Abstract
Interferon-γ inducible protein 10 (IP-10) involves inflammatory cell recruitment and cellular immune damage during virus infection. Although an increase of the peripheral IP-10 level is known in HBV-infected patients, the molecular basis of HBV infection inducing IP-10 expression has remained elusive. In the present study, we demonstrate that hepatitis B virus protein X (HBx) increases IP-10 expression in a dose-dependent manner. Transfection of the HBx-expressing vector into HepG2 cells results in nuclear translocation of NF-κB, which directly binds the promoter of IP-10 at positions from −122 to −113, thus facilitating transcription. The addition of the NF-κB inhibitor blocks the effect of HBx on IP-10 induction. In parallel, increase of NF-κB subunits p65 and p50 in HepG2 cells also augments IP-10 expression. Furthermore, we show that HBx induces activation of NF-κB through the TRAF2/TAK1 signaling pathway, leading to up-regulation of IP-10 expression. As a consequence, up-regulation of IP-10 may mediate the migration of peripheral blood leukocytes in a NF-κB-dependent manner. In conclusion, we report a novel molecular mechanism of HBV infection inducing IP-10 expression, which involves viral protein HBx affecting NF-κB pathway, leading to transactivation of the IP-10 promoter. Our study provides insight into the migration of leukocytes in response to HBV infection, thus causing immune pathological injury of liver.
Keywords: Chemotaxis, Cytokines/Chemokines, Gene/Promoters, Signal Transduction, Transcription/Promoter, Transcription/Target Genes, Viruses/Hepatitis
Introduction
Hepatitis B virus (HBV)3 infection is a main health problem worldwide by causing acute and chronic liver disease. Although there is no direct cytopathic effect on hepatocytes, HBV infection induces the infiltration of immune cells, leading to formation of necroinflammatory foci, thus mediating disease processes (1, 2). To date, migration of immune cells to the inflammatory site is known to be triggered by chemokine signaling (3).
Chemokines are a family of small chemotactic cytokines that contain between two and four highly conserved NH2-terminal cysteine amino acid residues. They function to recruit and activate immune cells to inflammatory sites through binding to a subset of G protein-coupled receptors (4). IFN-γ inducible protein 10 (IP-10, CXCL10) is a CXC chemokine that can be secreted by hepatocytes and sinusoidal endothelium in the liver of hepatitis patients (5, 6). By binding CXCR3 receptor, IP-10 exerts the chemoattracting effect on NK cells, activated T cells, and dendritic cells (7). In IP-10−/− mice, immune responses to infection by neurotropic mouse hepatitis virus are reduced, concomitant with decreased CD4+ and CD8+ lymphocyte trafficking to the brain and reduced production of inflammatory factors (8). In HBV transgenic mice, the blockade of IP-10 significantly decreases migration of mononuclear cells and the severity of liver lesions induced by HBV-specific cytotoxic T lymphocytes (9). Interestingly, mRNA and protein levels of IP-10 in PBMCs, sinusoidal endothelium, and plasma are all increased in patients with chronic hepatitis B (10). Therefore, the induction of IP-10 is an important event for development of hepatitis B. However, the molecular basis of how IP-10 is regulated for induction and function exertion still remains elusive so far.
The above analysis indicates that induction of IP-10 in hepatitis B patients may play an important role in driving leukocyte migration and causing the development of the immune-mediated injury. However, to date, the mechanism of how HBV induces IP-10 expression still remains elusive. It is known that the HBV genome encodes DNA polymerase, surface antigen (HBs), core protein (HBc), and nonstructural regulatory protein HBx (11). Among them, HBx is a pleiotropic protein involved in viral replication, signal transduction, and HBV-mediated carcinogenesis (12, 13). HBx is located at either the cytosol or the nucleus. The former may activate the signal transduction cascade, whereas the latter activates specific transcription factors (14). HBx does not bind DNA directly but transactivates multiple transcription factors including AP1 (15), NF-κB (16, 17), HIF-1 (18), and ATF/cAMP-response element-binding protein (19, 20). Among them, NF-κB has gained much attention due to its pivotal role in regulating an exceptionally large number of genes, particularly those involved in immune and inflammatory responses (21, 22).
Multiple viruses may regulate the expression of IP-10, such as HRV (23), Sendai virus (24), measles virus (25), rabies virus (26), severe acute respiratory syndrome coronavirus nsp-1 (27), and human immunodeficiency virus gp120 (28). This hints that HBV probably has the same effect. In this study, we hypothesize that HBx regulates expression of IP-10 through the NF-κB pathway. Our data show that the HBx protein increases IP-10 expression by promoting binding of NF-κB on the κB1 binding site of the 5′-untranslated region of IP-10 in a dose-dependent manner. The activation of NF-κB by HBx involves signal molecules TRAF2 and TAK1, thus leading to up-regulation of IP-10 expression. In turn, up-regulation of IP-10 mediates migration of peripheral blood lymphocytes (PBLs). These findings provide new insight into the regulatory mechanism of leukocyte recruitment and infiltration to the HBV infection site.
EXPERIMENTAL PROCEDURES
Patients
Twelve patients (8 male, 4 female; age range 32–65 years, average 48 ± 9) with chronic hepatitis B who underwent surgery or cancer treatment were selected from the Union Hospital of Tongji Medical College for IP-10 detection in liver tissues by real time PCR and immunohistochemistry. These patients were positive for HBsAg, HBeAg, anti-HBc, and HBV-specific DNA in serum. Other causes of chronic liver disease had been excluded by clinical and laboratory assessments. Seven patients with non-HBV infection that underwent surgery for cancer treatment were also selected. Five normal non-HBV-infected control liver tissues were obtained from a macroscopically normal area during liver hemangioma resection.
Cell Lines and Cell Culture
Human hepatocellular cell lines HepG2 and HepG2.2.15 (expressing the whole HBV proteins persistently) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 incubator. The HBx-expressing HepG2 cell line, transfected with the pCMV-HBx plasmid and that stably expressed the HBx protein, was cultured with 500 μg/ml of G418 and 10% fetal bovine serum at 37 °C in a 5% CO2 incubator.
RNA Extraction and Real Time PCR
Total RNA of fresh tissue samples was extracted using TRIzol (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized as described previously (29). Equal amounts of cDNA were submitted to PCR in the presence of the SYBR-PCR Master mix kit (Applied Biosystems, Foster City, CA) and real time PCR detection machine Mx3000PTM (Stratagene, La Jolla, CA). IP-10 specific primers were as follows: forward primer, 5′-GCCTCTCCCATCACTTCCCTAC-3′; reverse primer, 5′-GAAGCAGGGTCAGAACATCCAC-3′. The housekeeping gene GAPDH was used as an internal control.
Immunohistochemistry
Immunohistochemical staining was described previously (30). In brief, after deparaffinization and hydration, the slides were treated with endogenous peroxidase in 0.3% H2O2 for 30 min. Then, the sections were blocked for 2 h at room temperature with 1.5% blocking serum. Sections were incubated with anti-IP-10 antibody (Abcam, Cambridge, MA) overnight at 4 °C. Labeled horseradish peroxidase was applied for 30 min at room temperature, followed by application of diaminobenzidine solution until color developed. Slides were counterstained with hematoxylin. The results were observed under a light microscope.
Preparation of Promoter Constructs
A 1050-bp IP-10 promoter construct, corresponding to the sequence from −953 to +97 (relative to the transcriptional start site) of the 5′-flanking region of the human IP-10 gene was generated from human genomic DNA using forward 5′-TTTGCTAGCCAGTTATCACTGTTACTAGC-3′, and reverse 5′-AAACTCGAGGGCAGCAAATCAGAATGGCA-3′ primers. Using the (−953/+97) IP-10 construct as a template, several deletion constructs of the IP-10 promoter, including −534/+97, −239/+97, −191/+97, and −97/+97 were similarly generated by the corresponding forward primers: 5′-TTTGCTAGCACTTGCCAGTTCCAGATCTT-3′, 5′-TTTGCTAGCGAAACAGTTCATGTTTTGGAA-3′, 5′-TTTGCTAGCAAAAGAGGAGCAGAGGGAAAT-3′, and 5′-TTTGCTAGCGGTTTTGCTAAGTCAACTGTAA-3′. Point mutations in two NF-κB sites, NF-κB1 and NF-κB2, were generated in the (−534/+97) IP-10 construct using standard site-directed mutagenesis procedures. For NF-κB1, a mutant reverse primer 5′-GTTCCTGGtGAAGTCaCATGTTGCAGAC-3′ was annealed in combination with the previously described forward primer and subjected to PCR amplification. Meanwhile, a mutant forward primer 5′-GCAACATGtGACTTCaCCAGGAACAGCC-3′ was annealed in combination with the previously described reverse primer and PCR amplification. The amplified product was gel purified and ligated into pGL3-Basic vector. A point mutant construct for NF-κB2 was generated by the same strategy with mutated primers 5′-GGAGCAGAGtGAAATTaCGTAACTTGGA-3′ and 5′-CAAGTTACGtAATTTCaCTCTGCTCCTC-3′. All constructs were sequenced for success.
Vectors containing individual viral structural genes of HBV and NF-κB subunits were kindly provided by Dr. Wen-Jie Huang (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, People's Republic of China). The pNF-κB-Luc (Stratagene, La Jolla, CA) containing four copies of the binding sequence of NF-κB and firefly luciferase gene was used in NF-κB activity detection.
RNA Interference
A series of double-stranded small interfering RNA targeting HBs, HBc, and HBx protein of HBV had been cloned into a siRNA-expressing vector pGenesil-1. The validity and efficiency of these siRNA plasmids were identified by determining the mRNA and protein levels of the corresponding target gene after transfecting them or mock vector into HepG2.2.15 cells. For the assay, cells were plated at a density of 1 × 105 cell/well in a 24-well plate. After 24 h, cells were transfected 0.8 μg of siRNA plasmids per well or mock vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HBx-expressing HepG2 cells were plated at a density of 5 × 105 cells/well in a 6-well plate. TRAF2 siRNA or TAK1 siRNA (Santa Cruz, Santa Cruz, CA) per well was used to knock down TRAF2 or TAK1 expression in HBx-expressing HepG2 cells according to the manufacturer's instructions.
Transfections and Reporter Gene Assays
HepG2 cells were plated at a density of 1 × 105 cell/well in a 24-well plate. After 24 h, cells were transfected with 0.8 μg of the full-length gene of HBV (pBlue-HBV), different HBV protein expressing plasmids, and p50 or p65 expressing plasmids, respectively. For the luciferase assay, cells were co-transfected with 0.6 μg of expression vector plasmids, 0.18 μg of promoter reporter plasmids, and 0.02 μg of the pRL-TK plasmids. 5 h later, cells were washed and allowed to recover in fresh medium supplemented with 1% fetal bovine serum. 48 h later, luciferase activity was detected with the Dual Luciferase® Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. The relative luciferase activity was determined by a ModulusTM Laboratory Luminometer (Turner Biosystems, Sunnyvale CA), and transfection efficiency was normalized by Renilla luciferase activity.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were prepared as previously described (31) from HepG2 cell-transfected relative expression vector plasmids. Biotin end-labeled oligonucleotides were synthesized and annealed to obtain double-stranded DNA fragments. The oligonucleotide sequences of NF-κB1 were as follows: 5′-CATGGGACTTCCCCAGGAACAGC-3′ and 5′-GCTGTTCCTGGGGAAGTCCCATG-3′. EMSA was performed using the LightShift® Chemiluminescent EMSA kit (Pierce) according to the manufacturer's protocol. Briefly, the binding reactions were performed in 20-μl samples containing 1 μl of biotin-labeled oligonucleotides, 4 mg of nuclear extracts, 2 mg of poly(dI-dC), 2 μl of 10× binding buffer, 0.1 mm EDTA, and 10% glycerol. After incubation at room temperature for 20 min, samples were separated on 6% polyacrylamide gels with 0.5× TBE buffer and transferred to a nylon membrane (Amersham Biosciences) at 380 mA (∼100 V) for 15 min. A chemiluminescent detection of membranes was scanned by Image Station 4000R (Kodak, Rochester, NY). Specificity of the protein-DNA interaction was confirmed by competition with 20- or 100-fold of unlabeled probes of the same sequence, or the NF-κB1 mutated probes: 5′-CATGtGACTTCaCCAGGAACAGC-3′.
Chromatin Immunoprecipitation Assay (ChIP)
ChIP assays were carried out using a commercial ChIP assay kit (Upstate Biotechnology, Billerica, MA). Briefly, HepG2 cells transfected with relative plasmids were cross-linked to histones of DNA using 1% formaldehyde at 37 °C for 10 min. After washing with cold phosphate-buffered saline, cells were scraped into a conical tube and sonicated to shear the chromatins. The sonicated chromatins were incubated with anti-p65 antibody, anti-p50 antibody, or an isotype control IgG for 2 h. The chromatin-antibody complex was precipitated with protein A-agarose beads. The DNA isolated from the complex was subject to PCR amplification using the following primers flanking the NF-κB1 site in IP-10 promoter: 5′-AACTTGGAGGCTACAATAAA-3′ and 5′-GAGGAATGTCTCAGAAAACG-3′.
Confocal Laser Scanning Microscopy
HepG2 cells were cultured on coverslips at a density of 5 × 104 cells/coverslip. After transfecting the relative plasmids for 48 h, cells were fixed with ice-cold methanol for 10 min, blocked with 1% bovine serum albumin in phosphate-buffered saline for 1 h, and probed for 90 min at room temperature with anti-p65 antibody (Santa Cruz). After probing, the signal was visualized with fluorescein isothiocyanate-conjugated second antibody (Sigma). Nuclei were stained using 4′,6-diamidino-2-phenylindole (Sigma). Micrographs were acquired with a BX51 system equipped with DP70 (Olympus, Japan).
ELISAs
The amounts of IP-10 present in culture supernatants were determined using a specific rat anti-human IP-10 ELISA kit (eBioscience, San Diego, CA) according the manufacturer's instructions.
Western Blotting
Total proteins, nuclear proteins, and cytoplasmic proteins were prepared as previously described (32), separated by stacking gel and SDS-PAGE with a Tris glycine system at 100 V for 1 h, and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were blocked in 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween 20 for 2 h at room temperature. Then the membranes were incubated with specific anti-p65 antibody (Santa Cruz), anti-phospho-TAK1 (Thr184) antibody (Cell Signaling Technology, Boston, MA), or p-IKKα (Thr23) antibody (Santa Cruz) overnight at 4 °C. The membranes were washed three times and incubated with horseradish peroxidase-conjugated secondary antibody. Proteins were visualized by ECL Western blotting substrate (Themo Pierce, Rockford, IL).
Migration Assay
Migratory activity was quantified using 24-well Transwell inserts (5-μm pore size, Costar, Bethesda, MD). PBLs (1 × 105) in 200 μl of RPMI medium were added to the upper chamber. 1-ml of culture supernatant from the cells transfected with the relative plasmids was added to the lower chamber. To investigate the role of IP-10 in cell migration, 10 μg of neutralizing anti-IP-10 (Abcam, Cambridge, MA) isotype-matched control antibody was added 30 min prior to the migration assay. Supernatants of HepG2 cells transfected with pCMV-HBx following treated with the NF-κB inhibitor SN-50 (Upstate Biotechnology, Lake Placid, NY) were also investigated. The chambers were incubated for 4 h at 37 °C in 5% CO2, then the Transwell inserts were removed and migration cells were hematoxylin and eosin stained and counted.
Data Analysis
Data were expressed as mean ± S.D. of three determinations. Statistical analysis data were analyzed using the Statistical Package for Social Sciences (SPSS) software (version 13.0). Statistical comparisons were made using Student's t test, with p < 0.05 being considered significant.
RESULTS
Up-regulation of IP-10 Expression in HBV-infected Liver Tissues
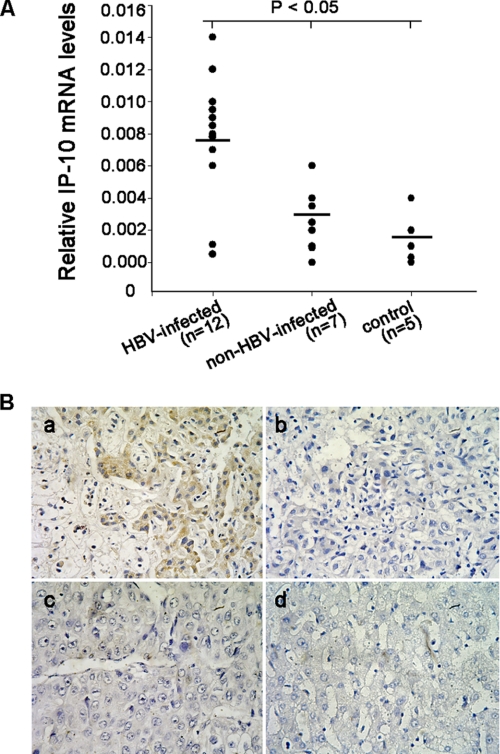
The up-regulation of IP-10 expression has been reported in the PBMCs, plasma, and liver of patients with chronic hepatitis B (10). To confirm the previous report, we analyzed the expression of IP-10 in HBV-infected liver tissue, and found that the mRNA levels of IP-10 were much higher in HBV-positive liver cancer tissues, compared with HBV-negative liver cancer tissues or normal liver tissues (Fig. 1A). The similar protein result was also confirmed by immunohistochemical staining (Fig. 1B). These data suggested that IP-10 is elevated in HBV-infected liver tissue, and may play an important role in HBV infection-induced hepatitis.
FIGURE 1.
IP-10 expression in human liver tissues. A, real time PCR detection of IP-10 mRNA expression in HBV-infected, non-HBV-infection, and normal control liver tissues. Results are mean ± S.D. of three experiments performed in duplicate and relative to the housekeeping gene GAPDH. Bars represent mean. B, detection of IP-10 protein expression in liver tissue by immunohistochemistry. a, immunoreactive IP-10 protein was mainly visualized on sinusoidal endothelium and hepatocytes in HBV-infected liver cancer tissue; b, isotype IgG was used as a negative control in HBV-infection liver cancer tissue; c, immunoreactive IP-10 protein was scarcely visualized in non-HBV-infection liver cancer tissue; d, in normal control liver tissues, the IP-10 protein could not be detected by immunohistochemistry.
HBx Induces IP-10 Gene Expression in HepG2 Cells
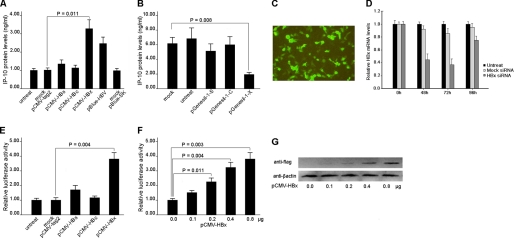
To evaluate the effect of HBV on IP-10 expression, HepG2 cells were transfected with the full-length HBV gene (pBlue-HBV) for 48 h and protein levels of IP-10 in the supernatants were measured. As shown in Fig. 2A, IP-10 protein was significantly increased in the pBlue-HBV-transfected group relative to control. We then evaluated the effect of different HBV proteins by transfecting pCMV-HBs, pCMV-HBc, and pCMV-HBx, respectively. The result showed that transfection of HBx, rather than HBs, or HBc-expressing vector increased IP-10 protein levels (Fig. 2A). Consistently, using siRNA technology, we found that knockdown of the HBx gene significantly decreased the protein levels of IP-10 in HepG2.2.15 cells (Fig. 2B). The efficiencies of transfection and gene silencing were determined in Fig. 2, C and D. Interestingly, we found that knockdown of either the HBs or HBc genes had no effect on IP-10 expression in HepG2.2.15 cells (Fig. 2B). Together, these data suggested that HBx is a critical regulator for IP-10 expression during HBV infection.
FIGURE 2.
HBx protein induces IP-10 expression. A, the full-length gene of HBV (pBlue-HBV) and plasmids expressing different HBV proteins were transfected into HepG2 cells, respectively. The protein levels of IP-10 were measured by the ELISA kit. Transfection efficiency was 35.8 ± 6.2%. Results are mean ± S.D. of three experiments performed in duplicate. Error bars represent S.D. siRNA-expressing vectors targeting different HBV proteins and mock plasmids were transfected into HepG2.2.15 cells, respectively. The supernatant protein levels of IP-10 were analyzed by the ELISA kit. The transfection efficiency was 41.6 ± 7.4%. The results are representative of three experiments. C, transfection efficiency of GFP-expressing HBx siRNA plasmids into HepG2.2.15 cells were observed under a fluorescence microscope. D, siRNA-expressing vectors targeting HBx proteins and mock plasmids were transfected into HepG2.2.15 cells, respectively. The HBx mRNA levels were analyzed by real time PCR after 48, 72, and 96 h. The mRNA levels of HBx in HepG2.2.15 cells transfected with mock plasmids were set to 1.0 and results relative to the housekeeping gene GAPDH. E, HepG2 cells were co-transfected with the IP-10 promoter luciferase reporter vector and different HBV protein-expressing vectors, respectively. Relative luciferase activity was determined by standard procedures. The luciferase activity of the mock pCMV-tag group was designated as 1.00. F, HepG2 cells were co-transfected with different doses of HBx-expressing vector and the reporter vector. 48 h later, relative luciferase activity was determined. G, HepG2 cells were transfected with different doses of pCMV-HBx-FLAG, in which the sequence for the FLAG epitope (DYKDDDDK) was tagged to the 3′ end of the HBx sequence, and the sequence of HBx-FLAG was inserted into the pCMV-tag2 vector. The increased protein levels of HBx were determined by Western blot with anti-FLAG antibody (Sigma).
To determine whether HBx induces IP-10 gene transcription through a promoter, a human IP-10 promoter reporter plasmid containing the sequence from −953 to +97 (relative to the transcriptional start site) of the 5′-flanking region was co-transfected with individual HBV protein-expressing plasmids (pCMV-HBs, pCMV-HBc, or pCMV-HBx) or mock plasmid (pCMV-tag2) into HepG2 cells. The results show that the IP-10 promoter was markedly activated by the HBx protein in a dose-dependent manner (Fig. 2, E and F). In line with this, a gradual increased level of HBx protein in HepG2 cells was confirmed by Western blot (Fig. 2G).
NF-κB Activity Is Required for HBx-induced IP-10 Expression
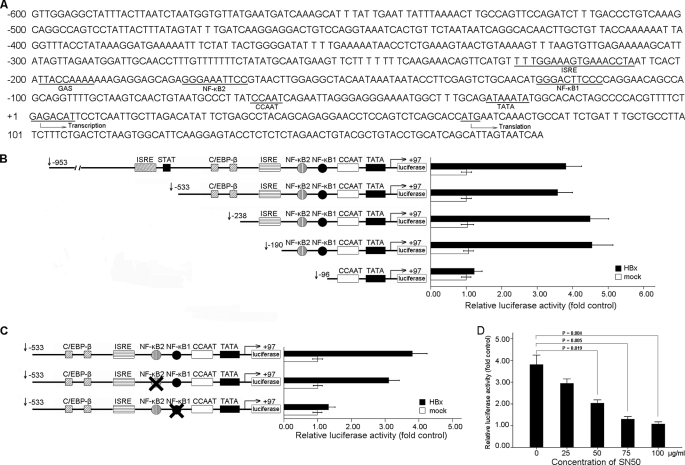
Analysis using the Genomatix MatInspector program and Gene2Promoter software revealed the presence of many consensus cis-elements including GAS, ISRE, AP-1, and NF-κB binding sites on the IP-10 promoter (Fig. 3A). To investigate the role of these cis-elements in regulating IP-10 expression by the HBx protein, a series of 5′ deletions of the IP-10 promoter were constructed and co-transfected with pCMV-HBx, respectively, into HepG2 cells. Luciferase activity was measured 48 h after transfection. As shown in Fig. 3B, the deletion from nt −953 to −190 did not affect HBx-induced luciferase activity, however, deletion to nt −96 significantly decreased HBx-induced luciferase activity, suggesting that the sequence between nt −190 to −96 is critical for activation of the IP-10 promoter by the HBx protein. Coincidently, two NF-κB binding sites (NF-κB1 and NF-κB2) were found in this region, and could be the reason for IP-10 activation. Thus, we mutated these two NF-κB sites by multiple rounds of PCR, and found that only mutation of NF-κB1 but not the NF-κB2 binding site effectively reduced HBx-induced activation of the IP-10 promoter (Fig. 3C). These data suggested that the NF-κB1 binding site is required for activation of the IP-10 promoter regulated by the HBx protein.
FIGURE 3.
The κB site in the IP-10 promoter involved in HBx-induced IP-10 expression. A, potential binding sites for cis-acting elements in the 5′-flanking region of the human IP-10 gene are shown. Gene2Promoter software revealed several transcription factor binding sites, including NF-κB, ISRE, and GAS. The transcription start site and translation start site are also indicated in the figure. B, effect of HBx protein on the truncated IP-10 promoter constructs. HepG2 cells were co-transfected with serial truncated IP-10 promoter constructs and pCMV-HBx, and relative luciferase activity was determined. The schematic constructs shown (left) and the bar graphs are the relative levels of luciferase activity in each of the transfected samples (right). C, effect of mutated κB sites on activity of the IP-10 promoter. HepG2 cells were co-transfected with pCMV-HBx and the construct with mutated to NF-κB1 or NF-κB2 sites. The relative luciferase activity was determined. D, NF-κB activity was necessary for HBx-induced IP-10 expression. HepG2 cells were co-transfected with pCMV-HBx and the reporter vector following treatment with the NF-κB inhibitor SN50 at different concentrations as indicated. After 48 h, luciferase activity was measured. Results are mean ± S.D. of three experiments performed duplicate. Error bars represent S.D.
To further determine the roles of NF-κB in activation of the IP-10 promoter by the HBx protein, HepG2 cells were treated with different concentrations of the NF-κB inhibitor SN50 after co-transfection with pCMV-HBx and the −953 to +97 IP-10 reporter plasmid. Results from the luciferase activity assay showed that blocking NF-κB by SN50 resulted in inhibition of the IP-10 promoter activity (Fig. 3D), suggesting that NF-κB activation is required to activate the IP-10 promoter by the HBx protein.
NF-κB Binds to the Promoter of HBx-induced IP-10 Directly
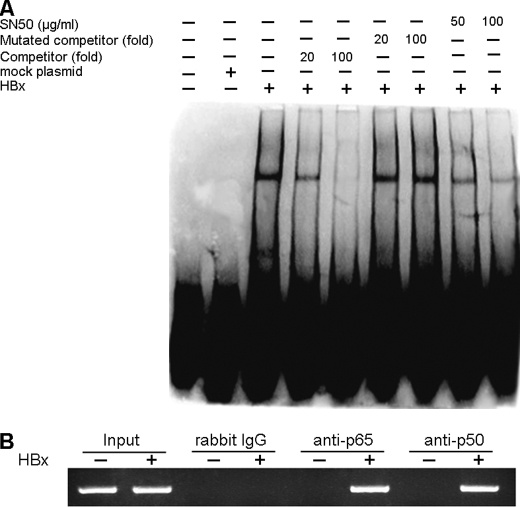
Next, we asked whether the activated NF-κB bound to the IP-10 promoter directly. For this purpose, we performed EMSA with a probe of biotin-labeled NF-κB1 binding oligonucleotides in the IP-10 promoter (−125 to −102), and found that the DNA binding activity of NF-κB was significantly increased in cells transfected with pCMV-HBx, compared with mock plasmid (Fig. 4A). To determine the specificity of the NF-κB binding activity, nuclear extracts were incubated with labeled NF-κB1 binding oligonucleotides in the presence of either an unlabeled wild type NF-κB binding probe or a mutated probe. As shown in Fig. 4A, the wild type NF-κB-binding oligonucleotides, but not the mutated oligonucleotides abrogated NF-κB complexes. Consistently, the addition of NF-κB inhibitor SN50 also inhibited the DNA binding activity of NF-κB (Fig. 4A). These data indicate that NF-κB binds to the NF-κB1 binding site in the IP-10 promoter.
FIGURE 4.
NF-κB subunits bind to the IP-10 promoter directly. A, EMSA showed direct binding of NF-κB to the NF-κB1 site of the IP-10 promoter. HepG2 cells were transfected with pCMV-HBx or mock plasmids for 48 h. Nuclear extracts were subjected to EMSA with biotin-labeled κB1 or κB2 oligonucleotides in the absence and presence of the indicated folds of unlabeled competitors or unlabeled mutated competitors. In addition, the NF-κB inhibitor SN50 was involved in the indicated case. B, the CHIP assay showed direct binding of p65 and p50 to the IP-10 promoter. Amplification of a 177-bp DNA fragment containing the NF-κB1 site in the IP-10 promoter and the input DNA are shown.
Using a comparable approach, we also performed a ChIP assay. Chromatin fragments were prepared from HepG2 cells transfected with pCMV-HBx and immunoprecipitated with antibody against either the p50 or p65 subunit. A 177-bp DNA fragment was amplified by PCR from DNA isolated from cells transfected with pCMV-HBx in the presence of anti-p50 or anti-p65 antibody, but they were not detected in the presence of mock plasmid or control antibody (Fig. 4B). Thus, these data suggested that NF-κB binds to the NF-κB1 binding site in the IP-10 promoter.
HBx Induces Translocation of NF-κB Subunits p65 into the Nucleus
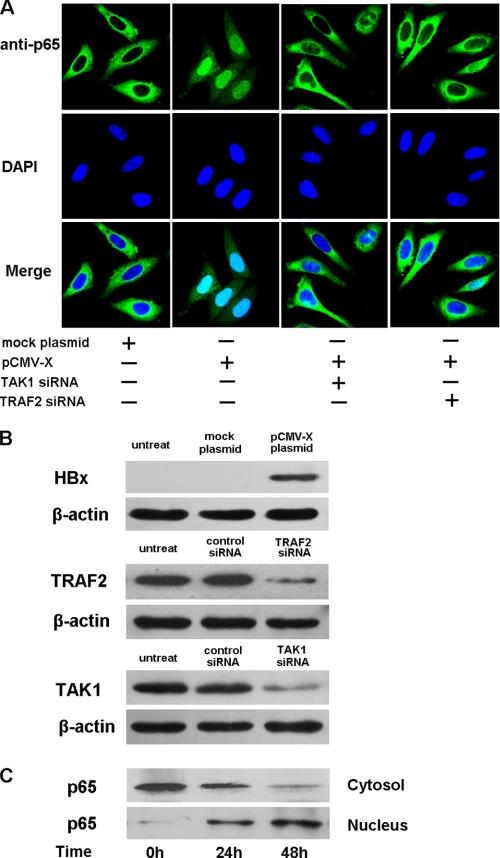
NF-κB is composed of homo- and heterodimers, typically p65 and p50 heterodimers. After the destruction of IκB, NF-κB is capable of translocating into the nucleus and transactivates target genes (33). We examined the effect of the HBx protein on translocation of p65 from the cytosol to the nucleus after transfecting pCMV-HBx into HepG2 cells. Nuclear translocation of p65 was defined by confocal microscopy. As shown in Fig. 5A, transfection with pCMV-HBx, but not the pCMV-tag, caused a remarkable translocation of the p65 subunit of NF-κB from the cytosol to the nucleus. In addition, we also tested the HBV-transfected HepG2.2.15 cell line. A similar result was observed; and knockdown of HBx expression by transfecting the siRNA impaired p65 translocation (data not shown). Moreover, at 0, 24, and 48 h post-transfection of pCMV-HBx, protein levels of p65 in cytosol and nucleus fractions were analyzed by Western blot using anti-p65 antibody. As shown in Fig. 5C, the levels of p65 protein decreased in the cytosol and increased in the nucleus in parallel with transfection times. However, this phenomenon was not observed in cells transfected with the pCMV-tag (data not shown). Together, these data suggest that HBx protein facilitates translocation of p65 from the cytosol to the nucleus.
FIGURE 5.
HBx protein induces the translocation of NF-κB subunits. A, HepG2 cells were transfected with HBx plasmids or co-transfected with HBx plasmids and TRAF2 or TAK1 siRNA for 48 h. Cells were then fixed, permeabilized, and stained with anti-p65 antibody followed by incubation with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, and immunofluorescence was monitored by confocal microscopy. B, the levels of ectopic HBx protein in cells transfected with pCMV-HBx-FLAG are shown by Western blot using the anti-FLAG antibody. The levels TRAF2 and TAK1 in cells transfected with the respective siRNA and controls are also shown by Western blots using the anti-TRAF2 antibody or anti-TAK1 antibody. C, levels of cytosolic and nuclear p65 protein were determined at the indicated times by Western blot using anti-p65 antibody after transfection with pCMV-HBx. DAPI, 4′,6-diamidino-2-phenylindole.
NF-κB Subunits p65 and p50 Increase the Transcriptional Activity of IP-10
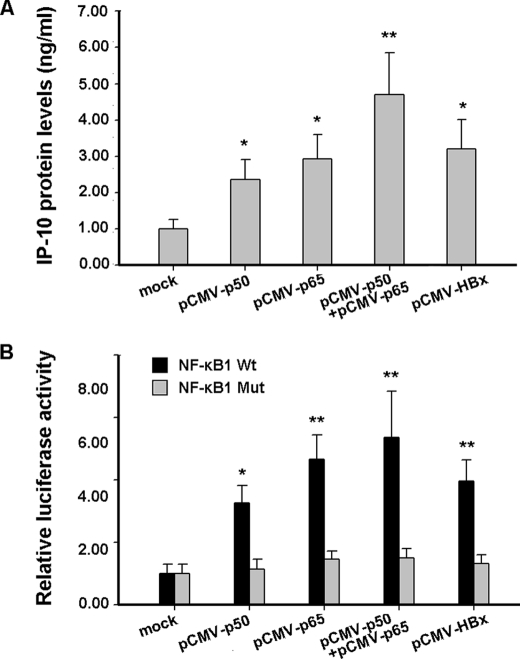
NF-κB is composed of homo- and heterodimers, typically p65 and p50 heterodimers. To investigate the effect of p50 and p65 subunits of NF-κB on transcriptional activation of IP-10, HepG2 cells were transfected with the plasmid expressing p65 or p50, and the protein levels of IP-10 were determined by ELISA. As shown in Fig. 6A, transfection of p50 or p65 proteins increased the IP-10 protein levels, and IP-10 reached the highest level when both p50 and p65 proteins were present. Furthermore, we co-transfected HepG2 cells with plasmids expressing p65 or p50 and IP-10 (−534/+97) reporter plasmids. Results from luciferase activity assays showed that transcriptional activity of IP-10 was higher in the presence of the p50 or p65 proteins compared to the control (Fig. 6B). The highest level was reached when both p50 and p65 proteins were present (Fig. 6B). However, mutating the NF-κB1 binding site in the IP-10 promoter abolished the effect of the p50 and/or p65 proteins on activation of the IP-10 promoter (Fig. 6B). Therefore, these data suggested that the p65/p50 heterodimer induces IP-10 expression.
FIGURE 6.
NF-κB subunits increase the IP-10 expression. A, HepG2 cells were transfected with p65 and/or p50 expression constructs. IP-10 protein levels in the supernatants were measured by the ELISA kit. Results are mean ± S.D. of three experiments performed in duplicate. B, HepG2 cells were co-transfected with (−534/+97) IP-10 or NF-κB1 Mut reporter constructs and p65 or p50 expression constructs, respectively. After 48 h, luciferase activity was measured. The luciferase activity of the cells co-transfected with pCMV-tag2 and the indicated reporter plasmids was set to 1.00, respectively. Results are mean ± S.D. of three experiments performed in duplicate. Error bars represent S.D. *, a statistically significant difference from mock plasmids (p < 0.05). **, a statistically significant difference from mock plasmids (p < 0.01).
HBx-induced IP-10 Expression Is Mediated by the TRAF2/TAK1/NF-κB Signaling Pathway
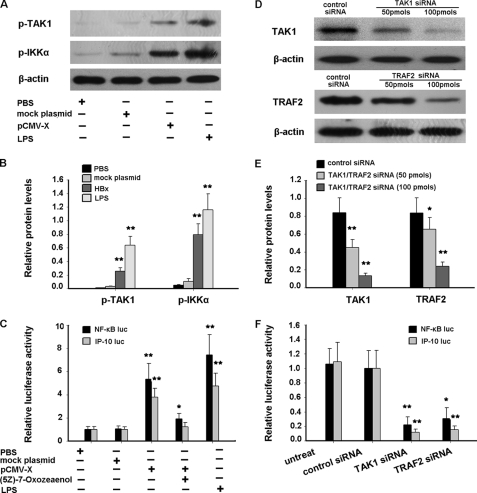
Mechanisms on the activation of NF-κB by HBx have been reported before (16, 34). However, the upstream molecular event still remains largely unknown. Numerous studies have demonstrated that NF-κB can be activated through the Toll-like receptor signaling pathway. Toll-like receptors are a type of pattern recognition receptors and recognize molecules that are broadly shared by pathogens but distinguishable from host molecules, collectively referred to as pathogen-associated molecular patterns (35). After binding to ligand, Toll-like receptor signaling is transducted and the scaffold intermediate kinase TAK1 is activated, which subsequently activates IKK, leading to activation of NF-κB. Because HBx is a viral protein, it can also be considered a pathogen-associated molecular pattern. In this regard, we hypothesized that HBx induced activation of NF-κB via activating TAK1. After transfection of the HBx plasmid into the HepG2 cell line, phosphorylation of TAK1 and IKKα was observed in the HBx plasmid group, but not in mock or phosphate-buffered saline groups (Fig. 7, A and B). (5Z)-7-Oxozeaenol is a specific inhibitor of TAK1 by competing with ATP to bind TAK1, thus inhibiting TAK1 activity (36). Therefore, we further used (5Z)-7-Oxozeaenol to block the activity of HBx-induced TAK1 in HepG2 cells. As shown in Fig. 7C, both NF-κB activity and IP-10 promoter activity were reduced by TAK1 inhibition. In parallel, knockdown of TAK1 by the siRNA also caused the decrease of NF-κB and IP-10 promoter activities (Fig. 7, D–F). Together, these data suggest that HBx indeed may activate NF-κB via activating TAK1/IKK.
FIGURE 7.
TRAF2/TAK1/NF-κB signaling pathway is involved in HBx-induced IP-10 expression. A, HepG2 cells were transfected with HBx expression plasmids or mock plasmids. After 48 h, phosphorylation of TAK1 and IKKα was detected by Western blot. HepG2 cells stimulated with 100 ng/ml of lipopolysaccharide (LPS) for 30 min were used as positive control. B, semi-quantitative analysis of p-TAK1 and p-IKKα protein levels was performed by Band-Scan software 5.0, and expression of β-actin was used as control. This quantitative analysis represented the respective Western blot of the above (A). C, HepG2 cells were co-transfected with NF-κB or IP-10 promoter luciferase reporter plasmids and HBx expression plasmids or mock plasmids with or without the TAK1-specific inhibitor (5Z)-7-Oxozeaenol (50 nm). After 48 h, luciferase activity was measured. HepG2 cells stimulated with 100 ng/ml of LPS for 30 min were used as positive control. The luciferase activity of the cells co-transfected with mock plasmids pCMV-tag2 and the indicated report plasmids was set to 1.00, respectively. D and E, the knockdown of TAK1 and TRAF2 genes. HepG2 cells were co-transfected with the HBx expression plasmid and TAK1 or TRAF2 siRNA for 48 h. The protein levels of TAK1 and TRAF2 were analyzed by Western blot, respectively (D), and quantified with Band-Scan software 5.0 against the internal control β-actin (E). F, HBx-expressing HepG2 cells were co-transfected with NF-κB or IP-10 promoter luciferase reporter plasmids and TAK1 siRNA or TRAF2 siRNA. 48 h later, luciferase activity was measured. The luciferase activity of cells co-transfected with mock siRNA and the indicated report plasmids was set to 1.00. In C and F, results are mean ± S.D. of three experiments performed in duplicate. Error bars represent S.D. *, p < 0.05, compared with mock plasmids. **, p < 0.01, compared with mock plasmids.
To further investigate the upstream signal molecule(s) that result in activation of TAK1, we concentrated on TRAF2 due to: 1) TRAF family members such as TRAF6 and TRAF2 that participate in the activation of TAK1; and 2) that HBx may activate TNFR1 (37) and TRAF2 is the adaptor for TNFR1 signaling. For this purpose, we knocked down the TRAF2 gene by transfecting TRAF2 siRNA into cells. The efficiency of TRAF2 siRNA was confirmed by Western blot (Fig. 7, D and E). Under such conditions, we measured the NF-κB and IP-10 promoter activity induced by HBx. The result showed that TRAF2 knockdown impaired NF-κB activity induced by HBx (Fig. 7F). Consistently, the knockdown of TRAF2 or TAK1 resulted in inhibition of nuclear translocation of the NF-κB subunit p65 (Fig. 5A). The levels of ectopic HBx protein as well as the levels TRAF2 and TAK1 in cells transfected with the respective siRNA and controls were shown by Western blots (Fig. 5B). Taken together, our data suggest that HBx-induced IP-10 expression might be mediated by the TRAF2/TAK1/NF-κB signaling pathway.
HBx-induced IP-10 Increases Migration of PBLs
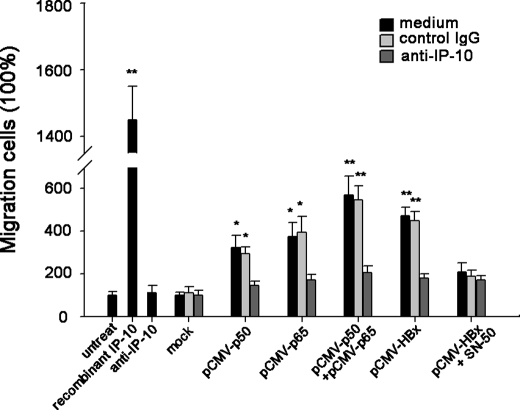
To define the functionality of the secreted IP-10, we performed a migration assay. With the 4-h time frame of the experiment, minimal migration of PBLs was observed in response to the supernatant from HepG2 cells transfected with mock plasmid; however, PBLs exhibited a decent increase of migratory activity in response to supernatants from cells transfected with plasmids expressing HBx, p50, or p65 (Fig. 8). The addition of neutralizing anti-IP-10 but not control antibody to the supernatants significantly impaired this migration (Fig. 8). Interestingly, we additionally found that treatment with the NF-κB inhibitor SN50 after transfection of pCMV-HBx could decrease the chemotactic activity (Fig. 8). Together, these data suggested that HBx-induced IP-10 may mediate the migration of PBLs and NF-κB activity is involved in this process.
FIGURE 8.
HBx-induced IP-10 expression increases the chemotaxis. Supernatants from HepG2 cells transfected with the indicated plasmid cells were added to the lower chamber of transwell plates, and PBLs were placed in the upper chamber. The lower chamber was in the presence of neutralizing anti-IP-10 antibody or control antibody. After a 4-h incubation, the number of migrated cells was counted in three randomly selected fields per well. Recombinant IP-10 (10 ng/ml) and antibody alone (10 μg/ml) were used as positive and negative controls, respectively. The number of migrated cells in response to supernatants from HepG2 cells transfected with the pCMV-tag was set to 100%. Results are mean ± S.D. of three experiments performed in duplicate. *, a statistically significant difference from mock plasmids (p < 0.05). Error bars represent S.D. **, a statistically significant difference from mock plasmids (p < 0.01).
DISCUSSION
During HBV infection, the increase of chemokine IP-10 is a critical step for inflammatory cell recruitment and hepatopathology. In the present study, we demonstrate the increase of IP-10 levels in HBV-infected patients, and elucidate the molecular mechanism of IP-10 expression through the viral regulatory protein HBx-activated NF-κB signaling pathway in the human hepatocellular cell line HepG2. We also define that HBx-induced IP-10 can attract inflammatory cells in vitro.
The regulation of IP-10 expression has been reported by various transcription factors and signal elements. For instance, the phosphorylation of STAT1 could transcriptionally activate IP-10 though ISRE-binding factors (38–40), and signal molecules such as PKC, PKA, Akt, and p38 MAPK may be involved the regulation (41–44). Regardless of these, the mechanism of the increase of IP-10 expression in individuals carrying HBV (10) so far remains elusive. Here, we demonstrate that HBx rather than HBs or HBc encoded by HBV activates IP-10 expression. Different from HBs or HBc, HBx profoundly influences various signaling pathways. The HBx protein interacts with and stimulates many kinases such as PKC, Jak1, IKK, phosphatidylinositol 3-kinase, SAPK (stress-activated protein kinase), and Akt/PKB (12, 37, 45–47). The 154 amino acids of the HBx protein contain regions important for transactivation, dimerization, p53 binding, and 14-3-3 protein binding (13). In this study, we find that HBx transactivates IP-10 by activating and translocating NF-κB to the nucleus. Although interferons α/β or γ are known as inducers for IP-10 via the STAT1 signaling pathway (38, 48), we confirm that the levels of IFN-α, IFN-β, IFN-γ mRNAs, and proteins are not changed in HepG2 cells after transfection of HBx vector.4 Thus, interferons are unlikely involved in HBx-induced IP-10 expression. Nevertheless, whether other signaling pathway(s) contribute to HBx-induced IP-10 expression still needs to be clarified, if considering that HBx targets multiple signaling pathways.
NF-κB can be homo- or heterodimers, made up of different members of the Rel family of proteins (p50/NF-κB1, p52/NF-κB2, c-Rel/Rel, p65/Rel-A, Rel-B, and Dorsal). Of these, the p50/p65 heterodimer (commonly referred to as NF-κB) is the most abundant and ubiquitous. In this study, we find the heterodimer of p50 and p65 involved in HBx-induced IP-10 expression, evaluated by EMSA and ChIP assays. These results are further confirmed by transfection of wild type p50 and p65 into HepG2 cells and followed an increase of IP-10 expression. Previous studies have reported that HBx activated NF-κB through multiple ways, including 1) HBx may induce the degradation of IκBα and the p105 via phosphorylation of these proteins (16); 2) HBx directly interacts with IκBα and IκBϵ, leading to translocation of NF-κB into the nucleus (34); 3) the HBx protein may activate hepatic NF-κB via TNFR1 (37); and 4) collaboration of HBx with hepatitis B virus X-associated protein is also capable of coactivating NF-κB signaling (49). In addition, several upstream signal transduction cascades defined to be involved in HBx-induced activation of NF-κB, including PKC, Src, Ras, and Akt have been identified (12, 14, 39, 45). In this study, we define another upstream signaling pathway. HBx may activate NF-κB activity though phosphorylation of TAK1 and IKK. Blockade of TAK1 activity by its specific inhibitor markedly inhibits NF-κB activity and IP-10 luciferase activity. As a pivotal factor for MAPK and NF-κB activation in response to the Toll-like receptor, interleukin-1R, and TNFR stimulation (40), TAK1 activation requires the participation of TRAF family members such as TRAF2 and TRAF6. Here, we further confirm that TRAF2 is required for HBx-induced NF-κB activation.
IP-10 shares receptor CXCR3 with CXCL9 (Mig) recruiting inflammatory cells to the sites of infected tissues and may exaggerate the immune-mediated injury. In the mouse model of hepatitis virus infection, treatment with either anti-IP-10 or anti-Mig antibody shows a decrease of T cell infiltration into the brain (41, 42). Similarly, in HBV transgenic mice, blockade of IP-10 significantly reduces the recruitment of host-derived inflammatory cells into the liver and the severity of liver damage (9). Those findings demonstrate that CXCR3 signaling is critical in leukocytic chemotaxis and the pathogenesis of liver disease after HBV infection. Interestingly, in the present study, we find that such chemotaxis activity of HBx-induced IP-10 requires NF-κB activity. Transfection of either p65 or p50 expression vectors to cells significantly increased the chemoattractive effect, whereas the addition of NF-κB inhibitors to the culture medium significantly decreased the chemotaxis, suggesting that NF-κB is a critical mediator for the resulting HBx, IP-10-induced chemotaxis. Blockade of the NF-κB pathway has served as a potential strategy to treat inflammatory diseases and tumors, such as rheumatoid arthritis, myeloma, and T-cell leukemia/lymphoma (43, 44, 50). Our findings further suggest that targeting the NF-κB pathway is a potential therapeutic strategy against HBV-related inflammatory damage, which not only blocks IP-10 generation but also impairs its chemotatic function.
In summary, the data reported here show for the first time that the HBx protein encoded by HBV induces IP-10 expression directly in hepatocytes, and binding of NF-κB to the NF-κB1 site in the IP-10 promoter is important for HBx-induced IP-10 expression. The underlying molecular mechanism involves TRAF2/TAK1-mediated signaling pathway. Our data also indicate that the NF-κB signaling pathway may play an important role in chemotaxis of the HBx-induced IP-10. These findings may shed light on the understanding of how leukocytes migrate into the liver and exaggerate host-derived immune responses, and probably provide a novel therapeutic target in severe acute or fulminant hepatitis.
Acknowledgment
We thank Dr. Wen-jie Huang for the HBV protein expression vectors.
This work was supported by the National Key and Basic Research Development Program of China Grant 2007CB512900, State Project on Major Infectious Diseases Prevention Grant 2008ZX10002-009, Program for New Century Excellent Talents in University Grant NCET-08-0219, and Special Research Foundation for Universities affiliated with China Ministry of Education Grant Z2009005.
Y. Zhou, S. Wang, J.-W. Ma, Z. Lei, H.-F. Zhu, P. Lei, Z.-S. Yang, B. Zhang, X.-X. Yao, C. Shi, L.-F. Sun, X.-W. Wu, Q. Ning, G.-X. Shen, and B. Huang, unpublished data.
- HBV
- hepatitis B virus
- IFN-γ
- interferon-γ
- HBs
- surface hepatitis B
- HBc
- core hepatitis B
- nt
- nucleotide
- PBL
- peripheral blood lymphocyte
- EMSA
- electrophoretic mobility shift assay
- ChIP
- chromatin immunoprecipitation
- MAPK
- mitogen-activated protein kinase
- siRNA
- small interfering RNA
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ELISA
- enzyme-linked immunosorbent assay
- CMV
- cytomegalovirus
- TNFR1
- tumor necrosis factor receptor 1
- TRAF
- TNFR-associated factor.
REFERENCES
- 1.Chisari F. V., Ferrari C. (1995) Annu. Rev. Immunol. 13, 29–60 [DOI] [PubMed] [Google Scholar]
- 2.Tiollais P., Charnay P., Vyas G. N. (1981) Science 213, 406–411 [DOI] [PubMed] [Google Scholar]
- 3.Luster A. D., Wang J., Zhao J. H., Wang P. P., Xiang G. J., Lee Y. H., Yun Y., Doria M., Klein N., Lucito R., Schneider R. J. (1998) N. Engl. J. Med. 338, 436–4459459648 [Google Scholar]
- 4.Rossi D., Zlotnik A. (2000) Annu. Rev. Immunol. 18, 217–242 [DOI] [PubMed] [Google Scholar]
- 5.Narumi S., Tominaga Y., Tamaru M., Shimai S., Okumura H., Nishioji K., Itoh Y., Okanoue T. (1997) J. Immunol. 158, 5536–5544 [PubMed] [Google Scholar]
- 6.Nishioji K., Okanoue T., Itoh Y., Narumi S., Sakamoto M., Nakamura H., Morita A., Kashima K. (2001) Clin. Exp. Immunol. 123, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neville L. F., Mathiak G., Bagasra O. (1997) Cytokine Growth Factor Rev. 8, 207–219 [DOI] [PubMed] [Google Scholar]
- 8.Dufour J. H., Dziejman M., Liu M. T., Leung J. H., Lane T. E., Luster A. D. (2002) J. Immunol. 168, 3195–3204 [DOI] [PubMed] [Google Scholar]
- 9.Kakimi K., Lane T. E., Wieland S., Asensio V. C., Campbell I. L., Chisari F. V., Guidotti L. G. (2001) J. Exp. Med. 194, 1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Zhao J. H., Wang P. P., Xiang G. J. (2008) Hepatobiliary Pancreat. Dis. Int. 7, 45–50 [PubMed] [Google Scholar]
- 11.Robinson W. S., Clayton D. A., Greenman R. L. (1974) J. Virol. 14, 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kekulé A. S., Lauer U., Weiss L., Luber B., Hofschneider P. H. (1993) Nature 361, 742–745 [DOI] [PubMed] [Google Scholar]
- 13.Diao J., Garces R., Richardson C. D. (2001) Cytokine Growth Factor Rev. 12, 189–205 [DOI] [PubMed] [Google Scholar]
- 14.Doria M., Klein N., Lucito R., Schneider R. J. (1995) EMBO J. 14, 4747–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn J., Su F., Doria M., Schneider R. J. (1996) J. Virol. 70, 4978–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su F., Schneider R. J. (1996) J. Virol. 70, 4558–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucito R., Schneider R. J. (1992) J. Virol. 66, 983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon E. J., Jeong C. H., Jeong J. W., Kim K. R., Yu D. Y., Murakami S., Kim C. W., Kim K. W. (2004) FASEB J. 18, 382–384 [DOI] [PubMed] [Google Scholar]
- 19.Maguire H. F., Hoeffler J. P., Siddiqui A. (1991) Science 252, 842–844 [DOI] [PubMed] [Google Scholar]
- 20.Williams J. S., Andrisani O. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 3819–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S., May M. J., Kopp E. B. (1998) Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 22.Karin M., Delhase M. (2000) Semin. Immunol. 12, 85–98 [DOI] [PubMed] [Google Scholar]
- 23.Spurrell J. C., Wiehler S., Zaheer R. S., Sanders S. P., Proud D. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 289, L85–L95 [DOI] [PubMed] [Google Scholar]
- 24.Le Goffic R., Mouchel T., Aubry F., Patard J. J., Ruffault A., Jégou B., Samson M. (2002) Endocrinology 143, 1434–1440 [DOI] [PubMed] [Google Scholar]
- 25.Nazar A. S., Cheng G., Shin H. S., Brothers P. N., Dhib-Jalbut S., Shin M. L., Vanguri P. (1997) J. Neuroimmunol. 77, 116–127 [DOI] [PubMed] [Google Scholar]
- 26.Nakamichi K., Saiki M., Sawada M., Takayama-Ito M., Yamamuro Y., Morimoto K., Kurane I. (2005) J. Virol. 79, 11801–11812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law A. H., Lee D. C., Cheung B. K., Yim H. C., Lau A. S. (2007) J. Virol. 81, 416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asensio V. C., Maier J., Milner R., Boztug K., Kincaid C., Moulard M., Phillipson C., Lindsley K., Krucker T., Fox H. S., Campbell I. L. (2001) J. Virol. 75, 7067–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S. R., Lee J. H., Kim P. H. (2001) Eur. J. Immunol. 31, 1706–1715 [DOI] [PubMed] [Google Scholar]
- 30.Kim S. J., Rabbani Z. N., Dewhirst M. W., Vujaskovic Z., Vollmer R. T., Schreiber E. G., Oosterwijk E., Kelley M. J. (2005) Lung Cancer 49, 325–335 [DOI] [PubMed] [Google Scholar]
- 31.Takenaka M., Preston A. S., Kwon H. M., Handler J. S. (1994) J. Biol. Chem. 269, 29379–29381 [PubMed] [Google Scholar]
- 32.Kim Y., Fischer S. M. (1998) J. Biol. Chem. 273, 27686–27694 [DOI] [PubMed] [Google Scholar]
- 33.Hayden M. S., Ghosh S. (2004) Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 34.Weil R., Sirma H., Giannini C., Kremsdorf D., Bessia C., Dargemont C., Bréchot C., Israël A. (1999) Mol. Cell. Biol. 19, 6345–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aderem A., Ulevitch R. J. (2000) Nature 406, 782–787 [DOI] [PubMed] [Google Scholar]
- 36.Ninomiya-Tsuji J., Kajino T., Ono K., Ohtomo T., Matsumoto M., Shiina M., Mihara M., Tsuchiya M., Matsumoto K. (2003) J. Biol. Chem. 278, 18485–18490 [DOI] [PubMed] [Google Scholar]
- 37.Kim W. H., Hong F., Jaruga B., Hu Z., Fan S., Liang T. J., Gao B. (2001) FASEB J. 15, 2551–2553 [DOI] [PubMed] [Google Scholar]
- 38.Wenzel J., Zahn S., Bieber T., Tüting T. (2009) Arch. Dermatol. Res. 301, 83–86 [DOI] [PubMed] [Google Scholar]
- 39.Kim H., Lee Y. H., Won J., Yun Y. (2001) Biochem. Biophys. Res. Commun. 286, 886–894 [DOI] [PubMed] [Google Scholar]
- 40.Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 41.Mahalingam S., Farber J. M., Karupiah G. (1999) J. Virol. 73, 1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu M. T., Chen B. P., Oertel P., Buchmeier M. J., Armstrong D., Hamilton T. A., Lane T. E. (2000) J. Immunol. 165, 2327–2330 [DOI] [PubMed] [Google Scholar]
- 43.Feldmann M., Maini R. N., Bondeson J., Taylor P., Foxwell B. M., Brennan F. M. (2001) Adv. Exp. Med. Biol. 490, 119–127 [DOI] [PubMed] [Google Scholar]
- 44.Harousseau J. L., Shaughnessy J., Jr., Richardson P. (2004) Hematology Am. Soc. Hematol. Educ. Program, 237–256 [DOI] [PubMed] [Google Scholar]
- 45.Chung T. W., Lee Y. C., Kim C. H. (2004) FASEB J. 18, 1123–1125 [DOI] [PubMed] [Google Scholar]
- 46.Diao J., Khine A. A., Sarangi F., Hsu E., Iorio C., Tibbles L. A., Woodgett J. R., Penninger J., Richardson C. D. (2001) J. Biol. Chem. 276, 8328–8340 [DOI] [PubMed] [Google Scholar]
- 47.Lee Y. H., Yun Y. (1998) J. Biol. Chem. 273, 25510–25515 [DOI] [PubMed] [Google Scholar]
- 48.Rotondi M., Lazzeri E., Romagnani P., Serio M. (2003) J. Endocrinol. Invest. 26, 177–180 [DOI] [PubMed] [Google Scholar]
- 49.Shamay M., Barak O., Doitsh G., Ben-Dor I., Shaul Y. (2002) J. Biol. Chem. 277, 9982–9988 [DOI] [PubMed] [Google Scholar]
- 50.Horie R., Watanabe T., Umezawa K. (2006) Drug News Perspect. 19, 201–209 [DOI] [PubMed] [Google Scholar]