Abstract
The proto-oncogenic Ras isoforms (H, N, and K) have a C-terminal CAAX motif and undergo the same post-translational processing steps, although they traffic to the plasma membrane through different routes. Previously, we have shown that overexpression of the deubiquitinating enzyme USP17 inhibits H-Ras localization to the plasma membrane. Now we report that whereas H-Ras and N-Ras were unable to localize to the plasma membrane in the presence of USP17, K-Ras4b localization was unaffected. EGF stimulation was unable to induce N-Ras membrane localization in USP17-expressing cells. In addition, N-Ras activity and downstream signaling through the MAPK MEK/ERK and PI3K/JNK pathways were blunted. However, we still detected abundant N-Ras localization at the ER and Golgi in USP17-expressing cells. Collectively, our data showed that the deubiquitinating enzyme USP17 blocks EGF-induced N-Ras membrane trafficking and activation, but left K-Ras unaffected.
Keywords: G Proteins, Protein/Intracellular Trafficking, Protein/Post-translational Modification, Protein/Translocation, Signal Transduction/G-proteins, Subcellular Organelles/Endoplasmic Reticulum, Subcellular Organelles/Golgi, Deubiquitination
Introduction
The Ras family of GTPases are signaling proteins involved in cell proliferation, differentiation, and apoptosis. Ras proteins act as molecular switches at the plasma membrane (PM)2 conveying signals from the external environment to the interior of the cell. For Ras to be functional it cycles from its inactive GDP-bound to the active GTP-bound state. The active Ras then binds to downstream effectors initiating signaling cascades. For example, binding to the effector Raf results in mitogen-activated protein kinase (MAPK) signaling and activation of ERK and JNK. Regulation of GTPase activity is critical, and constitutively active Ras mutants frequently contribute to the development of aggressive cancers (1).
Additionally, Ras can signal from the ER and Golgi without prior trafficking to the PM (2–5). Whereas PM activation of Ras is thought to be rapid and transient, ER/Golgi activation is delayed and sustained (2, 6). Differences in signals from the varying locations may be due in part to the differential localization of scaffolds. Indeed, KSR1 has been shown to act preferentially on ERK from the PM, whereas Sef-1 regulates ERK at the ER and Golgi (7, 8). Ras has also been shown to signal from the endosomes where H-Ras and N-Ras can be localized as a result of their ubiquitination (9).
To achieve membrane localization Ras must go through a series of post-translational modifications, with processing of the C-terminal CAAX box being a central event. The CAAX box cysteine is initially isoprenylated by farnesyl transferase (FTase) or geranylgeranyl transferase type I (GGTaseI) (K-Ras and sometimes N-Ras) (10). Prenylation is essential for trafficking Ras to the ER, where the intramembrane protease Ras-converting enzyme 1 (RCE1) cleaves the AAX and isoprenylcysteine methyltransferase (ICMT) methylates the prenylated cysteine (11, 12). However, the subsequent processing varies for different isoforms: H-Ras and N-Ras are further palmitoylated at the Golgi whereas K-Ras4b contains a polybasic stretch of lysines that behaves as a membrane targeting signal and does not traffic through the Golgi (13).
Ubiquitination and deubiquitination of proteins is an important regulatory mechanism altering the fate of the modified protein and disregulation of this process can have profound effects. To date, five families of deubiquitinating enzymes have been identified: ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), ovarian tumor proteases (OTUs), Josephins, and JAB1/MPN/MOV34 metallo-proteases (JAMMs) (14, 15). The USP family are cysteine proteases identified by histidine and cysteine boxes within their catalytic domain (15). USP17 is an immediate early-gene being cytokine induced and is highly expressed in many cancers (16, 17). We have previously shown that expression of USP17 blocks plasma membrane localization and activation of H-Ras at least in part by inhibition of its post-translational processing through modulation of RCE1 (18). Furthermore, the MAPK pathway was disrupted resulting in delayed cell growth.
Because N-Ras and K-Ras are frequently mutated in cancers, we asked whether USP17 expression would also result in the mislocalization of these isoforms and what effect there would be on downstream effectors. Using confocal microscopy we show that USP17 inhibits H-Ras and N-Ras, but not K-Ras4b membrane localization. This is true for both wild-type Ras and oncogenic mutants. However, Ras activation and MAPK signaling is decreased, but still present, in cells overexpressing USP17. We propose that this is due to H-Ras and N-Ras being localized at the ER and Golgi where Ras can still be activated and signal to the MAPK pathway. These findings suggest that USP17 regulates differential Ras isoform signaling from various intracellular platforms.
EXPERIMENTAL PROCEDURES
Plasmids
pDQ-EV (His), pDQ-USP17 (His), and pDQ-USP17CS (His) were kind gifts from Dr. Derek Quinn (Queen's University, Belfast). pEGFP-C3-HRas, pEGFP-C3-HRasG12V, pEGFP-C3-NRas, pEGFP-C3-NRasG12V, pEGFP-C3-KRas4b, and pEGFP-C3-KRas4bG12V were kind gifts from Dr. Ian Prior (Liverpool, UK). pSUPER-USP17shRNA (sequence GCAGGAAGATGCCCATGAA) was a kind gift from Prof. Rene Bernards (The Netherlands Cancer Institute, Amsterdam, The Netherlands), and scrambled shRNA was purchased from OriGene Technologies.
Cell Culture and DNA Transfections
HeLa cells (American Type Culture Collection (ATCC)) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% penicillin (10,000 units/ml)/streptomycin (10,000 μg/ml), and 1% l-glutamine (200 mm), grown at 37 °C in 5% CO2 humidified incubator. Cells were transfected with FuGENE™ 6 transfection reagent (Roche) according to the manufacturer's instructions. Cells were seeded between 0.5 × 106 and 5.0 × 106 cells for protein experiments or 0.20 × 105 on LabTek II, CC2-treated 4 chamber slides (Nalge Nunc) for microscopy experiments. The cells were transfected with 3 μg of plasmid DNA for protein experiments and biological assays or 0.25 μg of plasmid DNA for confocal microscopy experiments. For those experiments with EGF stimulation, cells were rested for 12 h in Dulbecco's modified Eagles medium without serum to minimize Ras activation. Cells were then stimulated with 100 ng/ml EGF (R&D) for the indicated times in the figures. Brefeldin A (Sigma) was used at 5 μg/ml for 2 h after serum-starving cells for 12 h.
Cell Lysis and Immunoblotting
Whole cell lysates and immunoprecipitations were generated and separated prior to immunoblotting as previously described (15). The following primary antibodies were used: anti-USP17 (Fusion Antibodies), anti-γ-tubulin (Sigma), mouse Pan-Ras (Calbiochem), as well as β-actin, pERK, ERK, pJNK, and JNK (all from Cell Signaling). Densitometry was performed using ImageJ software (NIH), and error bars represent standard error from different gel exposures. ANOVA statistics were carried out to compare Ras activity using Prism GraphPad.
Ras-RBD Pulldown Assay
HeLa cells were transfected with the indicated plasmids and stimulated with EGF as previously described. Ras RBD pulldown assays were carried out as previously described (18).
Confocal Microscopy
HeLa cells were seeded, fixed, and stained as previously described (18). Antibodies and costains used were as follows: mouse anti-USP17 (Fusion Antibodies), rabbit anti-calnexin (AbCam), rabbit anti-GM130 (AbCam), donkey anti-mouse or rabbit Cy5 (Jackson ImmunoResearch), donkey anti-mouse or rabbit TRITC (Jackson ImmunoResearch). Slides were viewed on a Leica Sp2 Confocal Microscope and images analyzed using Leica LAS AF software. The images presented in the same figures were captured using standardized setting and exposure times. Cell counts were from at least 100 cells in greater than three experiments unless otherwise noted. ANOVA statistics compares PM localization.
RNA Extraction and RT-PCR
RNA extractions and RT-PCR was carried out as previously described (17).
RESULTS
USP17 Regulates Ras Localization
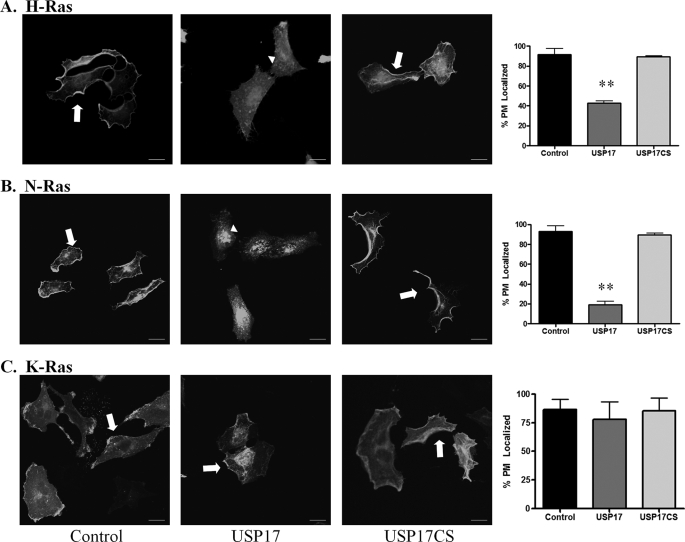
To characterize whether USP17 expression would affect the different Ras isoforms, we examined membrane localization at steady-state conditions in HeLa cells (Fig. 1). In the majority of control cells transfected with the Ras isoforms, H-Ras, N-Ras, and K-Ras were localized to the plasma membrane (Fig. 1, A–C, first panels, respectively; arrows). However, in the presence of USP17, H-Ras and N-Ras were mislocalized from the PM (Fig. 1, A and B, second panels, arrowheads). Quantification revealed a significant reduction in membrane localization in the presence of USP17 (Fig. 1, A and B, fourth panels). The majority of these cells exhibited clear cytosolic distribution with highly concentrated pools of Ras most evident in the perinuclear region. In sharp contrast, K-Ras was still localized to the plasma membrane in USP17-expressing cells (Fig. 1C, second and fourth panels). USP17CS (a catalytically inactive mutant of USP17)-transfected cells had similar Ras localization to control cells. To confirm USP17 and USP17CS expression, cells were stained with an antibody against USP17 (supplemental Figs. S1–S3). The experiments were repeated more than three times and at least 100 cells were blindly scored for membrane distribution by two individuals (Fig. 1, histograms, panel 4). These observations with H-Ras and N-Ras confirm our previous report that showed H-Ras was mislocalized in the presence of USP17 (18). We also previously demonstrated that depletion of USP17 with a shRNA results in constitutively active H-Ras following serum stimulation (18). Additionally, we now demonstrated that in USP17-depleted cells N-Ras was also constitutively activated whereas K-Ras was not affected (supplemental Fig. S4). This suggests that USP17 is differentially regulating the Ras isoforms and plays a role prior to its PM localization.
FIGURE 1.
USP17 inhibits H-Ras and N-Ras plasma membrane localization, but not K-Ras localization. HeLa cells were transfected with GFP-HRas (A), GFP-NRas (B), and GFP-KRas (C) along with USP17 or USP17CS. Cells were stained with a USP17 monoclonal antibody to confirm transfection (supplemental Figs. S1–S3) and imaged with confocal microscopy. Arrows indicate plasma membrane-localized Ras, and arrowheads perinuclear Ras localization. Scale bars are 20 μm. Quantification (n = 3) of plasma membrane localization for each Ras isoform is shown in the fourth panel. ** indicates p < 0.01 versus control.
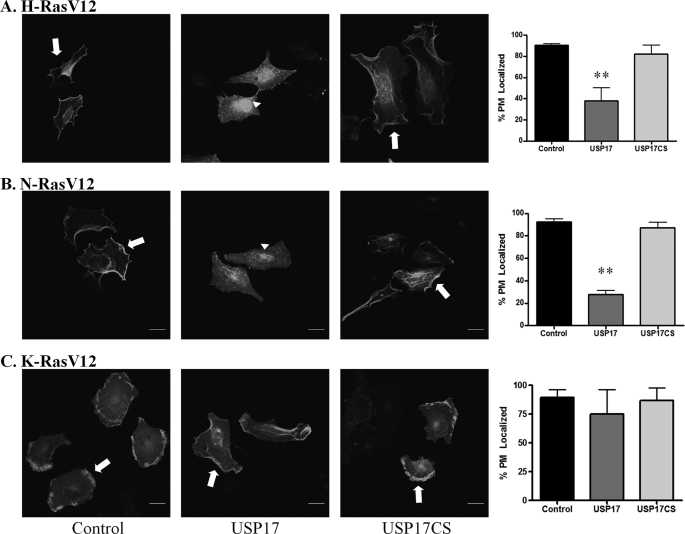
Oncogenic Ras has been reported in ∼30% of cancers and inhibition of Ras localization is an appealing therapeutic approach (19). It was important therefore to examine whether USP17 would also block the PM distribution in cells expressing constitutively active Ras, in which codon 12 (valine) was mutated to glycine. Similar to the results with wild-type Ras, the oncogenic Ras isoforms were predominantly localized to the plasma membrane in control and USP17CS-transfected cells (Fig. 2, A–C; first, third, and fourth panels). Again, USP17 inhibited oncogenic H-Ras and N-Ras localization to the plasma membrane but not the localization of mutant K-Ras (Fig. 2, second and fourth panels). USP17 and USP17CS expression was confirmed by staining with an antibody as in Fig. 1 (data not shown). These experiments were repeated more than three times and quantification revealed the difference in PM localization in USP17-expressing cells was statistically significant for H-Ras and N-Ras but not K-Ras (Fig. 2, fourth panel). Again, a predominant pool of perinuclear Ras was evident in USP17 cells. Taken together, USP17 could inhibit plasma membrane localization of both wild-type and oncogenic H-Ras and N-Ras but not K-Ras.
FIGURE 2.
USP17 inhibits oncogenic mutant H-Ras and N-Ras plasma membrane localization but not K-Ras localization. HeLa cells were transfected with GFP-HRasV12 (A), GFP-NRasV12 (B), and GFP-KRasV12 (C) along with control, USP17, or USP17CS. Cells were stained with USP17 monoclonal antibody to confirm transfection (not shown) and imaged with by confocal microscopy. Arrows indicate plasma membrane localized Ras, and arrowheads perinuclear localization. Scale bars are 20 μm. Quantification (n = 3) of plasma membrane localization is shown for each Ras isoform in the fourth panel. ** indicates p < 0.01 versus control.
EGF Stimulated Ras Activation in USP17 Cells
Previous data demonstrated that USP17 could inhibit H-Ras and N-Ras PM localization under steady-state conditions. In order for Ras to be fully activated, an external stimulus is required. Therefore, it was important to examine ligand-activated Ras. EGF stimulation results in Ras binding to its effectors, for example Raf, initiating downstream signaling cascades (20). This is most commonly associated with Ras plasma membrane localization. Because USP17 inhibited H-Ras and N-Ras localization to the plasma membrane, it was expected that Ras activation would also be decreased. Therefore, we examined the activity of N-Ras in the presence of USP17 using a Raf pulldown assay.
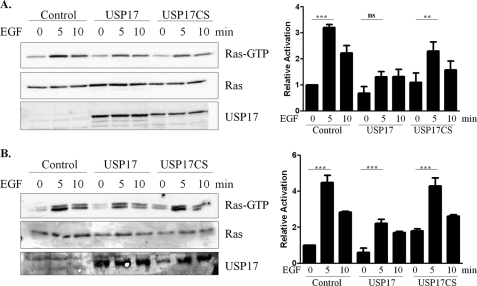
Transiently transfected HeLa cells were incubated in growth medium lacking serum for 12 h, then stimulated with EGF (100 ng/ml) for 5 or 10 min. Fig. 3A showed that EGF stimulated N-Ras activation, peaked 5 min after treatment, which was consistent with plasma membrane activation demonstrated previously (6). In the presence of USP17, N-Ras was still active but to a much lesser degree than that observed in control cells. Omerovic et al. (21) have demonstrated that in HeLa cells, N-Ras accounts for ∼50% of the total endogenous Ras protein and so the effect of USP17 on the endogenous Ras protein was also examined. As with overexpressed N-Ras, the endogenous protein was also activated in the presence of USP17, though markedly less than observed in control cells (Fig. 3B). Although USP17CS has a slight decrease in activation compared with control cells, the increase in activation following stimulation is still statistically significant. Densitometry of both the overexpressed N-Ras and endogenous Ras established that after 5 min EGF stimulation Ras activation in the presence of USP17 was nearly half that of control cells (Fig. 3, right panels). These results demonstrated that Ras activation was blunted in the presence of USP17.
FIGURE 3.
USP17 blunts N-Ras activation. A, HeLa cells were transfected with GFP-NRas along with control (empty vector), USP17, or USP17CS. B, HeLa cells were transfected with control (empty vector), USP17, or USP17CS. Cells were serum-starved then stimulated with 100 ng/ml EGF for 0, 5, or 10 min. To visualize active Ras, Raf pulldown assays (upper panels) and cell lysates (lower two panels) were analyzed by SDS-PAGE and blotted for pan-Ras or USP17. Densitometry of Ras activity is shown on the right. Statistics compare 0 to 5 min EGF treatment. *** indicates p < 0.001; **, p < 0.01; and ns for not significant.
USP17 Decreases N-Ras Localization to the PM but Not Perinuclear Structures
Because activation of Ras by EGF was markedly decreased in USP17-expressing cells, we assessed if Ras was being localized to intracellular compartments where activation may occur. Therefore, cellular localization of N-Ras following EGF stimulation was examined. Cells were grown in serum-free medium for 12 h, thereby markedly decreasing membrane localization of Ras (Fig. 4). As expected, the plasma membrane pool of N-Ras was rapidly induced by EGF stimulation for control and USP17CS-transfected cells (Fig. 4, A and C, respectively, arrows). This experiment was repeated in duplicate, and cell counts illustrate that 2 min after EGF stimulation, the percentage of membrane localized N-Ras increased from ∼10% to 70% for both control and USP17CS-transfected cells (Fig. 4D). However, this was not observed in USP17-transfected cells, where only a slight increase in PM localization was measured after EGF stimulation (Fig. 4, B and D). Ras localization at the intracellular organelles was also examined and quantified, but no change in distribution was observed in USP17-transfected cells (Fig. 4, arrowheads, quantification not shown). Data suggested that PM-localized Ras activation was rapid, whereas the ER/Golgi pools were stable and sustained. This is similar to reports by Ibiza et al. (22) that showed that a proportion of N-Ras was localized to the Golgi in cells under steady-state conditions.
FIGURE 4.
USP17 inhibits EGF-stimulated N-Ras localization to the PM. HeLa cells were transfected with GFP-NRas along with control (empty vector) (A), USP17 (B), or USP17CS (C). Cells were serum-starved then stimulated with EGF (100 ng/ml) for 0, 2, 5, or 10 min. Ras membrane distribution was visualized by confocal microscopy and quantification for PM Ras scored in duplicate experiments (D). Scale bars are 20 μm.
Ras Colocalizes with the ER and Golgi in USP17 Cells
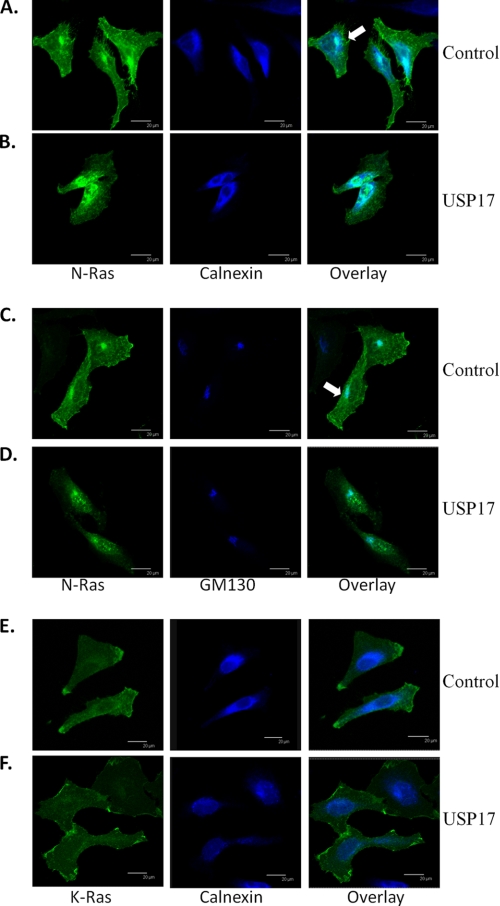
Given that Ras activation can occur at the ER and Golgi, we sought to determine whether N-Ras was still localized to the ER and Golgi in the presence of USP17. This may account for the N-Ras activation seen in Fig. 3. In control cells, N-Ras was distributed both at the plasma membrane and in the cytosol, which colocalized mostly with the ER marker calnexin. (Fig. 5A, arrow). Although N-Ras was not visualized at the plasma membrane in USP17-transfected cells, there was again a pool of internal N-Ras, which colocalized with the ER marker (Fig. 5B). We then examined the localization of N-Ras with the cis-Golgi marker GM130 and observed a similar trend, where N-Ras colocalized with the Golgi in both control and USP17-transfected cells (Fig. 5, C and D). Whereas intracellular pools of N-Ras predominantly co-localized with the ER and Golgi, there are likely additional cellular organelles that the isoforms localize to. This experiment was repeated more than three times, and consistently demonstrated that although the plasma membrane pool of N-Ras was disrupted in USP17-expressing cells, the ER and Golgi pools were still intact. Interestingly, although K-Ras is modified at the ER, it does not stably localize to intracellular structures (23). We also demonstrate that K-Ras does not co-localize with calnexin, and so does not stably interact with the ER (Fig. 5, E and F).
FIGURE 5.
N-Ras colocalizes with the ER and Golgi. HeLa cells were transfected with GFP-NRas along with control (empty vector) (A and C) or USP17 (B and D) or GFP-KRas with control and USP17 (E and F). Cells were stained with primary antibodies: calnexin for the ER (A, B, E, and F) or GM130 for the Golgi (C and D) and pseudo-colored blue. Representative images were captured by confocal microscopy. Scale bars are 20 μm.
USP17 Diminishes but Does Not Abolish Ras Signaling
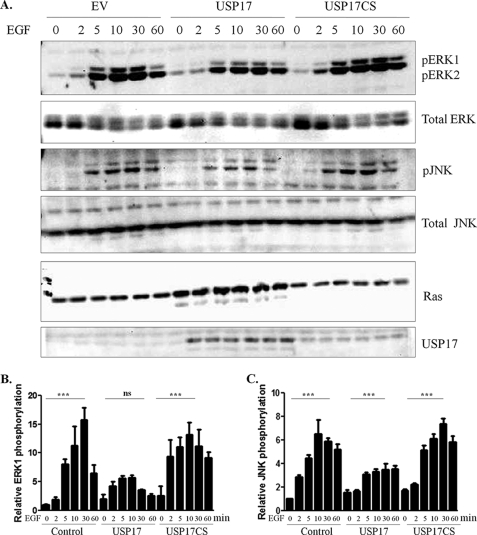
In the presence of USP17, we demonstrated that N-Ras was relocalized from the PM to the ER and Golgi, and that Ras activation by EGF stimulation was blunted. This suggested that expression of USP17 would inhibit downstream Ras signaling after stimulation. We, therefore, sought to determine whether USP17 inhibited MAPK activation. Because EGF-induced Ras activation at the plasma membrane occurs rapidly, whereas activation at endomembranes occurs later at 40–60 min (2), we stimulated cells with EGF over a 60-min time course. We detected pERK at 5 min after EGF (100 ng/ml) stimulation, with levels peaking at 30 min (Fig. 6, A, top panels, and B). Although phosphorylation of ERK1/2 was detected in the presence of USP17, the levels were decreased compared with controls. Furthermore, there was a marked decrease in p44 pERK1, but only a minor decrease in p42 pERK2. The increase in pERK1 at 30 min of EGF stimulation was statistically significant in control cells but not USP17-expressing cells. Given that p42 ERK1 is predominant at the endomembranes (such as the ER and Golgi), whereas p44 ERK1 is not, suggests USP17 differentially regulated the ERK isoforms.
FIGURE 6.
USP17 blunts Ras ERK and JNK MAPK signaling. A, HeLa cells were transfected with N-Ras along with control (empty vector), USP17, and USP17CS. Cells were serum-starved then stimulated with EGF (100 ng/ml) for the indicated times. Cell lysates were immunoblotted with pERK, pJNK, Ras, and USP17 antibodies. pERK and pJNK blots were reprobed for total ERK and total JNK, respectively. Densitometry of p44 pERK1 (B) and pJNK (C). Statistics compares 0 and 30 min of EGF treatment. *** indicates p < 0.001, and ns is not significant.
In addition, JNK can be phosphorylated in response to active Ras at endomembranes via PI3K activation (5). Following EGF stimulation, JNK phosphorylation increased, peaking at 30 min (Fig. 6, A, middle panels and C). Similar to the results with pERK, detectable pJNK levels were decreased in the presence of USP17 but not completely inhibited. Densitometry illustrated that pJNK expression was approximately half in USP17-transfected cells versus control and USP17CS-transfected cells (Fig. 6C). This modest increase of total pJNK following 30 min of EGF stimulation was significant. These results clearly indicated that MAPK activation was markedly reduced in the presence of USP17.
EGF Stimulates Ras Membrane Localization and Activity after Golgi Disruption
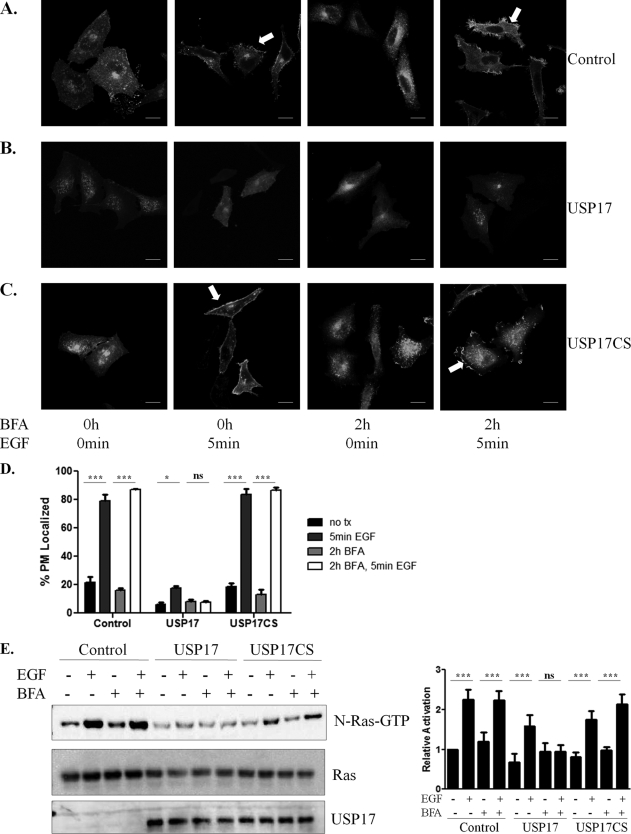
H-Ras and N-Ras predominantly traffic through the Golgi to the PM, but an alternative trafficking route has been suggested (25). Because plasma membrane localization of N-Ras was clearly reduced in USP17-expressing cells, we hypothesized that USP17 would also inhibit N-Ras transport to the PM via this alternate route. Brefeldin A (BFA) reversibly disrupts Golgi structure by interfering with COP I complex formation and so prevents vesicular trafficking (26). H-Ras and N-Ras trafficking to the PM via the Golgi have been demonstrated to be inhibited by BFA treatment (23, 28). Therefore, N-Ras localization in the presence of USP17 following BFA treatment and EGF stimulation was examined (Fig. 7, A–D).
FIGURE 7.
Inhibiting Golgi structure does not disrupt N-Ras PM localization and activation following EGF stimulation. HeLa cells were transfected with N-Ras along with control (empty vector), USP17, or USP17CS. Cells were serum-starved, treated with brefeldin A (BFA 5 μg/ml) for 2 h, then with EGF (100 ng/ml) for 5 min as indicated. A–C, representative confocal images of N-Ras localization following the indicated treatment. Arrows indicate PM-localized N-Ras; scale bars are 20 μm. D, quantification of membrane localization from three experiments. Raf-pulldown assays for overexpressed GFP N-Ras (upper panel) and whole cell lysates examined (lower two panels) for Ras or USP17 (E). Histogram on the right displays activated N-Ras. *** indicates p < 0.001, **, p < 0.01; *, p < 0.05; and ns stands for not significant.
In the presence of BFA treatment (5 μg/ml, 2 h), N-Ras was unable to traffic to the plasma membrane, similar to what has been observed in USP17-expressing cells. As expected, EGF stimulation alone (100 ng/ml 5 min) resulted in N-Ras localization to the plasma membrane in control cells, but not USP17-transfected cells as previously shown in Fig. 4 (Fig. 7, A–D). Furthermore, BFA treatment (5 μg/ml 2h) followed by EGF (100 ng/ml 5 min) stimulation resulted in increased cell membrane association in control cells but not USP17-expressing cells. These data illustrated that N-Ras membrane localization by EGF stimulation overcame Golgi disruption by BFA treatment. This suggested that when the Golgi was disrupted, N-Ras could traffic to the membrane via an alternate route, which could still be modulated by USP17. Furthermore, we saw that K-Ras localization was not affected by the presence of USP17 or disruption of the Golgi (data not shown).
To examine Ras activity, untransfected control or transfected cells were serum starved for 12 h, treated with BFA (5 μg/ml) for 2 h, then stimulated with EGF (100 ng/ml) for 5 min. As expected, Ras activity was increased following EGF treatment in the absence of BFA in control and USP17CS-transfected cells (Fig. 7E). However, Ras was not activated in USP17-expressing cells upon EGF stimulation alone or following the combination of BFA and EGF treatment. Thus, this confirms that when PM transport through the Golgi is inhibited and N-Ras is trafficked to the PM through an alternative route, ligand-induced N-Ras activation still occurred. However, the presence of USP17 also completely disrupts this.
DISCUSSION
Results presented in this study indicated that USP17 can inhibit H-Ras and N-Ras localization to the plasma membrane, but not K-Ras. Furthermore, USP17 is induced rapidly in response to EGF (data not shown) and its presence inhibits membrane localization blunting both N-Ras activation and EGF-induced MAPK signaling. N-Ras has been shown to signal from internal cellular compartments such as the ER and Golgi and the results presented here confirm this, because mislocalized N-Ras is still activated in response to EGF. Indeed, in control cells disruption of the Golgi with BFA resulted in Ras mislocalization and decreased activity, which could be overcome with EGF stimulation. However, in USP17-expressing cells N-Ras was still mislocalized from the PM suggesting that USP17 inhibits both the conventional transport route through the Golgi and the alternative route when the Golgi is inhibited. These results may have important implications in cancer because USP17 can inhibit specific Ras isoform localization and activation even in the presence of growth factors.
Ras mutations are found in ∼30% of all cancers with some cancers having much higher mutation rates (19). Therapeutic targets have predominantly focused on the prenylation enzymes FTase or GGTase I. However, a major problem encountered was that cancers treated with a specific prenylation inhibitor, such as a FTase inhibitor, were able to overcome this by being alternatively prenylated by the closely related enzyme GGTase I (29–31). Therefore, alternative approaches to inhibit Ras localization and downstream signaling are therapeutically attractive (32, 33).
USP17 has previously been shown to regulate H-Ras processing and activation (18). Because N-Ras and K-Ras undergo similar processing and activation we investigated the effect of USP17 on these isoforms as well. Here, we showed that similar to H-Ras, N-Ras was also mislocalized from the cell membrane, whereas K-Ras was not affected by the presence of USP17. In addition, identical H-Ras and N-Ras mislocalization was observed with constitutively active oncogenic mutants (i.e. USP17 mislocalizes H-Ras and N-Ras but not K-Ras). K-Ras is also processed by RCE1 and so a similar distribution should be expected. In keeping with our findings showing K-Ras at the plasma membrane in USP17-expressing cells, K-Ras has been demonstrated to be membrane-localized in RCE1-null cells (34, 35). Furthermore, USP17 expression decreases RCE1 activity by ∼50% (21). Additionally, K-Ras is crucial to the cells as the other isoforms are unable to compensate for a loss of K-Ras (36). The decreased activity of RCE1 may be enough to preferentially cleave K-Ras as opposed to H-Ras and N-Ras. Yet another possibility is that like many deubiquitinating enzymes, USP17 may have additional targets besides RCE1, which may differentially regulate transport of the Ras isoforms.
A major difference between the isoforms is that K-Ras does not need to go through the Golgi in order to be translocated to the PM. Instead K-Ras traffics to the membrane via microtubules from the ER, whereas H-Ras and N-Ras route by vesicular transport from the Golgi (23). Therefore, it would not be surprising if USP17 regulated transport and membrane localization via another mechanism.
Our results demonstrated that USP17 expression could inhibit MAPK signaling. Interestingly, USP17 preferentially inhibited the phosphorylation of the p44 ERK1 isoform over p42 ERK2. Although there is as yet no definite role of p44 ERK1, it has been visualized that the ERK isoforms may be localized to different regions of the cells (24). Total p42 ERK2 is localized to the cytosolic, nuclear and membrane fractions (which include the ER and Golgi as well as PM), whereas total p44 ERK1 is mainly found in the cytosol and nuclear fractions (24). This suggests that when N-Ras is properly localized to the PM it can signal downstream and activate both ERK1 and ERK2. However, when mislocalized, downstream signaling is altered and only the ERK2 isoform is phosphorylated. In addition, the ERK isoforms may have preferred scaffold proteins because ERK2, but not ERK1, was shown to associate with KSR-2 (37). This indicates that in the presence of USP17, when Ras is localized mainly to the ER and Golgi membranous organelles, ERK2 may be preferentially activated.
Recent reports clearly show that Ras can be active when localized to cellular organelles such as the ER and Golgi (5–7). Endomembrane activation was thought to be mainly due to Ras initially being activated at the PM, and then trafficked through retrograde pathways to the ER and Golgi. This would result in a delayed response in endomembrane activation compared with PM activation. However, Bivona et al. (38) showed that in the Jurkat T-cell line, H-Ras was activated at the Golgi following 5 min of TCR stimulation because of binding with a Golgi-specific GEF, not requiring preliminary PM localization or activation. Our observations showed that even in USP17-expressing cells, where PM localization was markedly diminished, Ras was activated as early as 5 min following EGF treatment. This suggests that in USP17-expressing cells, Ras activation at the ER/Golgi may occur independent of PM-activated Ras.
To examine N-Ras trafficking to the PM in more depth, cells were treated with Brefeldin A, which disrupts Golgi structure preventing COP I complex formation and so prevents conventional vesicular trafficking (26). After BFA treatment in control cells, N-Ras localization to the PM and activation was blunted although restored after 5 min of EGF stimulation. Previous work has demonstrated that when vesicular transport from the Golgi was disrupted, H-Ras was still trafficked to the PM via an alternative route, in which H-Ras formed mobile clusters not derived from perinuclear structures (25, 27). Interestingly, in USP17-expressing cells there are small intracellular concentrated pools of N-Ras, which may be transport clusters. Therefore, our data support this theory that when conventional trafficking routes to the plasma membrane are inhibited, there are alternative routes for Ras membrane localization.
Targeting oncogenic mutant Ras by inhibiting its post-translational modifications may be a key area of therapeutic intervention. Our data demonstrated that USP17 regulates H-Ras and N-Ras but not K-Ras localization. These results implicate USP17 as a novel regulator of differential trafficking of the Ras isoforms, making it an important protein for further study in relation to potential cancer therapy.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- PM
- plasma membrane
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- ER
- endoplasmic reticulum
- ANOVA
- analysis of variance
- USP
- ubiquitin-specific proteases
- EGF
- epithelial growth factor
- BFA
- Brefeldin A
- GFP
- green fluorescent protein.
REFERENCES
- 1.Malumbres M., Barbacid M. (2001) Nat. Rev. Cancer. 1, 222–231 [DOI] [PubMed] [Google Scholar]
- 2.Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., 2nd, Cox A. D., Philips M. R. (2002) Nat. Cell Biol. 4, 343–350 [DOI] [PubMed] [Google Scholar]
- 3.Caloca M. J., Zugaza J. L., Bustelo X. R. (2003) J. Biol. Chem. 278, 33465–33473 [DOI] [PubMed] [Google Scholar]
- 4.Perez de Castro I., Bivona T. G., Philips M. R., Pellicer A. (2004) Mol. Cell. Biol. 24, 3485–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matallanas D., Sanz-Moreno V., Arozarena I., Calvo F., Agudo-Ibáñez L., Santos E., Berciano M. T., Crespo P. (2006) Mol. Cell. Biol. 26, 100–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inder K., Harding A., Plowman S. J., Philips M. R., Parton R. G., Hancock J. F. (2008) Mol. Biol. Cell 19, 4776–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casar B., Arozarena I., Sanz-Moreno V., Pinto A., Agudo-Ibáñez L., Marais R., Lewis R. E., Berciano M. T., Crespo P. (2009) Mol. Cell. Biol. 29, 1338–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torii S., Kusakabe M., Yamamoto T., Maekawa M., Nishida E. (2004) Dev. Cell. 7, 33–44 [DOI] [PubMed] [Google Scholar]
- 9.Jura N., Scotto-Lavino E., Sobczyk A., Bar-Sagi D. (2006) Mol. Cell 21, 679–687 [DOI] [PubMed] [Google Scholar]
- 10.Wright L. P., Philips M. R. (2006) J. Lipid Res. 47, 883–891 [DOI] [PubMed] [Google Scholar]
- 11.Bergo M. O., Ambroziak P., Gregory C., George A., Otto J. C., Kim E., Nagase H., Casey P. J., Balmain A., Young S. G. (2002) Mol. Cell. Biol. 22, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergo M. O., Gavino B. J., Hong C., Beigneux A. P., McMahon M., Casey P. J., Young S. G. (2004) J. Clin. Invest. 113, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P. I. (2005) Science 307, 1746–1752 [DOI] [PubMed] [Google Scholar]
- 14.Komander D., Clague M. J., Urbé S. (2009) Nat. Rev. Mol. Cell Biol. 10, 550–563 [DOI] [PubMed] [Google Scholar]
- 15.Nijman S. M., Luna-Vargas M. P., Velds A., Brummelkamp T. R., Dirac A. M., Sixma T. K., Bernards R. (2005) Cell 123, 773–786 [DOI] [PubMed] [Google Scholar]
- 16.Burrows J. F., McGrattan M. J., Johnston J. A. (2005) Genomics 85, 524–529 [DOI] [PubMed] [Google Scholar]
- 17.Burrows J. F., McGrattan M. J., Rascle A., Humbert M., Baek K. H., Johnston J. A. (2004) J. Biol. Chem. 279, 13993–14000 [DOI] [PubMed] [Google Scholar]
- 18.Burrows J. F., Kelvin A. A., McFarlane C., Burden R. E., McGrattan M. J., de la Vega M., Govender U., Quinn D. J., Dib K., Gadina M., Scott C. J., Johnston J. A. (2009) J. Biol. Chem. 284, 9587–9595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubbert S., Shannon K., Bollag G. (2007) Nat. Rev. Cancer. 7, 295–308 [DOI] [PubMed] [Google Scholar]
- 20.Ren J. G., Li Z., Sacks D. B. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10465–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omerovic J., Hammond D. E., Clague M. J., Prior I. A. (2008) Oncogene 27, 2754–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibiza S., Pérez-Rodriguez A., Ortega A., Martínez-Ruiz A, Barreiro O., García-Domínguez C. A., Víctor V. M., Esplugues J. V., Rojas J. M., Sánchez-Madrid F., Serrador J. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10507–10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apolloni A., Prior I. A., Lindsay M., Parton R. G., Hancock J. F. (2000) Mol. Cell. Biol. 20, 2475–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballard-Croft C., Locklar A. C., Kristo G., Lasley R. D. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H658–H667 [DOI] [PubMed] [Google Scholar]
- 25.Zheng H., McKay J., Buss J. E. (2007) J. Biol. Chem. 282, 25760–25768 [DOI] [PubMed] [Google Scholar]
- 26.Bannykh S. I., Plutner H., Matteson J., Balch W. E. (2005) Traffic 6, 803–819 [DOI] [PubMed] [Google Scholar]
- 27.Rotblat B., Yizhar O., Haklai R., Ashery U., Kloog Y. (2006) Cancer Res. 66, 1974–1981 [DOI] [PubMed] [Google Scholar]
- 28.Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. (1999) Cell 98, 69–80 [DOI] [PubMed] [Google Scholar]
- 29.Blum R., Cox A. D., Kloog Y. (2008) Recent. Pat. Anticancer Drug Discov. 3, 31–47 [DOI] [PubMed] [Google Scholar]
- 30.Sousa S. F., Fernandes P. A., Ramos M. J. (2008) Curr. Med. Chem. 15, 1478–1492 [DOI] [PubMed] [Google Scholar]
- 31.Saxena N., Lahiri S. S., Hambarde S., Tripathi R. P. (2008) Cancer Invest. 26, 948–955 [DOI] [PubMed] [Google Scholar]
- 32.Konstantinopoulos P. A., Karamouzis M. V., Papavassiliou A. G. (2007) Nat. Rev. Drug Discov. 6, 541–555 [DOI] [PubMed] [Google Scholar]
- 33.Wong K. K. (2009) Recent. Pat. Anticancer Drug Discov. 4, 28–35 [DOI] [PubMed] [Google Scholar]
- 34.Roberts P. J., Mitin N., Keller P. J., Chenette E. J., Madigan J. P., Currin R. O., Cox A. D., Wilson O., Kirschmeier P., Der C. J. (2008) J. Biol. Chem. 283, 25150–25163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelson D., Ali W., Chiu V. K., Bergo M., Silletti J., Wright L., Young S. G., Philips M. (2005) Mol. Biol. Cell 16, 1606–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K., Ichise H., Nakao K., Hatta T., Otani H., Sakagami H., Kondo H., Katsuki M. (2008) Oncogene 27, 2961–2968 [DOI] [PubMed] [Google Scholar]
- 37.Liu L., Channavajhala P. L., Rao V. R., Moutsatsos I., Wu L., Zhang Y., Lin L. L., Qiu Y. (2009) Biochim. Biophys. Acta 1794, 1485–1495 [DOI] [PubMed] [Google Scholar]
- 38.Bivona T. G., Pérez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. (2003) Nature 424, 694–698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.