Abstract
In mammals, excess energy is stored in the form of triacylglycerol primarily in lipid droplets of white adipose tissue. The first step of lipolysis (i.e. the mobilization of fat stores) is catalyzed by adipose triglyceride lipase (ATGL). The enzymatic activity of ATGL is strongly enhanced by CGI-58 (comparative gene identification-58), and the loss of either ATGL or CGI-58 function causes systemic triglyceride accumulation in humans and mice. However, the mechanism by which CGI-58 stimulates ATGL activity is unknown. To gain insight into CGI-58 function using structural features of the protein, we generated a three-dimensional homology model based on sequence similarity with other proteins. Interestingly, the model of CGI-58 revealed that the N terminus forms an extension of the otherwise compact structure of the protein. This N-terminal region (amino acids 1–30) harbors a lipophilic tryptophan-rich stretch, which affects the localization of the protein. 1H NMR experiments revealed strong interaction between the N-terminal peptide and dodecylphosphocholine micelles as a lipid droplet-mimicking system. A role for this N-terminal region of CGI-58 in lipid droplet binding was further strengthened by localization studies in cultured cells. Although wild-type CGI-58 localizes to the lipid droplet, the N-terminally truncated fragments of CGI-58 are dispersed in the cytoplasm. Moreover, CGI-58 lacking the N-terminal extension loses the ability to stimulate ATGL, implying that the ability of CGI-58 to activate ATGL is linked to correct localization. In summary, our study shows that the N-terminal, Trp-rich region of CGI-58 is essential for correct localization and ATGL-activating function of CGI-58.
Keywords: Enzymes/Hydrolases, Enzymes/Lipid, Lipid/Lipase, Lipid/Triacylglycerol, Metabolism/Lipid, Protein/Binding/Lipid, Protein/Domains
Introduction
The protein CGI-58 (comparative gene identification-58; identical to ABHD5 (abhydrolase domain-containing protein 5)) plays an important role in mammalian fatty acid metabolism (1, 2). Interaction of CGI-58 with adipose triglyceride lipase (ATGL),2 the enzyme catalyzing the first step of lipolysis, enhances the hydrolytic activity of ATGL up to 20-fold (1, 3). CGI-58 is predicted to harbor an α/β-hydrolase domain. Typically, α/β-hydrolases exert their catalytic activity via a catalytic triad. In the case of CGI-58, however, the putative active site serine is replaced by an asparagine residue. Consequently, no triglyceride (TG)-hydrolyzing activity could be detected for the protein. Very recently, lysophosphatidic acid acyltransferase (LPAAT) activity of CGI-58 was discovered and kinetically characterized (4, 5). In humans, mutations in CGI-58 are associated with the development of neutral lipid storage disease with ichthyosis, which is also known as Chanarin Dorfman syndrome. These patients accumulate neutral lipids in various tissues and cell types, including granulocytes, and suffer from non-bullous congenital ichthyosiform erythroderma (2, 6, 7). Mutant forms of CGI-58 detected in patients with neutral lipid storage disease with ichthyosis harboring nonsense or missense mutations (recently reviewed in Ref. 8) are not competent for ATGL activation (1). CGI-58 mRNA is abundant in various tissues, including adipose tissue, liver, testis, neurons, muscle, and epidermis (1, 9). Interestingly, mRNA expression levels of CGI-58 and ATGL do not correlate in the tissues tested (1, 10–12). This may imply distinct or additional functions in various tissues. Patients with mutations in ATGL develop neutral lipid storage disease with severe cardiac myopathy and no ichthyosis (6). The phenotypic differences of patients with ATGL or CGI-58 deficiencies are indicative of an ATGL-independent function of CGI-58. Phenotypes associated with a mouse model for CGI-58 deficiency support the proposal that CGI-58 possesses an ATGL-independent function specifically important in skin metabolism (13). Newborn CGI-58-deficient mice suffer from systemic TG accumulation and severe hepatic steatosis, reflecting the important role of the protein in TG metabolism. Furthermore, these mice suffer from a severe skin permeability barrier defect and die within hours after birth. In contrast, skin of ATGL knock-out mice is normal (3). Despite the acknowledged importance of CGI-58 in lipolysis and skin function, the exact function of the protein remains unknown (14). CGI-58 was found to co-localize on the lipid droplet (LD) with LD-associated proteins of the PAT family. The PAT protein perilipin is only expressed in adipocytes and steroidogenic tissues. Perilipin is a structural protein that coats and protects the LD from lipolysis under basal conditions (15–18). Another member of the same protein family, adipose differentiation-related protein (ADRP), is widely expressed in various cell types (19–22). Interaction of CGI-58 with perilipin and ADRP was shown with co-immunoprecipitation, pull-down, and fluorescence complementation experiments (16, 17, 23–25). Interestingly, interaction of CGI-58 and perilipin is sensitive to hormonal stimulation. Activation of lipolysis disrupts the interaction of CGI-58 with perilipin and leads to the dissociation of CGI-58 from LDs (16, 23, 24, 26) and its colocalization with ATGL (24, 26). Currently, it is believed that this process regulates ATGL activity in adipocytes. In this study, we investigated the interrelation of CGI-58 function in ATGL stimulation and LD binding. We were interested in the potential contribution of the uncharacterized N terminus of CGI-58 to ATGL activation, localization, and CGI-58 LPAAT activity. Our results revealed that this N-terminal region of CGI-58 is crucial for stimulation of ATGL activity and does not affect LPAAT activity. Furthermore, our study demonstrates that this region is also important for correct localization of CGI-58 to the LD. The data therefore identify LD binding as a prerequisite for the stimulation of ATGL activity.
EXPERIMENTAL PROCEDURES
Three-dimensional Model of CGI-58
Three-dimensional homology models of murine CGI-58 were generated using SWISS-MODEL software packages (27) after sequence alignment of CGI-58 with the Aspergillus niger epoxide hydrolase (Protein Data Bank accession code 1qo7) (28) as template.
Cloning of CGI-58 and ATGL
Sequences containing the complete open reading frame of mouse CGI-58 (mCGI-58) and mouse ATGL (mATGL) were amplified by PCR using the Pwo SuperYield DNA polymerase kit (Roche Applied Science) and the FailSafeTM PCR system (Epicenter Biotechnologies, Madison, WI), respectively. Primers were designed to introduce endonuclease cleavage sites for subsequent cloning (supplemental Table 1). PCR products and target vectors were digested with corresponding restriction enzymes. Inserts coding for full-length or truncated mCGI-58 were ligated into the bacterial expression vector pGEX-6P2 (GE Healthcare), pSumo (kindly provided by Prof. Christoph D. Lima, Sloan-Kettering Institute), and eukaryotic expression vector pEYFP-N1 (BD Biosciences Clontech). Coding sequence for full-length mATGL was ligated into a pET21 vector modified with an N-terminal GB1 protein as solubility enhancer (29). Site-directed mutagenesis was performed using the QuikChange® site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions using primers listed in supplemental Table 1. The single mutant W21A resulted from incomplete mutagenesis during the production of the double mutant W21A/W25A. The triple mutant W21A/W25A/W29A was generated by site-directed mutagenesis of the double mutant W21A/W25A. All inserts were verified by sequence analysis.
Expression of Recombinant Proteins and Preparation of Cell Extracts
Strains BL21 and BL21 (DE3) were cultivated in selective LB media containing 50 μg/ml carbenicillin. Expression of mCGI-58 constructs in BL21 and mATGL in BL21 (DE3) were induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 16 and 20 °C, respectively. Bacterial cell extracts of mCGI-58 and mATGL were prepared by disrupting cells in buffer A (0.25 m sucrose, 1 mm EDTA, 1 mm dithiothreitol, 20 μg/ml leupeptin, 2 μg/ml antipain, 1 μg/ml pepstatin, 50 μg/ml lysozyme, pH 7.0) by sonication (Bandelin Sonoplus HD2070, Berlin, Germany). The cellular extracts were collected after centrifugation at 21,000 × g at 4 °C for 20 min. Expression of recombinant CGI-58 in COS-7 cells and preparation of cellular lysates was performed as described (30). Protein concentrations of cellular lysates were determined using a Bio-Rad protein assay kit and BSA as a standard, according to the manufacturer's protocol. Expression of the correct size of the proteins was confirmed by SDS-PAGE and/or Western blotting analysis.
Purification of GST-tagged mCGI-58 and Cleavage of the Fusion Protein
Bacterial cells were disrupted in buffer A, and the soluble GST-tagged proteins were bound to high affinity GST-resin (GE Healthcare) overnight and eluted with buffer B (50 mm Tris-HCl, pH 8.0, 100 mm KCl, 2 mm EDTA, 1 mm dithiothreitol, 0.05% Nonidet P-40) containing 1–15 mm reduced glutathione. Cleavage was performed overnight at 4 °C by adding PreScission Protease (GE Healthcare).
Purification of His-Sumo-tagged mCGI-58 and Truncated Constructs
Bacterial cells were disrupted in buffer containing 50 mm Tris-HCl, pH 7.5, 100 mm KCl, 1 mm dithiothreitol, 0.01% Nonidet P-40, 30 mm imidazole. Soluble His-tagged proteins were purified via affinity chromatography using prepacked His-Trap FF columns (GE Healthcare). CGI-58 constructs were eluted in buffer containing 50 mm Tris-HCl, pH 8.0, 100 mm KCl, 1 mm dithiothreitol, 0.05% Nonidet P-40, 250 mm imidazole.
Assay for TG Hydrolase Activity
Twenty μl of ATGL and 10 μl of CGI-58 lysates were incubated in a total volume of 100 μl of buffer A with 100 μl of substrate in a water bath at 37 °C for 60 min. TG substrate was prepared using triolein and radiolabeled triolein as tracer as described (12). As a control, incubations were performed under identical conditions using lysates expressing solely the N-terminal fusion proteins (GST or GB1). After incubation, the reaction was terminated by the addition of 3.25 ml of methanol/chloroform/heptane (10:9:7) and 1 ml of 0.1 m potassium carbonate, 0.1 m boric acid (pH 10.5). After centrifugation (800 × g, 15 min), the radioactivity in 1 ml of the upper phase was determined by liquid scintillation counting. Stimulation of mATGL was achieved by the addition of either lysates containing full-length or truncated GST-mCGI-58 variants, purified GST-tagged proteins, or purified GST-tagged proteins after treatment for GST cleavage.
Accumulation of TG in COS-7 Cells
COS-7 cells were co-transfected using Metafectene with expression vectors encoding His-tagged mATGL and yellow fluorescent protein (YFP)-tagged full-length or N-terminally truncated mCGI-58 variants. As a control, cells were transfected with His-tagged LacZ. After 24 h of transfection, cells were incubated in Dulbecco's modified Eagle's medium containing 10% fetal calf serum (Invitrogen), 400 μm oleic acid (complexed to BSA, 3:1), and 3H-labeled oleic acid (1 μCi/ml) (GE Healthcare) as tracer. After 18 h, cells were washed with phosphate-buffered saline. For determination of cellular triglyceride content, cellular lipids were extracted with hexane/isopropyl alcohol (3:2), dried, and then dissolved in chloroform. Lipids were separated on TLC (Merck silica gel 60) using hexane/diethylether/acetic acid (70:29:1) as solvent and visualized with iodine vapor. Triglycerides were isolated, and radioactivity was determined by liquid scintillation counting. For protein determination, cells were lysed with 0.1 m NaOH, 0.3 m SDS for 4 h, and protein content was measured using BCA reagent (Pierce) and BSA as a standard.
NMR Titrations
The Trp-rich peptide corresponding to residues VDSADAGGGSGWLTGWLPTWCP from Val10 to Pro31 of the N-terminal sequence of mCGI-58 was selected for NMR titration experiments. This peptide, termed WR1031, was obtained from Peptide Specialty Laboratories GmbH (Heidelberg, Germany). One mg of WR1031 was dissolved in 50 mm potassium phosphate buffer (pH 5.0) containing 10% D2O and 0.02% sodium azide. Deuterated dodecylphosphocholine (DPC-d38) (98%) was purchased from Cambridge Isotope Laboratories Inc. (Andover, MA). Deuterated detergent was used for two reasons: (a) deuterium is not NMR-active (i.e. it is invisible in the spectra, which consequently only display the resonance signals of protonated compounds (the peptide)), and (b) dipole-dipole couplings between the peptides in close contact with the detergent micelles are substantially reduced, which would otherwise lead to line-broadening and a decrease in spectral quality. A 750 mm stock solution of DPC-d38 micelles was prepared by adding the DPC-d38 powder to phosphate-buffered saline and treating the solution in an ultrasonic bath at room temperature for 10 min. Aliquots of this DPC-d38 micelles were added in six equal steps of 10 μl to the dissolved peptide to a final concentration of 68 mm (no significant further changes in the resonance signals were observed starting at a concentration of 24 mm DPC-d38). Before each addition, one-dimensional 1H- spectra with 64 scans each were recorded on a Bruker Avance DRX 500-MHz spectrometer equipped with an HX inverse probe with z axis gradients at 305 K.
Cellular Localization of mCGI-58 Mutants
For localization studies, COS-7 cells were transfected with expression vectors encoding full-length, N-terminally truncated mCGI-58 variants, and point mutants of mCGI-58 with C-terminal fusions of YFP. COS-7 cells were maintained in Dulbecco's minimal essential medium (Invitrogen) containing 10% fetal calf serum, penicillin/streptomycin under humidified atmosphere, 37 °C, and 5% CO2. COS-7 cells were seeded on glass coverslips in 6-well dishes (1.2 × 105 cells/well) and transfected with YFP-tagged full-length or truncated/mutated mCGI-58 constructs. Twenty-four h after transfection, cells were incubated for 20 h in Dulbecco's modified Eagle's medium containing fetal calf serum, supplemented with oleic acid (400 μm) complexed with a ratio of 3:1 to fatty acid-free BSA (Sigma) to increase LD formation. LDs were stained by the addition of 1 μg/ml BODIPY® 558/568 C12 (Invitrogen) and incubation for 30 min at 37 °C as described (31). Cells were then washed twice with phosphate-buffered saline, overlaid with Dulbecco's modified Eagle's medium, and analyzed. Microscopy was performed using a Leica SP2 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) with spectral detection and an HCX PL APO ×63 oil immersion objective, numerical aperture 1.32. YFP fluorescence was excited at 514 nm and detected at 520–540 nm. BODIPY® 558/568 C12 was excited at 543 nm and detected at 570–590 nm. Images represent single optical sections. From all cells, transmission images were also recorded.
Physical Interaction of CGI-58 and ATGL (ELISA)
For the detection of interacting proteins, ELISA plates (MaxiSorp, Nalge Nunc International, Rochester, NY) were coated with 1 μg of mATGL (partially purified using affinity resin) in coating buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl) overnight at 4 °C. The next day, wells were blocked with 5% BSA in coating buffer for 1 h at room temperature. Then 100 μg of protein/well of E. coli bacterial extracts containing GST-tagged mCGI-58, mCGI-58 mutants, or as control GST in coating buffer were added and incubated overnight at 4 °C. After washing with coating buffer containing 0.05% Tween 20 (wash buffer), rabbit anti-CGI antibody (provided by Dawn Brasaemble (Rutgers University)), diluted in coating buffer containing 0.5% BSA (1:3000), was added. Subsequent to three further washes, horseradish peroxidase-conjugated anti-rabbit antibody (Vector Laboratories Inc., Burlingame, CA) diluted in the same buffer (1:1000) was added. After washing three times with wash buffer, the absorbance of tetramethylbenzidine was determined at 450 nm using 620 nm as a reference wavelength.
Lysophosphatidic Acid Acyltransferase (LPAAT) Assay
The reaction mixture for LPAAT activity contained 1 μg of purified His-Sumo-tagged mCGI-58 in 50 mm Tris-HCl buffer, pH 7.5, 100 mm KCl, 50 μm LPA (or LPC), and 10 μm [1-14C]oleoyl-CoA (110,000 dpm/nmol) in a total volume of 100 μl. [1-14C]Oleoyl-CoA (59.3 mCi/mmol) and 1-oleoyl-LPA and phosphatidic acid were purchased from PerkinElmer Life Sciences and Avanti® Polar Lipids Inc. (Alabaster, AL), respectively. The reaction mixture was incubated at 30 °C for 10 min and terminated by extracting lipids with 1.2 ml of chloroform/methanol (2:1, v/v) and 400 μl of acidified water (2% phosphoric acid). Chloroform extracts containing total lipids were separated by TLC using silica gel 60 TLC plates (Merck) in chloroform/methanol/acetone/acetic acid/water (50:10:20:15:5 by volume) as solvent system (4). Spots co-eluting with the phosphatidic acid standard were excised and quantified by liquid scintillation counting.
RESULTS
Domain Organization and Homology Model of Murine CGI-58
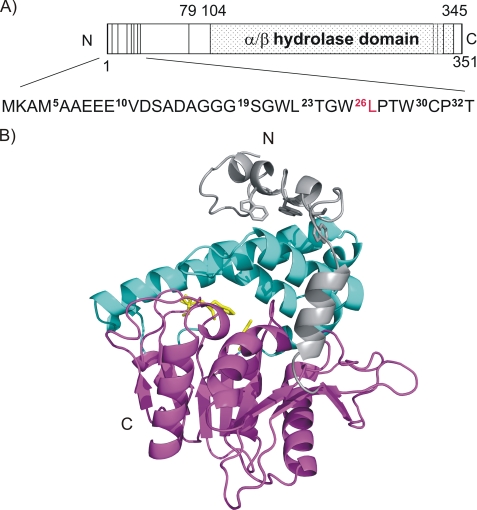
Human and mouse CGI-58 display 94% sequence identity and consist of 349 and 351 amino acids, respectively. To date, no experimental high resolution three-dimensional structural information for mCGI-58 is available. Based on sequence similarity, mCGI-58 is predicted to contain a domain with an α/β-hydrolase fold (PFAM PF00561, InterPro IPR000073) between residues Arg104 and Glu345 (Fig. 1A). Depending on the domain data base, the “α/β-hydrolase-related” region is predicted to extend from Gly62 to His348. Our three-dimensional model of mCGI-58 ranges from Ala5 to Val350 and is based on Aspergillus niger epoxide hydrolase (28) (19% identity, 41% similarity of 318 aligned residues) as template (Fig. 1B). The core of the homology model comprises a three-layer (αβα) sandwich with Rossman fold topology with the compact αβα sandwich starting with Val48 and ending with Val350. Proteins containing an α/β-hydrolase fold typically harbor a conserved motif of the consensus sequence Sm-X-Nu-X-Sm-Sm in the nucleophilic elbow, where Sm indicates a small amino acid, X is any amino acid, and Nu is the nucleophile. In CGI-58, Asn155 replaces the putative active site nucleophile. A large, primarily helical lid region (Pro180–Leu280) is inserted after strand β6 and covers the potential “active site.” An additional feature of the model is a long partly helical, partly unstructured region ranging from the N terminus to Cys47, which seems to cap the lid. The positioning of the lid and the N-terminal extension in the model has to be interpreted with great caution. No functional assignment of the corresponding N-terminal extension, which packs to the α/β-hydrolase core in the template A. niger epoxide hydrolase, is known.
FIGURE 1.
Domain organization and structural model of mCGI-58. A, domain organization of mCGI-58 and N-terminal truncations used in this work. The vertical lines indicate positions of truncated protein fragments as used in this study. The N-terminal region is shown in more detail. Superscripts refer to the different truncations of mCGI-58 used in this study; the last construct capable of stimulating mATGL activity is highlighted in red (L26). B, three-dimensional model of murine CGI-58 ranging from Ala5 to Thr349. The α/β-hydrolase core structure with an eight-stranded mostly parallel β-sheet is shown in magenta with “catalytic residues” highlighted as yellow sticks. The cap region covering the potential binding pocket is depicted in cyan, whereas the N-terminal extension is colored gray. Trp21, Trp25, and Trp29 in the Trp-rich region are depicted as gray sticks.
Heterologously Expressed Mouse ATGL and CGI-58 Are Functional Proteins
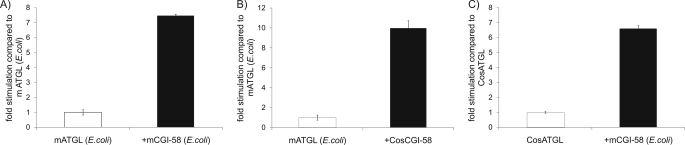
We investigated whether mCGI-58 and mATGL expressed in bacteria retain function. Expression of the GST-tagged mCGI-58 and His-tagged mATGL proteins in an E. coli host was confirmed by SDS-PAGE and by Western blotting analysis (data not shown). mATGL expressed in bacteria hydrolyzed TG, and that activity was stimulated to a similar extent by mCGI-58 expressed in E. coli as mCGI-58 expressed in COS-7 cells (Fig. 2, A and B). Moreover, the TG hydrolysis activity of mATGL expressed in COS-7 cells was enhanced to similar levels by heterologously expressed mCGI-58 (Fig. 2C).
FIGURE 2.
mATGL and mCGI-58 are active when expressed in bacteria. mATGL and mCGI-58 were expressed in COS-7 cells and E. coli. Cellular lysates were used in the TG hydrolase assay with [3H]triolein as substrate. For activation of mATGL, E. coli lysates containing mATGL were combined with E. coli lysates containing mCGI-58 (A) or COS-7 lysates containing mCGI-58 (B). In C, mATGL COS-7 lysates were combined with mCGI-58 E. coli lysates. Data are mean ± S.D. and represent three independent experiments.
Determination of the Minimal Fragment of mCGI-58 Required for mATGL Stimulation
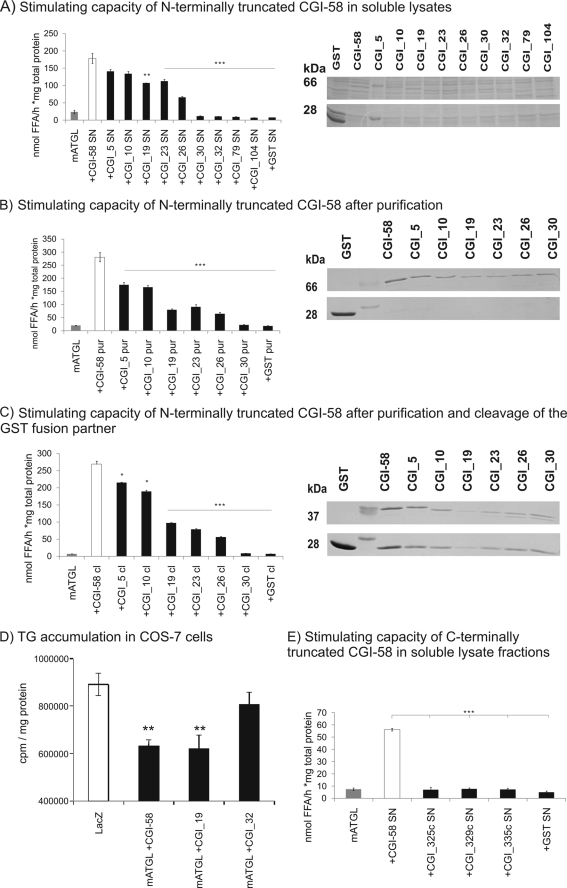
mCGI-58 fragments of various lengths (Table 1) were tested to determine the shortest region of CGI-58 competent to stimulate ATGL hydrolytic activity. The rationale was based on our three-dimensional homology model (Fig. 1B), suggesting that parts of CGI-58 outside the α/β-hydrolase domain could be important for the function of CGI-58. Truncation of the N-terminal part of mCGI-58 up to Leu26 led to only a partial loss of ATGL activation (Fig. 3A), whereas deletion of the first 29 amino acids completely abolished ATGL activating ability. To exclude the possible interference of other cellular proteins, we also tested affinity-purified truncated mCGI-58 variants. As shown in Fig. 3B, the purified mCGI-58 variants exhibited a comparable ability to activate ATGL. A loss of ATGL activation due to spatial restraints induced by the N-terminal GST fusion can be excluded because the same activation pattern was also observed after cleavage of the fusion protein GST (Fig. 3C). Taken together, our results indicate that the lack of the 30-residue N-terminal peptide of mCGI-58 prevents stimulation of ATGL activity. This was confirmed by measuring TG accumulation in COS-7 cells overexpressing wild-type (WT) CGI-58 or mutants N-terminally truncated at residues 19 and 32 (Fig. 3D). Wild-type CGI-58 and the mutant CGI_19 were able to significantly reduce TG accumulation in COS-7 cells, whereas when the CGI-58 mutant lacking 31 residues at the N terminus (CGI_32) was expressed, TG accumulation was not reduced (Fig. 3D). Furthermore, we observed that a stepwise deletion of amino acids Thr23–Cys30 is reflected in an increasingly defective capacity to stimulate ATGL (Fig. 3, A–C). Additionally, the three tested C-terminally truncated mCGI-58 variants completely lost their ability to activate the hydrolytic activity of ATGL as determined in in vitro TG hydrolase assays (Fig. 3E).
TABLE 1.
N-terminally and C-terminally truncated constructs and point mutants of mCGI-58
CGI-58 variants were cloned into GST- and YFP-tagged expression vectors for in vitro ATGL activation experiments and localization studies, respectively. Except where noted, constructs were tested only in an in vitro activation assay.
| CGI-58 mutants | Length |
|---|---|
| aa | |
| mCGI-58a | 1–351 |
| CGI_5 | 5–351 |
| CGI_10a | 10–351 |
| CGI_19a | 19–351 |
| CGI_23a | 23–351 |
| CGI_26a | 26–351 |
| CGI_30 | 30–351 |
| CGI_32a | 32–351 |
| CGI_79 | 79–351 |
| CGI_104 | 104–351 |
| CGI_335c | 1–335 |
| CGI_329c | 1–329 |
| CGI_325c | 1–325 |
| W29A | 1–351 |
| W21Aa | 1–351 |
| W21A/W25Aa | 1–351 |
| W21A/W25A/W29Aa | 1–351 |
a Construct was used for both in vitro activation and localization studies in cultured cells.
FIGURE 3.
The N-terminal region of CGI-58 is necessary for ATGL stimulation. A, stimulation of mATGL TG hydrolase activity by various cell lysates expressing mCGI-58 constructs (as indicated) using triolein as substrate. Expression of various GST-tagged mCGI-58 variants in the soluble fraction (SN) of bacterial cells was verified by SDS-PAGE. B, stimulation of TG hydrolase activity of mATGL by different GST affinity-purified mCGI-58 constructs. Purified proteins were analyzed by SDS-PAGE. C, stimulation of TG hydrolase activity of mATGL by different purified and cleaved mCGI-58 constructs. Purified and cleaved proteins were analyzed by SDS-PAGE. Representative assays (performed in triplicates) of three independent experiments are shown. Data are presented as mean ± S.D. (*, p < 0.05; **, p < 0.01; ***, p < 0.001). D, mATGL was co-expressed with YFP-tagged full-length mCGI-58, CGI_19, or CGI_32 in COS-7 cells. LacZ was expressed for comparison. To promote TG accumulation, cells were incubated with 400 μm [3H]oleate overnight, lipids were extracted and separated by TLC, and 3H content in the TG fraction was determined by liquid scintillation counting and normalized to cellular protein content. Data are presented as mean ± S.D. (**, p < 0.01). E, stimulation of mATGL TG hydrolase activity by various cell lysates expressing C-terminally truncated mCGI-58 constructs. Data are presented as mean ± S.D. ***, p < 0.001.
The N-terminal Region of mCGI-58 Binds to DPC Micelles
Next, we tested a peptide harboring the Trp-rich N-terminal sequence stretch of mCGI-58 (Val10–Pro31, WR1031), for its binding properties to phospholipid micelles using one-dimensional 1H NMR spectroscopy. This method is very sensitive to changes in the chemical environment of the observed nuclei (in our case, NH and aromatic protons of the peptide), as experienced in the case of an interaction between peptides with the added micelles. By comparing the one-dimensional 1H NMR spectra of the free peptide (Fig. 4, red trace) and in the presence of micelles (Fig. 4, cyan, purple, and blue traces), we observed drastic chemical shift changes in one-dimensional 1H NMR titration experiments, which clearly indicated that the peptide bound to micelles. Especially, shift changes of the Trp-HNϵ1 protons (Fig. 4, left) showed a distinct change of the chemical environment of these Trp residues upon interaction with this lipid droplet-mimicking system.
FIGURE 4.
One-dimensional 1H NMR titration experiments of the Trp-rich peptide with DPC micelles. Chemical shift changes observed in the amide region (right) and the Trp-HNϵ1 region (left) of the one-dimensional 1H NMR spectrum of LD-binding peptide domain in the presence of increasing amounts DPC-d38 micelles. The one-dimensional 1H NMR spectra of the free peptide in aqueous buffer (red) and the spectra resulting after the first two additions of DPC-d38 micelles (cyan and purple) are shown and demonstrate a large shift of the resonance peaks due the binding event. The spectra resulting after titration steps 3–5 are omitted in the figure for clarity because they are almost identical to the last point of the titration (depicted in blue), indicating that all peptide molecules are bound to micelles, and thus no additional changes in the chemical environment are observed. Red, peptide dissolved in aqueous buffer; cyan, purple, and blue, one-dimensional 1H NMR spectrum after the addition of 12, 24, and 68 mm DPC-d38 micelles.
Cellular Localization of Full-length and N-terminally Truncated mCGI-58 Variants
Localization of YFP-tagged variants of mCGI-58 was determined in lipid-laden COS-7 cells by laser-scanning microscopy. Full-length YFP-tagged mCGI-58 localized to LDs, as evident by the ringlike structures, which were obtained from the YFP emission scan (Fig. 5A, first column). To confirm LD localization, we stained LDs with BODIPY® C12 and overlaid the scans, which resulted in a perfect match (Fig. 5A, third column). Similar to WT mCGI-58, also N-terminally truncated mCGI-58 variants CGI_10, CGI_19, and CGI_23 localized to the LD as evident from ring structures and the merge with BODIPY® C12 staining (Fig. 5B). In this set of experiments, some images were scanned at lower magnifications (compare 5-μm scale bars of images). mCGI-58 truncated before residue 26 (CGI_26) localized mainly to the cytosol and exhibited only a weak co-localization with the LD. CGI_32 completely lost its ability to localize to the LD and was found exclusively in the cytosol (Fig. 5B). These results clearly demonstrated that the N-terminal extension of the α/β-hydrolase domain of CGI-58 is required for LD binding. The YFP-tagged mCGI-58 proteins behaved like the GST-tagged proteins in terms of their ability to stimulate ATGL hydrolase activity, suggesting that the YFP tag did not interfere with the biochemical function (data not shown).
FIGURE 5.
Live cell imaging of YFP-tagged wild type and N-terminally truncated/mutated mCGI-58 variants. Shown is subcellular localization of WT mCGI-58 (A), N-terminally truncated mCGI-58 variants (B), and Trp to Ala point mutations of mCGI-58 (C). COS-7 cells were transfected with wild type or truncated/mutated mCGI-58 variants and incubated overnight with 400 μm oleate to promote LD droplet formation. Cells were then incubated with BODIPY® 558/568 C12 for staining of LDs. YFP-specific (first column) and BODIPY® 558/568 C12-specific (second column) emissions were recorded simultaneously by confocal laser-scanning microscopy and overlaid (third column). The fourth column displays a transmission image of respective cells. Bars, 5 μm. Images were scanned at lower magnifications in Fig. 5B (compare scale bars of images).
Localization, Stimulation Capacity, and Protein-Protein Interaction of CGI-58 with Site-specific Mutations in the N-terminal Trp-rich Region
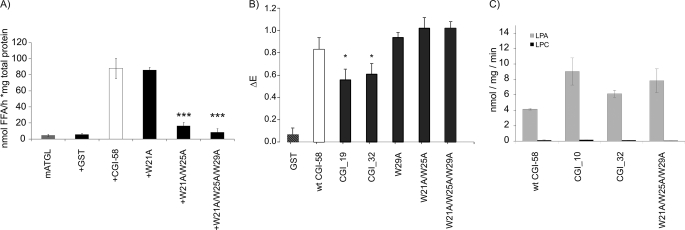
Next, we mutated the Trp residues in the N-terminal peptide to Ala residues and expressed them as YFP and GST fusion proteins. Trp-mutated YFP fusion proteins were used to determine the cellular localization of these proteins using lipid-laden COS-7 cells as the expression system and laser-scanning microscopy (Fig. 5C). The single mutant W21A retained its ability to localize to the LD (Fig. 5C, first row). The double mutant W21A/W25A localized to the LD and the cytosol because ring structures as well as dispersion of the fluorescence signal was detected (Fig. 5C, second row). Importantly, upon mutation of all three Trp residues (W21A/W25A/W29A) in this N-terminal region, mCGI-58 completely failed to localize to the LD and localized exclusively to the cytosol (Fig. 5C, third row). In the next set of experiments, we tested GST fusion proteins of these N-terminal Trp mutants for their ability to stimulate ATGL. Single mutant W21A showed ATGL stimulation comparable with that of WT mCGI-58. Residual stimulation capacity for ATGL activity was determined for the double mutant W21A/W25A, whereas the triple mutant W21A/W25A/W29A completely failed to activate ATGL (Fig. 6A). These results further corroborate our findings that the function of CGI-58 as co-activator for ATGL is closely correlated to its ability to localize to the LD. To investigate whether N-terminally truncated or Trp-mutated CGI-58 variants activate ATGL poorly because they fail to interact with this protein or because they do not localize to the LD, we performed ELISA experiments to test the interaction of WT CGI-58, the truncation mutations CGI_19 and CGI_32, and the point mutants W29A, W21A/W25A, and W21A/W25A/W29A with ATGL. Interestingly, all CGI-58 variants tested supported interaction with ATGL in the ELISA, yet both truncated CGI-58 variants, CGI_19 and CGI_32, showed about 30% reduced ATGL binding capacity (Fig. 6B). Given that CGI_32 failed to activate ATGL in vitro, whereas this was not the case for CGI_19, the extent of diminished ATGL binding and diminished ATGL activation did not correlate directly. Furthermore, all CGI-58 Trp mutants showed identical ATGL binding capacity, although they all were compromised in activating ATGL. These results indicated that not ATGL binding capacity but the ability to localize to the LD determines whether or not CGI-58 mutants were able to activate ATGL in vitro. Next, we also tested whether the CGI-58 truncated variants, CGI_10 and CGI_32, and the triple mutant W21A/W25A/W29A were also compromised in LPAAT activity. As shown in Fig. 6C, LPAAT activities of all N-terminal deletions and the triple-mutant of CGI-58 were equivalent to wild type. In agreement with published data, no acyltransferase activity was observed using lysophosphatidylcholine as substrate (4, 5).
FIGURE 6.
Trp site directed mutations affect the capacity of CGI-58 to activate ATGL TG hydrolase activity but not to physically interact with ATGL. A, stimulation of mATGL TG hydrolase activity by cell lysates expressing mCGI-58 Trp mutations. The soluble fraction of Trp point mutations of mCGI-58 (GST-tagged, expressed in E. coli) were tested for their activation capacity on bacterially expressed mATGL using [3H]triolein as substrate. B, protein-protein interaction of ATGL with truncations and mutations of mCGI-58 were tested by ELISA. Plates were coated with mATGL and incubated with cell extracts containing GST-tagged proteins, and the amounts of retained CGI-58 were determined using CGI-58-specific antiserum. C, mCGI-58, N-terminal truncation mutants, and the triple mutant W21_25_29A were tested for acyltransferase activity using LPA (gray) or lysophosphatidylcholine (black) as substrates. Data are presented as mean ± S.D. (A and B) or mean ± S.E. (C); *, p < 0.05; ***, p < 0.001.
DISCUSSION
In eukaryotes, TGs are stored as neutral lipids in cytoplasmic LD. Upon increased energy demand, stored TGs are mobilized from LDs in a process termed lipolysis. Research in the past few years increasingly supports the idea that LDs are highly dynamic organelles. Cellular LDs are variable in size but generally consist of a hydrophobic core of neutral lipids (mainly TG and/or steryl esters), which are surrounded by a monolayer of phospholipids (phosphatidylcholine and phosphatidylethanolamine) (32, 33). The surface of intracellular LDs is the site of many key regulatory processes in neutral lipid metabolism. Depending on the eukaryotic species, the outer monolayer also contains different structural proteins and enzymes. These proteins often possess the ability to translocate between the cytosol and the lipid droplet depending on the metabolic state of the cell.
In the present study, we tried to reduce this complex system to basic players in the first step of lipolysis. Therefore, we used bacterial cell lysates and purified proteins, which are devoid of LD and LD-associated proteins, for TG hydrolase assays. The TG substrate was offered by emulsifying triolein and phosphatidylcholine/phosphatidylinositol in an aqueous buffer system. This experimental setup renders the experiment independent of co-localizations or direct interactions of CGI-58 with mammalian LD-binding proteins. Therefore, it serves as a useful “filter,” which only monitors the ability of CGI-58 to act as a co-activator for ATGL. Our results demonstrate that the stimulating function of CGI-58 on ATGL is independent of any other eukaryotic LD-associated protein. From earlier TG hydrolase assays performed with COS-7 lysates, it could be inferred that the presence of perilipin is not essential for the function of CGI-58 (1).
Furthermore, we observed that the ability of CGI-58 to stimulate ATGL requires almost the entire protein chain. The three-dimensional homology model allows for N-terminally truncated proteins to still adopt a compact folded domain structure. C-terminal truncations are expected to interrupt the integrity of the α/β-hydrolase domain. This is in complete agreement with our observations; the tested C-terminal truncations of CGI-58 were incapable of stimulating ATGL. N-terminal deletions up to residue Leu26 stimulated ATGL although to a lower extent compared with WT CGI-58.
Deletion of residues Met1–Cys30 resulted in complete loss of stimulatory capacity. These findings were also confirmed by studies in cultured cells. Accordingly, TG accumulation was observed in COS-7 cells expressing CGI-58 with the N-terminal truncation at residue 32. Interestingly, loss of the N terminus did not abolish LPAAT activity. Therefore, it can be assumed that the N terminus of CGI-58 is not essential for this specific activity. The catalytic site for LPAAT activity is assumed to be conveyed by the residues His329 and Asp334, which reside within an HX4D motif often observed in acyltransferases.
The N-terminal extension contains an unusual Trp-rich amino acid region, which is conserved in many orthologs of CGI-58. Membrane-associated and integral membrane proteins are characterized by a significantly higher amount of aromatic residues, especially tryptophans, at the interface between the lipid core and the aqueous phase. Aromatic residues and especially tryptophan residues play a critical role in binding of a protein to membranes (34, 35). Tryptophan residues can adopt a position where they form hydrogen bonds with the lipid headgroups while their hydrophobic rings are immersed in the hydrophobic part of the lipid chain (36). Consequently, the Trp-rich region in CGI-58 leads to the idea that this 10-amino acid-long stretch might serve as an LD binding region and could thus affect the subcellular localization of CGI-58. Our results obtained by one-dimensional 1H NMR spectroscopy showed that a peptide containing this Trp-rich stretch within amino acids Gly19 and Pro32 is capable of binding to phospholipid micelles. Previously, an LD binding pocket was suggested to reside within residues Met69–Gly87 of CGI-58 based on the presence of hydrophobic residues in this region (4, 37). Within this region, residues Met69–Thr78 contain mainly polar and charged residues, which reside in a surface-exposed loop in our three-dimensional homology model. The hydrophobic stretch 79PLVLLHGFG87 is highly similar in A. niger epoxide hydrolase (PIALLHGWPG) (28), which was used as template for homology modeling. However, this hydrophobic stretch is located within a central β-strand of the α/β-hydrolase domain of CGI-58, which has α-helices packing against it on each side of the strand. Therefore, it seems likely that these residues are involved in forming the hydrophobic core of the protein rather than being responsible for LD binding.
Our localization studies demonstrate that the Trp-rich stretch of mCGI-58 is essential for localization of the protein in COS-7 cells. Although wild-type CGI-58 localized to the LD, a truncated version starting with Thr32 failed to localize to the LD. Our results are in excellent agreement with a previous study by Yamaguchi et al. (17) and provide an explanation for their observations. In their localization studies in 3T3-L1 cells, rat CGI-58 with point mutation E9K still localized to the LD (17). Mutants 1–5 had large C-terminal deletions and failed to localize to the LD. Mutant 6, ranging from residue 19 to 351 exhibited cell type-dependent co-localization with LD binding proteins or a diffuse cytosolic pattern. Mutants 7–11 had large N-terminal and partial C-terminal deletions and failed to localize to the LD. According to our three-dimensional model, protein fragments with large truncations are devoid of their native fold and consequently lose their biological function.
Interestingly, the localization of CGI-58 depends on the cell type, the abundance of LD-binding proteins, and the hormonal and nutritional state of the cell. The localization in preadipocytes is debated and reported to show a diffuse cystosolic pattern or co-localization with ADRP (16–18). However, upon expression of perilipin in these preadipocytes, CGI-58 localized to the LD (16). In differentiated adipocytes, CGI-58 is localized to the LD under basal, nonstimulated conditions (16, 17, 26, 38). CGI-58 was reported to diffuse into the cytoplasma upon hormonal stimulation (16, 17, 38). Other fluorescence studies reported CGI-58 to be localized to the LD in different cell lines, including CHO-K1, HeLa cells (17), and cardiomyocytes (25). In addition to the localization on the LD, Brown et al. (39) also reported CGI-58 to be localized to the Golgi apparatus and the endoplasmic reticulum in various cells. Mass spectrometry analysis of Chinese hamster ovary cells and human epithelial cells identified ADRP and CGI-58 as protein components of the LD (21, 40). Our localization experiments were performed in COS-7 cells. There, LDs are supposed to be coated by LD-associated proteins like ADRP but not perilipin. In our study, we could clearly demonstrate the importance of the N-terminal region for the localization of CGI-58 to LDs. Interestingly, all tested CGI-58 mutants are able to interact with ATGL, while not all tested CGI-58 mutants are able to localize to the LD. These results further emphasize the significance of the localization of CGI-58 to the LD to exert its activation capacity on ATGL. It can be speculated that the presence and possible interaction with other LD proteins can influence CGI-58 on the LD in multiple ways (e.g. orientation of CGI-58 on the LD, conformational changes, and exposure of different protein-protein interaction areas). Many previous localization studies of CGI-58 mutants did not test the co-activating capability because this function of CGI-58 was discovered after the studies were published (1). This study demonstrates a correlation between the co-activating function and localization of CGI-58 on LDs. This is in excellent agreement with a very recent study by Granneman et al. (24) demonstrating that the protein kinase A-induced activation of lipolysis leads to an increase in the interaction of CGI-58 and ATGL mainly at the LD. A complete loss of stimulating capacity of CGI-58 was observed upon truncation of the N-terminal Trp-rich region, highlighting the significance of this peptide stretch. Interestingly, this region corresponds exactly to the residue requirements of CGI-58 for correct localization on the LD. A mutant of CGI-58 in which the three Trp residues were replaced by Ala residues lost the ability to localize to the LD and to stimulate ATGL, clearly indicating the importance of the Trp residues. In conclusion, our data demonstrate that the correct localization of CGI-58 on the LD is a prerequisite for its capability to stimulate the hydrolytic activity of ATGL and that the N-terminal Trp-rich region plays an important role in the biological function of CGI-58.
Supplementary Material
Acknowledgments
We thank Klaus Zangger (Institute of Chemistry, University of Graz) for sharing expertise in investigation of micelle-bound peptides by NMR spectroscopy and Ellen Zechner for critical reading of the manuscript.
This work was supported by grants from “GOLD: Genomics of Lipid-Associated Disorders,” which is part of the Austrian Genome Project “GEN-AU Genome Research in Austria” and is funded by the Austrian Federal Ministry of Science and Research and DK Molecular Enzymology, which is funded by Austrian Science Fund Grant FWF W901-B05.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- ATGL
- adipose triglyceride lipase
- LD
- lipid droplet
- TG
- triacylglycerol
- WT
- wild-type
- LPAAT
- lysophosphatidic acid acyltransferase
- mCGI-58
- mouse CGI-58
- mATGL
- mouse ATGL
- GST
- glutathione S-transferase
- YFP
- yellow fluorescent protein
- BSA
- bovine serum albumin
- DPC
- dodecylphosphocholine
- ELISA
- enzyme-linked immunosorbent assay.
REFERENCES
- 1.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. (2006) Cell Metab. 3, 309–319 [DOI] [PubMed] [Google Scholar]
- 2.Lefèvre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J. L., Weissenbach J., Ozgüc M., Lathrop M., Prud'homme J. F., Fischer J. (2001) Am. J. Hum. Genet. 69, 1002–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A. K., Ramakrishnan G., Chandramohan C., Rajasekharan R. (2008) J. Biol. Chem. 283, 24525–24533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Moran G., Caviglia J. M., McMahon D., Rothenberg A., Subramanian V., Xu Z., Lara-Gonzalez S., Storch J., Carman G. M., Brasaemle D. L. (2010) J. Lipid Res. 51, 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer J., Lefèvre C., Morava E., Mussini J. M., Laforêt P., Negre-Salvayre A., Lathrop M., Salvayre R. (2007) Nat. Genet. 39, 28–30 [DOI] [PubMed] [Google Scholar]
- 7.Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E289–296 [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi T., Osumi T. (2009) Biochim. Biophys. Acta 1791, 519–523 [DOI] [PubMed] [Google Scholar]
- 9.Akiyama M., Sakai K., Takayama C., Yanagi T., Yamanaka Y., McMillan J. R., Shimizu H. (2008) Am. J. Pathol. 173, 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lake A. C., Sun Y., Li J. L., Kim J. E., Johnson J. W., Li D., Revett T., Shih H. H., Liu W., Paulsen J. E., Gimeno R. E. (2005) J. Lipid. Res. 46, 2477–2487 [DOI] [PubMed] [Google Scholar]
- 11.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 13.Radner F. P., Streith I. E., Schoiswohl G., Schweiger M., Kumari M., Eichmann T. O., Rechberger G., Koefeler H. C., Eder S., Schauer S., Theussl H. C., Preiss-Landl K., Lass A., Zimmermann R., Hoefler G., Zechner R., Haemmerle G. (2010) J. Biol. Chem. 285, 7300–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. (2009) J. Lipid Res. 50, 3–21 [DOI] [PubMed] [Google Scholar]
- 15.Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 16.Subramanian V., Rothenberg A., Gomez C., Cohen A. W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M. P., Brasaemle D. L. (2004) J. Biol. Chem. 279, 42062–42071 [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T., Omatsu N., Matsushita S., Osumi T. (2004) J. Biol. Chem. 279, 30490–30497 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T., Omatsu N., Omukae A., Osumi T. (2006) Mol. Cell Biochem. 284, 167–173 [DOI] [PubMed] [Google Scholar]
- 19.Brasaemle D. L., Barber T., Kimmel A. R., Londos C. (1997) J. Biol. Chem. 272, 9378–9387 [DOI] [PubMed] [Google Scholar]
- 20.Brasaemle D. L., Barber T., Wolins N. E., Serrero G., Blanchette-Mackie E. J., Londos C. (1997) J. Lipid Res. 38, 2249–2263 [PubMed] [Google Scholar]
- 21.Umlauf E., Csaszar E., Moertelmaier M., Schuetz G. J., Parton R. G., Prohaska R. (2004) J. Biol. Chem. 279, 23699–23709 [DOI] [PubMed] [Google Scholar]
- 22.Londos C., Brasaemle D. L., Schultz C. J., Segrest J. P., Kimmel A. R. (1999) Semin. Cell Dev. Biol. 10, 51–58 [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. (2007) J. Lipid Res. 48, 1078–1089 [DOI] [PubMed] [Google Scholar]
- 24.Granneman J. G., Moore H. P., Krishnamoorthy R., Rathod M. (2009) J. Biol. Chem. 284, 34538–34544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granneman J. G., Moore H. P., Mottillo E. P., Zhu Z. (2009) J. Biol. Chem. 284, 3049–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granneman J. G., Moore H. P., Granneman R. L., Greenberg A. S., Obin M. S., Zhu Z. (2007) J. Biol. Chem. 282, 5726–5735 [DOI] [PubMed] [Google Scholar]
- 27.Arnold K., Bordoli L., Kopp J., Schwede T. (2006) Bioinformatics 22, 195–201 [DOI] [PubMed] [Google Scholar]
- 28.Zou J., Hallberg B. M., Bergfors T., Oesch F., Arand M., Mowbray S. L., Jones T. A. (2000) Structure 8, 111–122 [DOI] [PubMed] [Google Scholar]
- 29.Zhou P., Lugovskoy A. A., Wagner G. (2001) J. Biomol. NMR 20, 11–14 [DOI] [PubMed] [Google Scholar]
- 30.Schweiger M., Schoiswohl G., Lass A., Radner F. P., Haemmerle G., Malli R., Graier W., Cornaciu I., Oberer M., Salvayre R., Fischer J., Zechner R., Zimmermann R. (2008) J. Biol. Chem. 283, 17211–17220 [DOI] [PubMed] [Google Scholar]
- 31.Bai J., Pagano R. E. (1997) Biochemistry 36, 8840–8848 [DOI] [PubMed] [Google Scholar]
- 32.Murphy D. J., Vance J. (1999) Trends Biochem. Sci 24, 109–115 [DOI] [PubMed] [Google Scholar]
- 33.Zweytick D., Athenstaedt K., Daum G. (2000) Biochim. Biophys. Acta 1469, 101–120 [DOI] [PubMed] [Google Scholar]
- 34.Gelb M. H., Cho W., Wilton D. C. (1999) Curr. Opin. Struct. Biol. 9, 428–432 [DOI] [PubMed] [Google Scholar]
- 35.Han S. K., Kim K. P., Koduri R., Bittova L., Munoz N. M., Leff A. R., Wilton D. C., Gelb M. H., Cho W. (1999) J. Biol. Chem. 274, 11881–11888 [DOI] [PubMed] [Google Scholar]
- 36.Yau W. M., Wimley W. C., Gawrisch K., White S. H. (1998) Biochemistry 37, 14713–14718 [DOI] [PubMed] [Google Scholar]
- 37.Akiyama M., Sawamura D., Nomura Y., Sugawara M., Shimizu H. (2003) J. Invest. Dermatol. 121, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 38.Granneman J. G., Moore H. P. (2008) Trends Endocrinol. Metab. 19, 3–9 [DOI] [PubMed] [Google Scholar]
- 39.Brown J. M., Chung S., Das A., Shelness G. S., Rudel L. L., Yu L. (2007) J. Lipid Res. 48, 2295–2305 [DOI] [PubMed] [Google Scholar]
- 40.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. (2004) J. Biol. Chem. 279, 3787–3792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.