an IC50 of the effects of rapamycin on EC migration that was increased ∼1000-fold while the ability of rapamycin to inhibit p70S6K phosphorylation was maintained. Thus, as in SMCs, there are p27Kip1-dependent and p27Kip1-independent mechanisms through which rapamycin regulates EC migration.
Initiation of cell migration is dependent on the proper adjustment of the balance of Rho GTPase activities within the cell (27). A gradient forms within the cell, which drives the formation of new cellular protrusions at the leading edge where Rac activity is high and maintains cell adhesions for traction toward the rear where Rho activity is high. In ECs and VSMCs, pharmacological inhibition of Rho in HUVECs and VSMCs inhibits their migration, suggesting that Rac activity exceeds Rho activity under normal conditions (28). Thus, to form the adhesions necessary to produce motility, there must be an increase in Rho activity for cell migration to occur in ECs. Besson et al. (20) have proposed a model where elevated p27Kip1 protein levels increase motility in cells in which the balance between Rho and Rac activity is shifted toward Rho (e.g. fibroblasts) and decrease motility in cells such as ECs and VSMCs where the balance is shifted toward Rac. Supporting this model, forced overexpression of p27Kip1 in HUVECs and in VSMCs has been demonstrated to inhibit their migration (29–31) In this study, we demonstrated that rapamycin pretreatment inhibits EC migration and differentiation through an increase in p27Kip1 protein levels that results in an inhibition of RhoA activation.
Although our data demonstrate the importance of the phosphorylation of Thr187 of p27Kip1 in the regulation of migration by mTOR, a clear link between mTOR and this phosphorylation event is not known. Our data suggest that the antimigratory effects of rapamycin may result from inhibition of mTORC2 as prolonged exposure to rapamycin leads to inhibition of mTORC2 formation and diminished Akt phosphorylation in HUVECs (11). This finding is in agreement with a previous finding that inhibition of mTORC2 blocks HUVEC migration stimulated by prostaglandin E2 (32). In contrast to that report, which identified mTORC2 as a signaling intermediary, our data describe the ability of mTOR inhibition to block Rho activation by VEGF. The target of p27Kip1, cyclin-dependent kinase 2, is also the kinase known to phosphorylate Thr187 initiating its degradation (33). The mechanisms regulating the balance between inhibition of cyclin-dependent kinase 2 by p27Kip1 and the initiation of p27Kip1 degradation are still being elucidated. Spy1, a member of the Speedy/RINGO family of cyclin-dependent kinase activators, has been shown to enhance phosphorylation of p27Kip1 by binding cyclin-dependent kinase 2 and preventing its inhibition by p27Kip1 (34). Alternatively, the Src family kinase Lyn and the Bcr-Abl oncogene phosphorylate p27Kip1 at its Tyr88 residue (35, 36). This phosphorylation blocks the ability of p27Kip1 to inhibit cyclin-dependent kinase 2 but does not block its association, facilitating the phosphorylation of p27Kip1 by cyclin-dependent kinase 2. Similarly, the tyrosine phosphatase SHP-2 reduces this phosphorylation in response to stimulation with granulocyte colony-stimulating factor in promyelocytic leukemia cells (37). Whether one of these mechanisms plays a role in the regulation of p27Kip1 by mTOR warrants further study.
The first-generation drug-eluting stents are highly effective at blocking neointimal hyperplasia but also exhibit reduced stent endothelialization. Longer term clinical studies have demonstrated that late stent thrombosis reduces the benefit of drug-eluting stents over bare metal stents (3–6). Although our studies focused on rapamycin, it is likely that the current analogs of rapamycin will exhibit similar properties given their similar mechanisms of action. Rapamycin and its analogs are also being investigated clinically as anticancer agents, with encouraging results reported in the treatment of advanced renal cell carcinoma (38) and mantle cell carcinoma (39). The basis for these studies was in part demonstration that inhibition of mTOR blocked angiogenesis through a decrease in expression of VEGF while also having direct effects on VEGF stimulation of EC proliferation and morphogenesis (15, 16). Our current study describes those direct effects, demonstrating that rapamycin is able to inhibit migration and differentiation through an increase in p27Kip1 protein levels.
In summary, we have demonstrated the ability of rapamycin to inhibit EC migration and differentiation in a p27Kip1-dependent manner. Prolonged rapamycin treatment inhibits mTORC2 and increases p27Kip1 levels through an inhibition of p27Kip1 phosphorylation at Thr187. Increasing p27Kip1 leads to an inhibition of RhoA activation and actin cytoskeletal reorganization. These data further elucidate the link between mTOR and cell migration.
Supplementary Material
Acknowledgment
We thank Dr. James M. Roberts (Fred Hutchinson Cancer Research Center, Seattle, WA) for the gift of the p27Kip1T187A knock-in mice.
This work was supported, in whole or in part, by National Institutes of Health Award P20RR018766 from the National Center for Research Resources. This work was also supported by Grants 0665320B and 0855316E from the Greater SE Affiliate of the American Heart Association.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods” and Figs. S1–S3.
- mTOR
- mammalian target of rapamycin
- EC
- endothelial cell
- VEGF
- vascular endothelial cell growth factor A
- VSMC
- vascular smooth muscle cell
- HUVEC
- human umbilical vein endothelial cell
- HCAEC
- human coronary artery endothelial cell
- siRNA
- small interfering RNA.
REFERENCES
- 1.Holmes D. R., Jr., Leon M. B., Moses J. W., Popma J. J., Cutlip D., Fitzgerald P. J., Brown C., Fischell T., Wong S. C., Midei M., Snead D., Kuntz R. E. (2004) Circulation 109, 634–640 [DOI] [PubMed] [Google Scholar]
- 2.Finn A. V., Joner M., Nakazawa G., Kolodgie F., Newell J., John M. C., Gold H. K., Virmani R. (2007) Circulation 115, 2435–2441 [DOI] [PubMed] [Google Scholar]
- 3.Mauri L., Hsieh W. H., Massaro J. M., Ho K. K., D'Agostino R., Cutlip D. E. (2007) N. Engl. J. Med. 356, 1020–1029 [DOI] [PubMed] [Google Scholar]
- 4.Spaulding C., Daemen J., Boersma E., Cutlip D. E., Serruys P. W. (2007) N. Engl. J. Med. 356, 989–997 [DOI] [PubMed] [Google Scholar]
- 5.Stone G. W., Moses J. W., Ellis S. G., Schofer J., Dawkins K. D., Morice M. C., Colombo A., Schampaert E., Grube E., Kirtane A. J., Cutlip D. E., Fahy M., Pocock S. J., Mehran R., Leon M. B. (2007) N. Engl. J. Med. 356, 998–1008 [DOI] [PubMed] [Google Scholar]
- 6.Kastrati A., Mehilli J., Pache J., Kaiser C., Valgimigli M., Kelbaek H., Menichelli M., Sabaté M., Suttorp M. J., Baumgart D., Seyfarth M., Pfisterer M. E., Schömig A. (2007) N. Engl. J. Med. 356, 1030–1039 [DOI] [PubMed] [Google Scholar]
- 7.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Cell 110, 177–189 [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar P. T., Hay N. (2007) Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 9.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 10.Hresko R. C., Mueckler M. (2005) J. Biol. Chem. 280, 40406–40416 [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Mol. Cell 22, 159–168 [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 14.Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 15.Del Bufalo D., Ciuffreda L., Trisciuoglio D., Desideri M., Cognetti F., Zupi G., Milella M. (2006) Cancer Res. 66, 5549–5554 [DOI] [PubMed] [Google Scholar]
- 16.Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., Bruns C. J., Zuelke C., Farkas S., Anthuber M., Jauch K. W., Geissler E. K. (2002) Nat. Med. 8, 128–135 [DOI] [PubMed] [Google Scholar]
- 17.Nourse J., Firpo E., Flanagan W. M., Coats S., Polyak K., Lee M. H., Massagué J., Crabtree G. R., Roberts J. M. (1994) Nature 372, 570–573 [DOI] [PubMed] [Google Scholar]
- 18.Luo Y., Marx S. O., Kiyokawa H., Koff A., Massagué J., Marks A. R. (1996) Mol. Cell. Biol. 16, 6744–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koff A., Polyak K. (1995) Prog. Cell Cycle Res. 1, 141–147 [DOI] [PubMed] [Google Scholar]
- 20.Besson A., Gurian-West M., Schmidt A., Hall A., Roberts J. M. (2004) Genes Dev. 18, 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J., Marx S. O., Chen H. J., Poon M., Marks A. R., Rabbani L. E. (2001) Circulation 103, 2967–2972 [DOI] [PubMed] [Google Scholar]
- 22.Malek N. P., Sundberg H., McGrew S., Nakayama K., Kyriakides T. R., Roberts J. M. (2001) Nature 413, 323–327 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M., Inoue K., Warabi E., Minami T., Kodama T. (2005) J. Atheroscler. Thromb. 12, 138–142 [DOI] [PubMed] [Google Scholar]
- 24.Rasband W. S. (1997–2007) Image J, National Institutes of Health, Bethesda, MD [Google Scholar]
- 25.Braun-Dullaeus R. C., Mann M. J., Seay U., Zhang L., von Der Leyen H. E., Morris R. E., Dzau V. J. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1152–1158 [DOI] [PubMed] [Google Scholar]
- 26.Roqué M., Reis E. D., Cordon-Cardo C., Taubman M. B., Fallon J. T., Fuster V., Badimon J. J. (2001) Lab. Invest. 81, 895–903 [DOI] [PubMed] [Google Scholar]
- 27.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 28.Nakayama M., Amano M., Katsumi A., Kaneko T., Kawabata S., Takefuji M., Kaibuchi K. (2005) Genes Cells 10, 107–117 [DOI] [PubMed] [Google Scholar]
- 29.Díez-Juan A., Andrés V. (2003) Circ. Res. 92, 402–410 [DOI] [PubMed] [Google Scholar]
- 30.Castro C., Díez-Juan A., Cortés M. J., Andrés V. (2003) J. Biol. Chem. 278, 4482–4490 [DOI] [PubMed] [Google Scholar]
- 31.Goukassian D., Díez-Juan A., Asahara T., Schratzberger P., Silver M., Murayama T., Isner J. M., Andrés V. (2001) FASEB J. 15, 1877–1885 [DOI] [PubMed] [Google Scholar]
- 32.Dada S., Demartines N., Dormond O. (2008) Biochem. Biophys. Res. Commun. 372, 875–879 [DOI] [PubMed] [Google Scholar]
- 33.Bloom J., Pagano M. (2003) Semin. Cancer Biol. 13, 41–47 [DOI] [PubMed] [Google Scholar]
- 34.McAndrew C. W., Gastwirt R. F., Meyer A. N., Porter L. A., Donoghue D. J. (2007) Cell Cycle 6, 1937–1945 [DOI] [PubMed] [Google Scholar]
- 35.Chu I., Sun J., Arnaout A., Kahn H., Hanna W., Narod S., Sun P., Tan C. K., Hengst L., Slingerland J. (2007) Cell 128, 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimmler M., Wang Y., Mund T., Cilensek Z., Keidel E. M., Waddell M. B., Jäkel H., Kullmann M., Kriwacki R. W., Hengst L. (2007) Cell 128, 269–280 [DOI] [PubMed] [Google Scholar]
- 37.Tossidou I., Dangers M., Koch A., Brandt D. T., Schiffer M., Kardinal C. (2008) Cell Cycle 7, 3858–3868 [DOI] [PubMed] [Google Scholar]
- 38.Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., Staroslawska E., Sosman J., McDermott D., Bodrogi I., Kovacevic Z., Lesovoy V., Schmidt-Wolf I. G., Barbarash O., Gokmen E., O'Toole T., Lustgarten S., Moore L., Motzer R. J. (2007) N. Engl. J. Med. 356, 2271–2281 [DOI] [PubMed] [Google Scholar]
- 39.Witzig T. E., Geyer S. M., Ghobrial I., Inwards D. J., Fonseca R., Kurtin P., Ansell S. M., Luyun R., Flynn P. J., Morton R. F., Dakhil S. R., Gross H., Kaufmann S. H. (2005) J. Clin. Oncol. 23, 5347–5356 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.