Abstract
Polarity proteins promote the asymmetric organization of cells by orienting intracellular sorting mechanisms, such as protein trafficking and cytoskeletal assembly. The localization of individual polarity proteins in turn is often determined by association with factors that mediate contact with other cells or the substratum. This arrangement for the Par and Crb apical polarity complexes at the tight junction is disrupted by the adaptor protein Amot. Amot directly binds the scaffolding proteins Patj and Mupp1 and redistributes them and their binding partners from the plasma membrane to endosomes. However, the mechanism by which Amot is targeted to endosomes is unknown. Here, a novel lipid binding domain within Amot is shown to selectively bind with high affinity to membranes containing monophosphorylated phosphatidylinositols and cholesterol. With similar lipid specificity, Amot inserts into and tubulates membranes in vitro and enlarges perinuclear endosomal compartments in cells. Based on the similar distribution of Amot with cholesterol, Rab11, and Arf6, such membrane interactions are identified at juxtanuclear endocytic recycling compartments. Taken together, these findings indicate that Amot is targeted along with associated apical polarity proteins to the endocytic recycling compartment via this novel membrane binding domain.
Keywords: Adaptor Proteins, Cell Junctions, Cell Migration, Membrane Proteins, Membrane Trafficking
Introduction
Epithelial cells are morphologically plastic and transition between the epithelial and mesenchymal phenotypes. Epithelial cells organize into a luminal facing apical pole and a basal pole that is directed toward the stroma. These poles are separated in the plasma membrane (PM)3 by lateral attachments to adjacent cells at the tight junction (TJ) (1). Mesenchymal cells lack intercellular attachments and exhibit a flattened shape with a migrational front and a trailing back side (2). In both morphologies, the unequal distribution of lipid and protein components requires the Par and Crb apical polarity protein complexes (3). The core constituents of the Par complex, Par-3·Par-6·atypical protein kinase C and Cdc42, were identified in Caenorhabditis elegans as essential factors for asymmetric cell division (4), whereas Crb proteins, Crb·Pals1 (protein associated with Lin7)·Patj (protein associated with tight junction), were identified in Drosophila as factors that direct vectorial cuticle secretion (5, 6). Both complexes concentrate on the cytosolic face of the tight junction (7–9) in epithelial cells, whereas they distribute to the migratory front of mesenchymal cells (2). Underlying their requirement for the formation and maintenance of cellular polarity is their interdependent (10) direction of vectorial processes, such as protein trafficking (11, 12), cytoskeletal dynamics (13–15), and lipid metabolism (16).
Recently, the Par complex was recognized in a genome-wide screen in C. elegans for roles in protein recycling (12). This function is probably conserved because Par-6 and Cdc42 regulate the recycling of receptors, such as MHC1 (major histocompatibility complex class I) protein, out of the juxtanuclear endosomal recycling compartment (ERC) in mammalian cells (12, 17, 18). Although it has not been shown that Par or Crb proteins associate with the ERC, several studies suggest this. For instance, the endocytosis of Crb is required to maintain the size of the apical membrane (19), whereas expression of constitutively active Cdc42 induces the accumulation of Par and Crb proteins at an enlarged endosomal compartment and the misregulation of E-cadherin trafficking (20). Likewise, in MDCK cells, Pals1 expression is necessary for proper E-cadherin trafficking (21), and proteins in the Par and Crb complexes redistribute to enlarged endosomes upon expression of the adaptor protein Amot/angiomotin (22). However, the lack of information regarding how such endosomes are recognized has hindered studies that define the relative importance of apical polarity proteins at this setting.
The adaptor protein Amot links cellular migration to the reorganization of polarity proteins. Amot was initially recognized for roles in the migration of endothelial cells (23–25). Although Amot expression is low in most differentiated epithelial cells (25, 26), it is strongly up-regulated during epithelial migration programs during developmental processes, such as trophoblast extension (27) and anterior visceral endoderm formation (22, 28). Amot is essential for this latter process because its genetic deletion in mice results in embryonic lethality from an inability of epithelial cells in the anterior visceral endoderm to migrate (28). Expression of the 80-kDa isoform of Amot (Amot80) specifically promotes migration presumably via selective ability to induce the loss of TJ integrity by redistributing components of the Par and Crb complex to endosomes versus the longer 130-kDa isoform, which has no effect (22, 29). Association of Par and Crb polarity proteins is mediated through a C-terminal PDZ binding motif that directly binds PDZ domains in Patj and its paralog Mupp1 (22, 26). However, the mechanism by which Amot recognizes endosomes or the identity of these compartments is unknown.
This study finds that Amot via a novel lipid binding domain directly binds and remodels recycling endosomes enriched in monophosphorylated phosphatidylinositols (PIPs) and cholesterol. Consistent with in vitro binding, the N-terminal region within Amot as well as full-length Amot associate with endosomal membranes that are marked by probes of PI(4)P and cholesterol. The ability to tubulate liposomes in vitro as well as to remodel/enlarge tubulating vesicles in cells suggests that this domain directly regulates membrane trafficking. Based on the co-localization of Amot with Arf-6, Rab11, and cholesterol, a significant fraction of such membrane binding and remodeling occurs at the ERC (30, 31). Such membrane binding, in turn, enhances the binding of Amot to Par and Crb proteins and promotes their redistribution to the ERC.
EXPERIMENTAL PROCEDURES
Reagents
Expression plasmids were constructed using Creator (Clontech)-based vectors described previously (32). Vectors v1759 and v3101 encode N-terminal monomeric Cerulean (Piston-Vanderbilt) fluorescent protein (CFP) tags. Vectors v2041 and v3102 encode N-terminal monomeric citrine/yellow (from R. Tsien, University of California, San Diego) fluorescent protein (YFP) tags. Vectors v1662 and v2916 encode an N-terminal tag that is a variant of the Clontech Discosoma sp. red fluorescent protein (DsRed2) with 43 point mutations that reduce toxicity, ablate dimerization, and increase brightness (32), referred to here as DsRed. Descriptions of YFP-Par-3, YFP Patj, 3× FLAG AmotΔC, 3× FLAG Amot80, 3× FLAG Amot80Δ Amot coiled-coil homology (ACCH), Amot ACCH, DsRed Amot80, YFP Amot80, and YFP AmotΔACCH were described previously (22). The open reading frames for Rab5, -7, -11, and -13 were purchased from Open Biosystems and subcloned into the indicated Creator-based vectors with their stop codon intact. Other constructs were gifts: CFP Akt PH (from T. Meyer (Stanford)), YFP Akt PH (from T. Meyer), YFP Plcδ1 (from T. Balla, National Institutes of Health), and GST FAPP1-PH (from O. Gozani, Stanford). The FAPP1 PH domain was subcloned into v3101, v3102, and v2916. Antibodies were obtained from the following sources: Par-3 (Chemicon), Rab11 (from K. Dunn, Indiana University School of Medicine), tubulin (Sigma), and Amot (22). Chemicals were purchased and diluted into working stocks as follows: filipin (Sigma) dissolved in DMSO at 5 mg/ml; BODIPY ceramide (Molecular Probes) dissolved at 0.25 mm in water; BODIPY cholesterol dissolved to 1 mm in water. Amplex Red Assays (Sigma) were performed as described by the manufacturer.
Imaging
All epifluorescence imaging was acquired on a Zeiss AxioObserverZ1 microscope. Live imaging utilized a c-apochromat ×40 water immersion objective (Zeiss), whereas fixed images were obtained with a Plan Apochromat ×63 oil immersion objective (Zeiss). Images were processed with Zeiss Axiovision 4.7. Confocal images and all optical sections were acquired as structured light via an Apotome (Zeiss). Live cells were cultured and imaged in 35-mm dishes with embedded coverslips from MatTek at 5% CO2 at 37 °C in an environmental chamber. Sample preparation for imaging live transwell and fixed samples was performed as described (33). Filipin staining of cells was carried out on paraformaldehyde-fixed cells in PBS containing 0.01 mg/ml filipin at room temperature while agitating for 1 h. BODIPY ceramide was incubated with cells for 30 min at 4 °C with 1 μm ceramide-bovine serum albumin in Hanks' buffered salt solution/HEPES. BODIPY cholesterol was incubated with cells in complete medium at 0.5 μm for 14 h; cells were then washed twice in complete medium before imaging. Antibodies were used at the following dilutions: α-Par-3, 1:500; α-Rab11, 1:1000; α-tubulin, 1:100; α-Amot, 1:200; α-Arf6, 1:500.
Cell Culture and Immunoprecipitation
MDCK Type II cells (gift from K. Mostov, University of California, San Francisco) and 293 (ATCC) cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. Exogenous protein expression was achieved either stably by selection of bulk populations of cells expressing the indicated cDNAs or by transient transfection with polyethylenimine (Aldrich) or Lipofectamine 2000 (Invitrogen) of MDCK cells. Immunoprecipitation from PLC-extracted postnuclear supernatants was carried out as described previously (22).
Liposome Binding Assay
The liposome sedimentation assays were performed as described (34). Briefly, solutions of phosphatidylcholine (POPC), phosphatidylethanolamine (POPE), phosphatidylserine (POPS) (Avanti Polar Lipids), and dipalmitoyl phosphoinositides (Cayman Chemical) dissolved in CHCl3·MeOH·H2O (65:25:4) were mixed and dried down under nitrogen. The lipids were resuspended in 20 mm Tris, pH 7.4, with 0.16 m KCl and 1 mm dithiothreitol and incubated at 65 °C for 1 h. The lipids were then frozen in liquid nitrogen and thawed at 37 °C for three cycles. Liposomes were then mixed with proteins in 100-μl reactions to yield solutions containing 1 mm total lipid and 5 μm proteins. Reactions were incubated for 20 min at room temperature, and then liposomes were collected by centrifugation for 20 min at 25,000 × g at room temperature. Supernatants were removed, and pellets were resuspended in 100 μl of Laemmli buffer, resolved by SDS-PAGE, and developed with Coomassie dye.
Surface Plasmon Resonance (SPR) Measurements
All SPR measurements were performed at 25 °C as described previously (35). Equilibrium SPR measurements were done at a flow rate of 5 μl/min. 85 μl of protein in respective buffers was injected to give an association time of 1020 s, whereas the dissociation was monitored for 500 s or more. After sensorgrams were obtained for five or more different concentrations of each protein within a 10-fold range of Kd (see Table 1), each of the sensorgrams was corrected for refractive index change by subtracting the control surface response from it. Each data set was repeated three times to calculate an S.D. value. The maximal response (Req, saturation value) for each protein concentration was determined from each plot. Req values were then plotted versus protein concentrations (C), and the Kd value was determined by a nonlinear least squares analysis of the binding isotherm using the equation, Req = Rmax/(1 + Kd/C) (36).
TABLE 1.
Lipid binding properties of the ACCH domain
| Lipid | Kd | Changea |
|---|---|---|
| nm | -fold | |
| PM mimetic + PI(4)P (PC/PE/PS/PI/cholesterol/PI(4)P), 9:35:22:9:22:3) | 4.4 ± 1 | 1 |
| POPC/POPE/PI(3)P (75:20:5) | 18 ± 4 | 4 |
| POPC/POPE/PI(4)P (75:20:5) | 16 ± 3 | 4 |
| PM mimetic (PC/PE/PS/PI/cholesterol, 12:35:22:9:22) | 22 ± 4 | 5 |
| POPC/POPE/PI (60:20:20) | 95 ± 8 | 22 |
| POPC/POPE/PI(5)P (75:20:5) | 120 ± 10 | 27 |
| POPC/POPE/PI(3,4)P2 (75:20:5) | 300 ± 40 | 68 |
| POPC/POPE/PI(3,5)P2 (75:20:5) | 360 ± 50 | 82 |
| POPC/POPE/POPS (60:20:20) | 540 ± 40 | 120 |
| POPC/POPE/POPAb (60:20:20) | 610 ± 60 | 140 |
| POPC/POPE/PI(4,5)P2 (75:20:5) | NDc | |
| POPC/POPE/PI(3,4,5)P3 (75:20:5) | ND |
a Decrease of affinity relative to the Kd of the ACCH domain for plasma membrane + PI(4)P.
b1-Palmitoyl-2-oleoyl phosphatidic acid.
c ND, not determined.
Monolayer Measurements
The monolayer penetration of the ACCH domain into the phospholipid monolayer of different lipid compositions was measured in terms of the change in surface pressure (π) using a 1-ml circular Teflon trough and wire probe connected to a Kibron MicroTrough X (Kibron, Inc., Helsinki, Finland). A lipid monolayer containing various combinations of phospholipids (see “Results”) was spread onto the subphase composed of 10 mm HEPES, pH 7.4, containing 0.16 m KCl, until the desired initial surface pressure (π0) was reached. The signal stabilized quickly (∼5 min), and 10 μg of protein was injected into the subphase through a hole in the wall of the trough. The surface pressure change (Δπ) was then monitored for 45 min because the Δπ value reached a maximum after 30 min in all experiments. Δπ is inversely proportional to π0, and an extrapolation of Δπ versus π0 yields the critical surface pressure (πc) that specifies an upper limit of π0 that a protein can penetrate the membrane (35).
Electron Microscopy
Liposome samples (1 mg/ml) were incubated in the presence or absence of protein (0.5–2 μm) in low salt buffer for 15 min at 25 °C. The sample (8 μl) was applied to a carbon-Formvar-coated copper grid (EMS) and incubated for 2 min. Excess liquid was carefully removed by using a wet absorbent tissue (Kimwipes, Kimberly Clark). The grids were incubated three times with 2% filtered uranyl acetate solution for 10 s and dried by air and then under a heat lamp. Negative staining was performed at 25 °C. Membrane morphologies were examined on an FEI Magellan scanning electron microscope with the electron energy set to 15 kV. Representative images were taken with a direct magnification of ×72,000–202,000.
Dynamic Light Scattering
Dynamic light scattering measurements were performed using a particle size analyzer (Beckman Coulter N4 Plus submicron particle size) with a 10-milliwatt helium-neon laser at a wavelength of 632.8 nm. Particle sizes were measured for a total reaction volume of 500 μl at 23 °C. Protein was incubated with liposomes for 5 min and then monitored for an experimental run time of 2 min.
Vesicle Dye Leakage Measurements
The release of 5-carboxyfluorescein dye encapsulated into liposomes of different compositions was monitored in the presence of protein using an Aminco Bowman Series 2 spectrofluorometer. Liposomes for these experiments were prepared as described for SPR measurements with the addition of 10 mm 5-carboxyfluorescein in the resuspension buffer. Following extrusion of the vesicles through a 100-nm membrane, unincorporated 5-carboxyfluorescein was removed from the solution via gel filtration chromatography. Experiments were performed via the addition of ACCH, epsin N-terminal homology (ENTH), or Triton X-100 to the respective solution of liposomes in a fluorescence cuvette. The change in fluorescence was then monitored (scanned every 10 s) for up to 5 min. The relative dye leakage (increase in fluorescence) was plotted versus time. Control experiments indicate the specificity for membrane-tubulating proteins ACCH and ENTH to disrupt vesicles in accordance with their lipid specificity and affinity.
RESULTS
Definition of the ACCH Domain
The NH2-terminal 240 residues of Amot80 has been predicted to encode a coiled-coil fold with positively charged residues that are positionally conserved with the BAR domain of amphiphysin (22). However, this region in Amot is most similar to a broad collection of coiled-coil domains with undefined function in proteins encoded throughout metazoans. Specifically, this domain shares over 68% (80% conservation) and 56% identity (70% conservation) with regions in junction-enriched and -associated protein (JEAP/AmotL1) and human MAGI-1-associated coiled-coil TJ protein (Mascot/AmotL2), respectively (Fig. 1A and supplemental Fig. S1A). This domain also shares 50% or more conservation of amino acids with at least one predicted protein sequence in all metazoan genomes compared (Fig. 1B and supplemental Fig. S1, B and C). Because this family of related sequences has not been recognized or named by domain-predicting algorithms such as SMART (37) or PFAM (38), we term them ACCH (Amot coiled-coil homology) domains. The ACCH domain is the most conserved region within predicted Amot homologues in vertebrates and is the only region that shares conservation with predicted invertebrate homologs of Amot (HomoloGene, NCBI). Although these putative ACCH domains in fish and invertebrates are predicted by PFAM to be SMC_N domains, their sequence identity (BLAST-NCBI) is higher with human Amot ACCH. That such ACCH domains share similar function is strongly supported by the rescue of Amot depletion in zebrafish by human AmotL1 (25).
FIGURE 1.
The ACCH domain defines a novel domain family encoded across metazoan genomes. A, the protein sequences of human Amot80 (NM_133265), Amot130 (130-kDa isoform of Amot; NM_001113490), JEAP/AmotL1 (NM_130847), and Mascot/AmotL2 (NM_016201) were aligned. The percentage identity between residues that collectively are predicted to form a coiled-coil fold (the region of highest identity between these proteins) and that are suggested here to compose the human ACCH domain members was computed by blast2seq (NCBI). B, putative proteins from the indicated metazoan genomes were predicted to encode ACCH domains based on their having a region of at least 200 residues that shared more conservation with human Amot ACCH than with any other region in any human protein. These proteins were iteratively aligned by blast2seq and by ClustalW alignments to human Amot. The conservation of residues between ACCH domains (ClustalW) are depicted at the left. The conservation between residues within the entire proteins with human Amot130 are depicted at the right (computed using Blast2seq/ClustalW alignments). A heat map indicates the percentage conservation. C, MDCK cells stably expressing YFP-tagged Amot80 were seeded on transwell filters, cultured for 2 days, and imaged live. A single confocal image is depicted. D, MDCK cells transiently transfected with a vector that expresses YFP AmotΔACCH were cultured and imaged. A single confocal image is depicted (top), and the y-z axis from reconstructed confocal sections from the region outlined in the red box is shown in the bottom image.
The ACCH domain is required for the association of Amot with membranes in live cells. A mutant of Amot lacking the ACCH domain (amino acids 1–242) diffusely localizes in fixed and detergent-permeabilized cells (22). However, these treatments can often interfere with membrane association, so we confirmed that this region drives the association of Amot with membranes in live cells. For this purpose, the localization of YFP-tagged full-length Amot and AmotΔACCH was examined in MDCK cells. YFP AmotΔACCH dispersed throughout the cytosol, as opposed to full-length Amot, which localized at the PM, at intracellular junctions, and at intracellular membranes (Fig. 1, C and D). Thus, the peripheral association of Amot with membranes (26) is probably mediated by the ACCH domain.
The ACCH Domain Specifically Binds to and Penetrates Membranes Containing Monophosphorylated Phosphatidylinositols
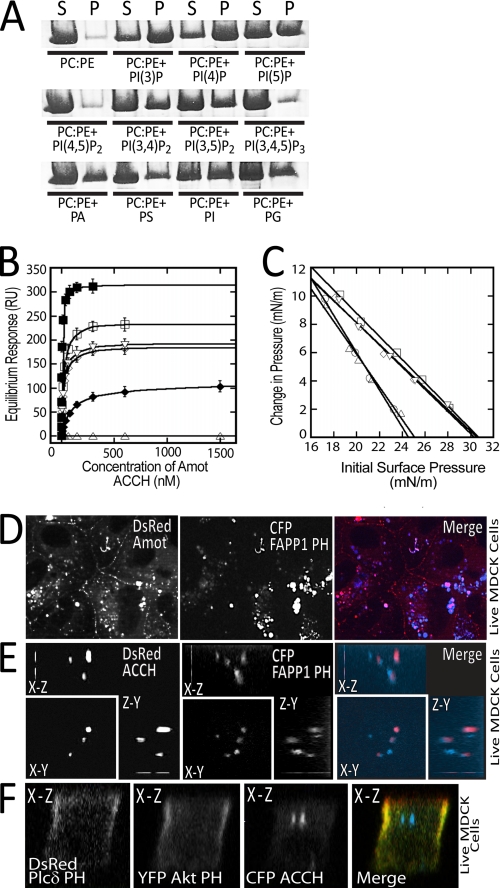
A liposome sedimentation assay was performed to test the association of the ACCH domain with different lipids. For this purpose, the region encompassing this domain (amino acids 1–242) in Amot80 was expressed as a GST fusion, and the untagged protein was recovered following thrombin cleavage from glutathione-Sepharose resin (supplemental Fig. S2A). This protein was incubated with large unilamellar vesicles composed of POPC and POPE plus a range of anionic lipids. The distribution of the unbound versus the membrane-associated Amot ACCH was determined following separation of supernatant (unbound) and pellet (bound to large unilamellar vesicles) fractions by centrifugation. Following resolution by SDS-PAGE, proteins were visualized with Coomassie dye. This revealed that the ACCH domain most strongly associated with vesicles containing the monophosphorylated phosphatidylinositols PI(4)P and PI(3)P and very weakly associated with POPC/POPE, POPC/POPE/PI(4,5)P2, or POPC/POPE/PIP3. Moderate affinity was observed for vesicles containing phosphatidic acid (PA), POPS, phosphatidylinositol (PI), phosphatidylglycerol, PI(5)P, PI(3,4)P2, and PI(3,5)P2 (Fig. 2A).
FIGURE 2.
The ACCH domain selectively binds and penetrates membranes containing monophosphorylated phosphatidylinositols. A, the purified human Amot ACCH domain was incubated with liposomes of the indicated compositions (5% PIs or 20% PA, PS, PI, or PG). The supernatant (S) and pellet (P) following sedimentation was resolved by SDS-PAGE and visualized by Coomassie dye. B, the saturation responses (Req) (see supplemental Fig. S2B) at each ACCH domain protein concentration from SPR sensorgrams were plotted versus protein concentration for different liposomes (PM + PI(4)P (■), PM (□), POPC/POPE/PI4P (75:20:5) (▿), POPC/POPE/PI(3)P (75:20:5) (◇), POPC/POPE/PI(5)P (75:20:5) (♦), and POPC/POPE/PI(4,5)P2 (75:20:5) (▵)). The Kd was determined for a minimum of seven different protein concentrations by nonlinear least squares analysis of the binding isotherm using the equation, Req = Rmax/(1 + Kd/C). At least three replicates were done to calculate an S.D. value. C, insertion of the ACCH domain into POPC/POPE (80:20) (○), PM (□), POPC/POPE/PI(4)P (75:20:5) (▿), POPC/POPE/PI(3)P (75:20:5) (◇), and POPC/POPE/PI(4,5)P2 (75:20:5) (▵) monolayers as a function of π. 10 μg of ACCH was injected into the subphase of a monolayer of the indicated initial surface pressure. The Δπ was then monitored for 45 min to construct the curves and determine πc, the x intercept. D, MDCK cells stably expressing DsRed Amot80 and transiently expressing CFP-tagged FAPP1-PH were imaged live. The merge of the Amot (red) and FAPP1-PH (blue) is depicted on right. E, three-dimensional reconstructions of the x-y, x-z, and y-z dimensions of a live MDCK cell grown on a transwell filter expressing Amot ACCH (red) and CFP FAPP1-PH (blue). F, MDCK cells were transfected with vectors that express citrine Akt-PH (green, PIP3 probe), DsRed PLCδ PH (red, PIP2 probe) and cerulean Amot ACCH (blue) (left). Cells were then cultured on a transwell filter for 48 h and then imaged live. The x-z cut section from reconstructed confocal images is shown. mN, millinewtons.
To better gauge the selectivity and affinity of the ACCH domains for different lipids, we quantitatively assessed binding to liposomes of varying composition in real time by SPR. Sensorgrams for POPC/POPE/PI (60:20:20) vesicles obtained for a range of protein concentrations are illustrated in supplemental Fig. S2, B and C. The Kd values for ACCH association with different liposomes were determined by nonlinear least squares analysis of plots of equilibrium binding values (saturation response values) versus protein concentration. Amot ACCH directly binds liposomes with a composition resembling the PM (39) with a Kd of 22 nm, which is enhanced 4-fold by the addition of 5 mol % PI(4)P. Further, although the affinity of Amot for POPC/POPE liposomes is undetectable, the addition of PI, PI(4)P, PI(3)P, or PI(5)P results in binding with affinities of 95, 16, 18, and 120 nm, respectively. However, up to a 10 μm concentration of the ACCH domain had no detectable binding to vesicles containing PI(4,5)P2 or PIP3 (Fig. 2B and Table 1). This difference in lipid specificity is not due to compromised lipids because other proteins, such as the PH domain of Akt, bound normally to these liposomes (data not shown). Moreover, the ACCH domain of Amot displays moderate affinity for bulk anionic lipids, such as PS and PA, that are enriched in the PM and endosomes.
The ACCH domain probably achieves high affinity binding to membranes by inserting into the bilayer. Penetration of a lipid monolayer by Amot ACCH was measured in a Kibron microtrough in the presence of chemically defined phospholipids at fixed surface concentrations as described previously (40). Phospholipid monolayers at the air/water interface serve as a highly sensitive tool to measure the membrane-penetrating ability of protein. The effects of Amot ACCH on the surface pressure of each monolayer were extrapolated for the capacity to penetrate a bilayer on the shared properties of monolayers and bilayers (40, 41). The increases in surface pressure (Δπ) of monolayers containing PI(4)P but not PI(4,5)P or PIP3 induced by Amot ACCH indicate that this domain spontaneously and specifically binds and penetrates the hydrocarbon region of such monolayers (Fig. 2C). Such increases are generally inversely proportional to the initial surface pressure (π) (42) and were therefore extrapolated by the Δπ versus π0 plot to yield the critical surface pressure (πc), which specified the upper limit of π0 that allowed protein penetration.
Proteins generally penetrate cell membranes and phospholipid monolayers with a comparable πc of 31 millinewtons/meter or more (43). Thus, the penetration of PIP-containing phospholipid monolayers by Amot ACCH with πc > 31 millinewtons/meter indicates that it probably penetrates cell membranes with a similar specificity (36). The low ability of Amot ACCH to penetrate membranes containing PIP2 is consistent with the selectivity of the ACCH domains for PIPs.
Amot and Amot ACCH specifically associate with cellular membranes enriched in PI(4)P. The intracellular binding of Amot ACCH with PI(4)P was measured based on its co-localization with the PH domain of FAPP1 (FAPP1-PH), which binds selectively to this lipid (44). MDCK cells that stably expressed DsRed-tagged Amot80 were transfected with a vector encoding CFP-tagged FAPP1-PH. Following 24 h, these two proteins were visualized at common perinuclear intracellular compartments (Fig. 2D). Similarly, polarized MDCK cells grown on transwell filters showed DsRed-tagged Amot ACCH co-distributed with CFP-tagged FAPP1-PH at enlarged intracellular compartments (Fig. 2E). In both cases, roughly 50% of the FAPP1-PH did not overlap with Amot or Amot-ACCH. This probably represents the previously ascribed distribution of FAPP1-PH at endosomes and the Golgi (45).
Conversely, Amot ACCH does not distribute to membranes that bind probes of PIP2 or PIP3. MDCK cells simultaneously expressing CFP-tagged Amot ACCH, YFP-tagged Akt-PH (probe of PIP3), and DsRed-tagged Plcδ1-PH (probe of PIP2) were grown on collagen-coated filters in a transwell dish for 54 h and then imaged live. Amot ACCH concentrated at intracellular membranes that extend as a column along the apical to basal axis of the cell with no apparent co-localization with PLCδ1-PH or Akt-PH (Fig. 2F and supplemental Fig. S3A and Movie 1). The sizes of such structures were larger than those induced by Amot80, indicating that the ACCH domain exhibits increased activity when removed from the context of the full-length protein. Cellular polarity was unlikely to be grossly perturbed because the PLCδ1-PH and Akt-PH domains asymmetrically concentrated at the apical and basal regions of the PM as reported previously (16).
Cholesterol Enhances Binding of the ACCH Domain to Liposomes
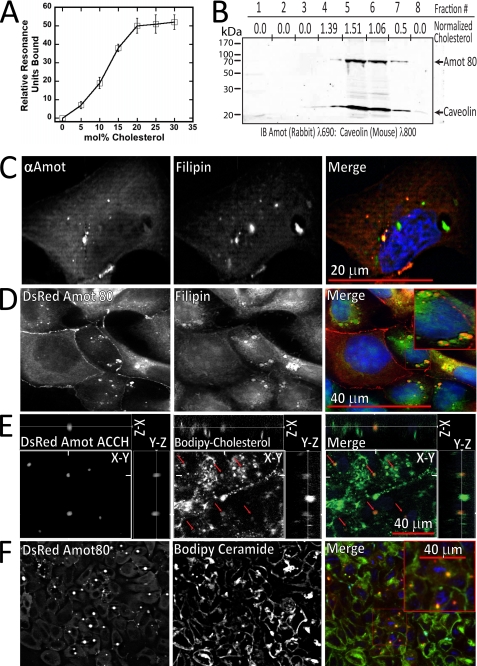
To directly determine the impact of cholesterol on the membrane binding properties of Amot ACCH, the binding of purified protein to liposomes with increasing cholesterol content was measured using SPR. The affinity of the ACCH domain for liposomes increased over 10-fold as 5 mol % increments of cholesterol were added at the expense of POPC to POPC/POPE (80:20) vesicles (Fig. 3A). Although binding saturates at roughly 30% cholesterol, the addition of PI or PIP further promotes binding (Table 1). Thus, cholesterol cooperatively drives binding of Amot ACCH to membranes with PIP.
FIGURE 3.
The ACCH domain preferentially binds membranes enriched in cholesterol. A, relative response values (relative to POPC/POPE (80:20) control surface) were measured using SPR for 100 nm ACCH binding to POPC/POPE/cholesterol (80 − x:20:x) liposomes, where x equals the cholesterol concentration in each measurement. The S.D. value was calculated from five or more measurements for each respective cholesterol concentration. B, cellular membranes were prepared from MDCK cells expressing 3× FLAG-tagged Amot. Membranes were then separated by density using an Optiprep gradient, and the cholesterol content and the relative levels of Amot and caveolin levels in each fraction are depicted. The relative amounts of cholesterol were determined using an Amplex Red® kit (Sigma), whereas Amot and caveolin were detected by immunoblot analysis. C, fixed MDCK cells were permeabilized and immunostained for Amot (red) and for cholesterol as measured by binding to filipin (green) and with DAPI stain (blue). Confocal images of Amot (left), filipin (middle), and the merge showing DAPI stain (blue) (right) are shown. D, MDCK cells stably expressing DsRed Amot80 (red) were stained with filipin (green) and with DAPI stain (blue) and visualized as indicated. E, MDCK cells expressing DsRed Amot ACCH (red) were cultured on transwell filters with BODIPY cholesterol (green) and stained for nuclei with DAPI (blue). Live cells were then imaged as a single en face confocal section (main panel) and as x-y as well as x-z reconstructed z-stacks. F, MDCK cells stably expressing DsRed Amot80 were incubated with BODIPY ceramide and imaged live where a confocal section is depicted with DsRed Amot80 (left) and BODIPY ceramide (middle), and the merge is shown with Amot (red), BODIPY ceramide, and DAPI stain (blue) (right).
Amot and Cholesterol Partition at Common Intracellular Membranes
The relatively low buoyant density of cholesterol-rich membranes allows their biochemical enrichment by flotation on a density gradient (46). MDCK cells stably expressing low levels of 3× FLAG-tagged Amot80 were lysed, and light membranes were enriched by the OptiPrep method (47). Immunoblot analysis indicates that Amot co-fractionates with the cholesterol binding protein caveolin, whereas the majority of cholesterol is present in these same fractions as measured by an Amplex Red assay (Fig. 3B).
Amot highly distributes with cholesterol enriched membranes in fixed MDCK cells. The localization of endogenous Amot was analyzed in MDCK cells cultured on glass slides for 24 h that were then fixed with paraformaldehyde and extracted with 0.1% Triton X-100. The distribution of Amot was detected by immunostaining with an antibody against Amot, whereas cholesterol was detected by staining with filipin, a fluorescent polyene antibiotic that specifically binds cholesterol (48) (Fig. 3C). This revealed that Amot was almost exclusively found in filipin-stained membranes. Filipin, however, was often seen in membranes lacking Amot. Similarly, DsRed-tagged Amot80, when expressed at low levels, co-distributed at enlarged intracellular structures with filipin at perinuclear compartments (Fig. 3D). High resolution images indicate that Amot resides in a limited area within the larger cholesterol-enriched compartments (see Fig. 3E). When expressed at high levels, DsRed Amot80 is visualized throughout the membranes of highly enlarged perinuclear compartments in which almost all of the intracellular non-extracted cholesterol appears to be distributed (supplemental Fig. S3B).
Amot ACCH distributes with a small fraction of cholesterol in live MDCK cells. MDCK cells transfected with a vector expressing DsRed-tagged Amot ACCH were cultured on transwell filters with BODIPY cholesterol, a fluorescent analog that mimics natural cholesterol (49). Amot ACCH was mainly visualized in these live cells at intracellular compartments with BODIPY cholesterol. However, unlike filipin staining, only the small fraction of BODIPY cholesterol was represented at Amot ACCH-positive compartments (Fig. 3E).
Based on the differences between the fraction of detected cholesterol in Triton X-100-extracted and live MDCK cells, the ability of Amot to preferentially bind cholesterol in lipid rafts was examined. Cholesterol partitions into ordered membranes that are resistant to detergent extraction and are referred to as “lipid rafts” (50). This was accomplished by determining the localization of stably expressed DsRed-Amot80 in polarized MDCK cells with exogenously introduced BODIPY ceramide as it preferentially inserts into lipid rafts (51, 52). The concentration of BODIPY ceramide in Amot-containing compartments further indicates that Amot mainly associates with cholesterol in ordered membranes (Fig. 3F).
The Amot ACCH Domain Preferentially Binds Curved Membranes and Induces Tubulation of Vesicles
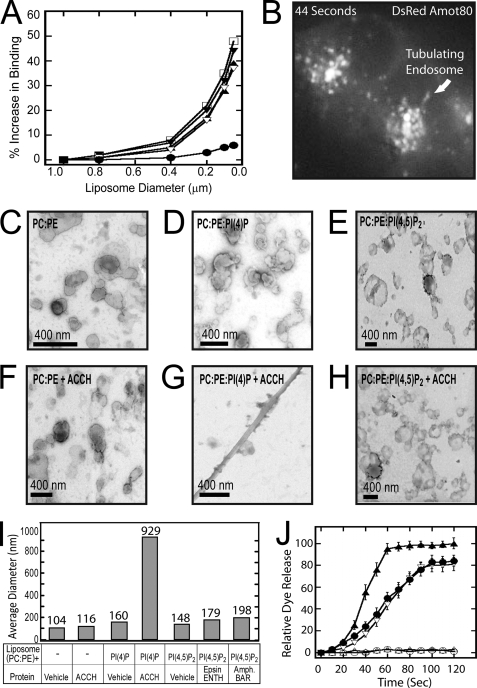
The binding of Amot ACCH to membranes is enhanced in POPC/POPS (phosphatidylserine)-composed liposomes of increasing positive curvature (small diameter) by as much as 50% (Fig. 4A). Although increased curvature also improved binding to the PM-mimetic (Table 1) as well as PI(4)P (supplemental Fig. S4A)-containing vesicles, it did not improve binding to PC/PE-, PC/PE/PI(4,5)P2-, or PC/PE/PIP3-containing vesicles. That the C2 domain of protein kinase Cα did not show an increase in binding to POPC/POPE/POPS vesicles with increasing curvature (supplemental Fig. S4A) indicates that changes in binding by Amot ACCH are not a result of some nonspecific property, such as a total increase in available membrane surface area. This underscores the specificity of the ACCH domain for anionic-containing membranes, especially those containing PI(4)P but not PIP2 or PIP3. This is also consistent with Amot associating with cholesterol-rich membranes as this lipid concentrates in curved membranes (46). Binding by the curvature-independent ENTH domain (53) under all conditions indicates that membranes used in these experiments were intact and indicates the specific nature of the curvature-dependent association of the ACCH domain (Fig. 4A).
FIGURE 4.
The ACCH domain selectively binds curved membranes and tubulates liposomes containing PI(4)P. A, liposomes with the indicated diameters were analyzed by SPR as described in the legend to Fig. 2 for binding of 100 nm Amot ACCH to POPC/POPE/PI(4)P (75:20:5) (▿), PM (□), POPC/POPE/POPS (60:20:20) (▴), and POPC/POPE/PI (60:20:20) (▾). Also, binding of 100 nm epsin ENTH was monitored to POPC/POPE/PI(4,5)P2 (75:20:5) (●) liposomes of the indicated diameters. B, MDCK cells that stably express DsRed Amot80 were imaged live at 2-s intervals for 80 s. A single frame from this sequence shows Amot marking highly dynamic and often tubulating endosomes (for the complete sequence, see supplemental Movie 2). C–H, liposome samples (1 mg/ml) prepared by extrusion through 100-nm membranes in POPC/POPE (80:20) (C), POPC/POPE/PI(4)P (75:20:5) (D), and POPC/POPE/PI(4,5)P2 (E) were applied to carbon-Formvar-coated copper grids (EMS), and membrane morphologies were examined on an FEI Magellan scanning electron microscope at an electron energy of 15 kV. Representative images were taken with a direct magnification of ×72,000–202,000. The scale bar in each panel is 400 nm. To assess the ability of the ACCH domain to induce lipid curvature changes, liposomes in POPC/POPE (80:20) (F), POPC/POPE/PI(4)P (75:20:5) (G), and POPC/POPE/PI(4,5)P2 (75:20:5) (H) were incubated with 5 μm ACCH domain for 15 min at 25 °C. The sample (8 μl) was then applied to the grids and negatively stained and imaged as described above. I, the size distributions of base POPC/POPE (80:20) liposomes with the indicated lipid ligand (POPC/POPE/PI(4)P (75:20:5) or POPC/POPE/PI(4,5)P2 (75:20:5) were prepared by 100-nm extrusions and assessed by dynamic light scattering analysis. Subsequently, liposomes of the indicated composition were incubated with the respective membrane-tubulating proteins. Reactions were carried to saturation, and then the average diameter of the membranes in each sample was determined by dynamic light scattering analysis. Each bar represents the mean diameter for the respective lipid. J, leakage of 5-carboxyfluorescein dye from liposomes of the indicated compositions by the addition of Amot ACCH protein (POPC/POPE (○), POPC/POPE/PI(4)P (▿), and POPC/POPE/PI(4,5)P2 (▵)) or ENTH domain (POPC/POPE/POPI(4,5)P2 (●)). Triton X-100 disruption of vesicles and subsequent leakage was used as a positive control (▴). At least three replicates were done to calculate an S.D. value for each time point.
Amot distributes with highly dynamic tubulating endosomes in live MDCK cells with low apical/basal polarity. To visualize whether Amot distributes at sites of potential membrane curvature (i.e. actively trafficking structures), it was expressed as an N-terminal chimera with DsRed. Imaging of the live cells over 1.5 min at 2-s intervals revealed that Amot80 associates with highly dynamic membranes that move into and out of perinuclear dense regions as tubules and vesicles (Fig. 4B and supplemental Movie 2). The proclivity of Amot-positive vesicles to merge (supplemental Fig. S4B) and/or assume tubulated structures indicates that the Amot ACCH domain is positioned to mediate such events in cells.
Amot ACCH specifically tubulates lipid vesicles containing PI(4)P and PI(3)P. To visualize the effect of Amot ACCH on remodeling lipid vesicles, we directly imaged membranes using scanning electron microscopy. Incubation of 1 μm ACCH domain with liposomes composed of POPC/POPE, POPC/POPE/POPS, or POPC/POPE/PI(4,5)P2 produced no visible changes. In consonance with lipid specificity for binding and penetration, the ACCH domain formed tubules from vesicles composed of POPC/POPE with PI(4)P or PI(3)P (Fig. 4, C–H, and supplemental Fig. S4, C–F). Incubation of POPC/POPE/PI(4,5)P2 vesicles with the ENTH domain induced tubules of ∼500 nm (supplemental Fig. S4, G and H), consistent with previous reports (54). Although Amot ACCH and epsin ENTH produced tubules with a diameter of ∼40 nm, the tubules induced by Amot were much longer, at several μm.
Dynamic light scattering data indicate that Amot ACCH induces a greater average increase in mean membrane structure size versus amphiphysin BAR or epsin ENTH domains. The roughly similar shape and diameter of tubules induced by Amot ACCH, epsin ENTH, and amphiphysin BAR indicates that differences in dynamic light scattering result from changes in average tubule length, assuming that conversion of liposomes to tubules occurs at equal efficiencies for each protein. To analyze Amot ACCH using this method, liposomes with a mean particle size of 100 nm were generated by extrusion and tested for mean diameter. Incubation of Amot ACCH with 100-nm liposomes composed of POPC/POPE/PI(4)P but not POPC/POPE results in an increase in average diameter of over 10-fold. In contrast, the epsin ENTH and amphiphysin BAR domains alter the mean diameter of POPC/POPE/PI(4,5)P2 liposomes by less than 2-fold (Fig. 4I and supplemental Fig. S4J).
Amot ACCH deforms available liposomes with the same efficiency as the epsin ENTH domain. To measure the fraction of available liposomes that epsin ENTH and Amot ACCH can deform, we measured their effects on the leakage of a membrane-impermeable dye from liposomes (Fig. 4J). Vesicles containing 5-carboxyfluorescein were mixed with protein, and the release of dye over time was taken as an indication of membrane-compromising events. This method allows the sensitive measurement of membrane disruption in real time. The ENTH domain induces saturable leakage of dye at nanomolar concentrations only from PI(4,5)P2-containing vesicles, concordant with its known tubulating activity (55). Dye leakage induced by Amot ACCH occurs with PI(4)P containing liposomes within 20 s. Similar to epsin ENTH, dye release saturates at around 100 s. Amot ACCH, however, had no effect on dye release from POPC/POPE- or POPC/POPE/PI(4,5)P2-containing vesicles. The similar levels of total leakage of ∼80% (at saturation) induced by both domains therefore indicate that Amot ACCH deforms roughly the same percentage of available liposomes as epsin ENTH. Such membrane disruption probably is not a general property of membrane binding because the C2 domain of protein kinase Cα and the PX domain of p40phox induced no detectable dye leakage from PS- or PI(3)P-containing vesicles under conditions where both domains bound liposomes (supplemental Fig. S4I). These data therefore indicate that Amot ACCH induces tubules of a greater length on average versus epsin ENTH instead of utilizing available liposomes more efficiently.
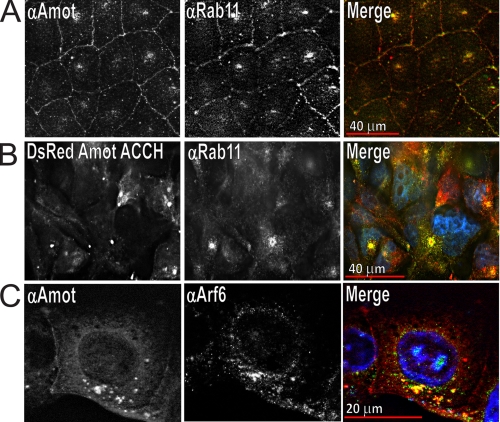
Amot Co-localizes in MDCK Cells with Rab11 and Arf6
To identify the intracellular compartment that Amot binds, its co-localization with well characterized trafficking markers was examined. Vesicles recycling bulk cargo to the PM bind Rab11 (56), whereas endosomes returning TJ-associated cargo, such as occludin, associate with Rab13 (57). Endogenous Amot partially distributes to endosomes with Rab11 in MDCK cells that are flattened and are in a migratory configuration (Fig. 5A and supplemental Fig. S5A). Such targeting is probably driven by the ACCH domain because DsRed-tagged Amot ACCH distributes with Rab11 similarly (Fig. 5B). Consistent with such compartments mediating the trafficking of TJ-associated proteins, DsRed-tagged Amot80 partially distributes in live MDCK cells with an N-terminally YFP-tagged Rab13 (supplemental Fig. S5B). Amot80 and Rab11, however, did not co-distribute in cells grown on transwell filters that had assumed an epithelial morphology (supplemental Fig. S5C). Consistent with Amot compartments having little recycling activity in highly polarized cells, these structures were more enlarged and showed little trafficking activity (data not shown). Taken together, Amot mainly associates with Rab11 in migratory or low apical/basal polarized cells but not noticeably in more apically basal polarized cells.
FIGURE 5.
Amot and Amot ACCH similarly distribute in MDCK cells at perinuclear endosomes with Rab11 and Arf6. A, MDCK cells were plated for 18 h, fixed with paraformaldehyde, and immunostained with a rabbit polyclonal antibody directed against Amot (left) and mouse monoclonal antibody directed against Rab-11 (middle) and DAPI stain to visualize nuclei. The merger of Amot (red), Rab11 (green), and DAPI stain (blue) is shown on the right. B, MDCK cells stably expressing DsRed Amot ACCH were plated for 24 h before being fixed with paraformaldehyde and immunostained for Rab11 and stained with DAPI. Left, DsRed Amot ACCH; middle, Rab11; right, merge of Amot (red), Rab11 (green), and DAPI (blue). C, MDCK cells were plated for 18 h and then immunostained for endogenous Amot (left) and Arf-6 (middle). The merger of Amot (red), Arf6 (green), and DAPI (blue) is shown on the right.
Amot was not found to distribute with other endosomal populations. Specifically, Amot was analyzed for co-localization with Rab5, which marks vesicles carrying newly internalized cargo (58), and with Rab7, which associates with late endosomes that carry cargo designated for the lysosome (59). In all cases, Amot was never found to significantly distribute to vesicles or remodel vesicles carrying Rab5 or Rab7 (supplemental Fig. S5, D–F).
The frequent proximity of Amot at Rab11-positive compartments around the nucleus in tubulating endosomes suggests an association with vesicles that traffic into and out of the ERC (60, 61). The ERC resides near the nucleus and is mainly denoted by the presence of the small GTPase Arf6 (31) and to a lesser extent Rab11 and EEA1 (31, 62, 63). Such membranes form tubular structures enriched in cholesterol (30), PIP2 (63), and PI(3)P (61). To identify whether Amot distributes with the ERC, MDCK cells were immunostained for endogenous Amot and Arf6. This revealed that both proteins concentrated in shared vesicles that were mainly adjacent to the nucleus (Fig. 5C). Further, expression of DsRed Amot80 resulted in highly enlarged perinuclear compartments to which it strongly co-distributed with exogenously expressed Arf6 YFP in live cells (supplemental Fig. S5G) as well as with endogenous Arf6 in fixed cells (supplemental Fig. S5H).
The enlarged compartments seen upon Amot expression are unlikely to represent the accumulation of misfolded proteins at aggresomes. When the proteasome is overwhelmed or dysfunctional, misfolded proteins concentrate at aggresomes. Aggresomes strongly stain for ubiquitin and co-localize with tubulin at the centrosome (64). However, Rab11 and Arf6, which reside in Amot-positive compartments, are not generally found in aggresomes. Conversely, DsRed Amot80 was generally not observed with tubulin or ubiquitin even at very high levels of expression of Amot (supplemental Fig. S5, I–K). The enrichment of the same lipid ligands that drive Amot ACCH association and tubulation of liposomes, indicates that the ACCH domain drives these endosomal reconfigurations via its ability to deform membranes. Further, the specific recruitment of apical proteins to this compartment by full-length Amot but not Amot ACCH or AmotΔCOOH, which induce similarly enlarged endosomes, indicates that Amot mediates membrane-remodeling events in conjunction with selective protein recruitment via a PDZ domain/PDZ interaction motif (22) (supplemental Fig. S6A).
The ACCH Domain of Amot Promotes Binding to Par-3 and Mupp1 and Is Necessary for Amot to Target These Proteins to Endosomes
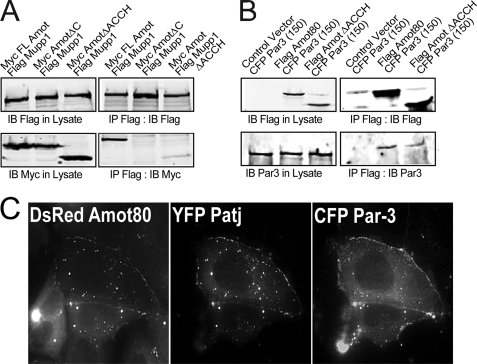
Amot expression was previously shown to redistribute Par-3 and Pals1 to endosomes and to induce the loss of TJ integrity (22). Although direct binding of Amot to Patj is through the C terminus of Amot, the moieties that place these proteins at similar intracellular locales is unknown. Full-length Amot precipitates Mupp1 (Fig. 6A) and Par-3 (Fig. 6B) preferentially over mutants of Amot that lack the ACCH domain (AmotΔACCH). Thus, the ACCH domain probably promotes such interactions by placing Amot on membranes that also contain these polarity proteins.
FIGURE 6.
The ACCH domain promotes the interaction of Amot with polarity proteins at endosomal membranes. A, the relative association of Myc-tagged Amot80, AmotΔCOOH (which lacks 4 C-terminal residues), and AmotΔACCH (which lacks residues 1–242) with FLAG-tagged Mupp1 was examined. HEK 293T cells co-transfected with the indicated vectors were used for immunoprecipitations with anti-FLAG (M2) antibody. Immunoblots (IB) of lysates (left) and immunoprecipitants (IP) (right) using the indicated antibodies against FLAG (M2) and Myc (9E10) are shown. B, the relative association of FLAG-tagged Amot80 or AmotΔACCH with CFP-tagged Par-3 was examined. HEK 293T cells co-transfected as indicated were used for immunoprecipitation with anti-FLAG antibody. Immunoblots of lysates (left) and immunoprecipitants (right) with antibodies against FLAG and Par-3 are shown. C, a single en face confocal image of live MDCK cells showing the co-distribution of exogenously expressed DsRed Amot80 (left), YFP Patj (middle), and CFP Par-3 (right). MDCK cells were transfected with vectors expressing these three proteins and imaged live after removing calcium from the media. The movement of these proteins over 5 min is shown in supplemental Movies 3, A–C.
Amot/Patj-positive endosomes join with Par-3 as it moves off of the PM upon loss of intercellular contacts. The partial silencing of Amot results in a delay in the loss of TJ integrity upon the removal of calcium from the media (22). However, the events whereby Amot coordinates with the loss of intercellular contacts is not known. To examine the relationship of Amot-positive endosomes upon the loss of intercellular contacts, DsRed Amot, YFP Patj, and CFP Par-3 were simultaneously imaged over time in MDCK cells detaching from neighboring cells. This was accomplished by co-transfecting MDCK cells with vectors expressing each of these tagged proteins. Following 24 h, cells were switched into calcium-free media, and after an additional 1 h, they were imaged live over 5 min. DsRed-tagged Amot and YFP-tagged Patj almost perfectly co-distribute on endosomes that moved toward the PM as it detached from the opposing cell (Fig. 6C and supplemental Movies 3, A–C). Par-3 moved as a wave off of this membrane, where it is apparently captured by Amot/Patj-positive endosomes. Such retargeting is dependent on the ACCH domain because DsRed-tagged AmotΔACCH has no impact on the intracellular localization of Par-3 (supplemental Fig. S6B) as has been reported previously (22). Thus, Amot requires both the ACCH domain and its C-terminal PDZ binding motif to efficiently bind polarity proteins and to subsequently target them to endosomes.
DISCUSSION
Amot encodes the prototypical member of a novel and conserved family of predicted membrane binding functionalities collectively termed ACCH domains. Unlike most lipid binding moieties, this domain stably binds a selective lipid distribution and consequently induces membrane tubules that are demonstrably elongated when compared with those produced by amphiphysin BAR or epsin ENTH domains. These properties probably explain the impact of Amot expression in cells where it associates with tubulating endosomes and promotes enlargement of the ERC. This also indicates a context whereby full-length Amot directly interfaces apical polarity proteins with this compartment (12, 62).
The ACCH Domain Family Shares Properties with N-BAR and ENTH Domains
BAR domains are coiled-coil structures that dimerize to form a surface that binds curved membranes and often induce membrane curvature (65). The ACCH domain of Amot, like BAR domains, is composed of a coiled-coil fold of ∼200 residues that binds membranes rich in anionic lipids. Further, Amot ACCH selectively binds curved membranes, suggesting that it shares the concave structure of N-BAR domains (66). Because Amot contains hydrophobic residues at its N terminus similarly to the amphipathic α-helices within amphiphysin and endophilin (53, 67) that mediate penetration (53), this region within the ACCH domain may also mediate membrane penetration. In many cases, penetration is necessary for biological activity (68), including altering membrane curvature to enable membrane trafficking (66). Multiple modes of membrane penetration, a prerequisite of membrane tubulation, have been discovered for ENTH and BAR domains (66, 69, 70). Although the mechanism of insertion of the ACCH domain is unknown, it will probably require novel aspects that generate the lipid curvature changes necessary for the formation of highly elongated tubules.
The Membrane Binding Properties of the ACCH Domain Are Consistent with Functions in Cholesterol-dependent Recycling of Endosomes
The affinity of the ACCH domain for anionic phospholipids with a preference for PI(4)P and PI(3)P is a rare property among membrane-deforming BAR and BAR-like domains and has only been shown for one other protein, arfaptin (53). The molecular basis of exclusion of PI(4,5)P2 and PI(3,4,5)P3 by the ACCH domain and perhaps arfaptin is yet to be determined. This will require integration of structural or computational data with a variety of lipid binding studies.
The interaction of Amot ACCH with monophosphorylated PIs is consistent with a function at endosomal compartments. The ACCH domain excludes PI(4,5)P2 and PIP3, which concentrate at apical and basal PM (71) while preferentially binding PI(4)P and PI(3)P, which are highly represented in the trans-Golgi and endosomes (45, 72). Based on the inhibition of membrane recycling produced by perturbing proteins that bind or produce PI(4)P, Amot probably shares a similar role. For instance, expression of the PH domain of FAPP1, the loss of expression of the phosphatidylinositol transfer protein (73, 74), or silencing of the Type IIIB PI(4) kinase (75) all block endosomal movement to the PM. Although the association of Amot with PI(3)P may implicate it in early endosomal trafficking (76), the lack of co-localization of Amot with Rab5 and the presence of EEA1 in the ERC (62) argue against this. Thus, the strong affinity of the ACCH domain for PI(4)P and to a lesser extent PI(3)P is most consistent with Amot functioning in protein recycling.
To our knowledge, Amot ACCH is the only reported BAR-like domain that selectively associates with cholesterol-enriched membranes. Thus, ACCH domains are probably essential for many curvature-inducing events in ordered membranes that exchange between the apical PM and the ERC. Cholesterol concentrates with ordered sphingolipids and sterols in microdomains (46, 77) of varying lipid curvatures (78), mainly at intracellular tubules and vesicles that make up the ERC (79–81) and that exchange with the apical PM (82). Consistently, Amot, through its ACCH domain, distributes with cholesterol at the PM and at endosomes in live and fixed cells. The significantly lower fraction of BODIPY cholesterol that distributes with Amot in live cells versus filipin in detergent-permeabilized cells indicates that Amot preferentially sorts with ordered membranes that are resistant to extraction by detergent (82). Consistently, Amot partially co-distributes with BODIPY ceramide in cells and co-fractionates with membranes containing caveolin (50, 52).
The Biophysical Properties of the ACCH Domain Indicate the Membrane Composition of the ERC and a Mechanism by Which It Is Remodeled
The localization of Amot to the ERC sheds light onto the lipids that are enriched in this compartment. Endogenous Amot partially distributes with Rab11 and Arf6, both markers of the ERC (31, 62, 63). Consistent with the lipid binding properties of the ACCH domain, probes of cholesterol and PI(4)P also mark Amot-positive perinuclear endosomes. Although cholesterol is highly represented in the ERC (83), to our knowledge, this is the first report that PI(4)P is also enriched there. The consistent co-distribution of Amot with lipids in cells to which the ACCH domain binds in vitro posits that the lipid binding profile of this domain indicates the composition of membranes in the ERC.
Changes in ERC morphology produced by perturbing the function of ERC-resident proteins may be mediated by Amot. Expression of the PI(3)kinase (62), a GTP binding-defective mutant of Arf6 (84), or a mutant of syndecan that does not bind PIP2 produces a single engorged ERC that resembles those produced by Amot (61). Similarly, depletion of Arf6 (85) or expression of Rab11 (86) promotes the accumulation of cholesterol in an enlarged ERC. Because none of these proteins directly deform membranes, it is unclear how such treatments alter ERC structure. We suggest that such remodeling may in part result from their cooperatively providing the coincidence of PI(4)P/PI(3)P and cholesterol that in turn recruits Amot and enhances the tubulation activity of the ACCH domain. This would then define the region within the membrane where changes in membrane curvature promote a trafficking event.
The requirement for Par proteins in ERC function (12) indicates that they may play a part in its role in cellular migration (87–89). This study identifies that the ACCH domain recognizes anionic lipids embedded in “lipid rafts” in the ERC. Thus, in the context of the TJ-associated proteins Amot, JEAP, and MASCOT, it may explain their shared ability to promote cellular migration (90, 91) by directing them to this compartment. This is predicted to promote the loss of epithelial architecture by both redistributing associated proteins, such as Pars, to the ERC and by direct remodeling of this compartment to prevent recycling to the PM.
Supplementary Material
This work was supported by an Indiana University School of Medicine Biomedical research grant, American Cancer Society Grant IRG-84-002-22, and the Notre Dame Integrated Imaging Facility (to R. V. S.) and by the Showalter Foundation, American Cancer Society Grant IRG-81-003-20, and United States Department of Defense Grant W81XWH (to C. D. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and Movies 1, 2, and 3A–3C.
- PM
- plasma membrane
- TJ
- tight junction
- YFP
- yellow/citrine fluorescent protein
- CFP
- blue/cerulean fluorescent protein
- PLC
- phospholipase C
- MDCK
- Madin-Darby canine kidney
- PI
- phosphatidylinositol
- PIP
- monophosphorylated phosphatidylinositol
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- PG
- phosphatidylglycerol
- PA
- phosphatidic acid
- ENTH
- epsin N-terminal homology
- BAR
- Bin-amphiphysin-Rvs domain
- ERC
- juxtanuclear endosomal recycling compartment
- POPS
- phosphatidylserine
- POPC
- phosphatidylcholine
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycerophosphoethanolamine
- SPR
- surface plasmon resonance
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- PH
- pleckstrin homology
- DAPI
- 4′,6-diamidino-2-phenylindole.
REFERENCES
- 1.Nelson W. J. (2003) Nature 422, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson W. J. (2009) Cold Spring Harb. Perspect. Biol. 1, a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin K., Fogg V. C., Margolis B. (2006) Annu. Rev. Cell Dev. Biol. 22, 207–235 [DOI] [PubMed] [Google Scholar]
- 4.Kirby C., Kusch M., Kemphues K. (1990) Dev. Biol. 142, 203–215 [DOI] [PubMed] [Google Scholar]
- 5.Tepass U., Knust E. (1993) Dev. Biol. 159, 311–326 [DOI] [PubMed] [Google Scholar]
- 6.Tepass U., Theres C., Knust E. (1990) Cell 61, 787–799 [DOI] [PubMed] [Google Scholar]
- 7.Lin D., Edwards A. S., Fawcett J. P., Mbamalu G., Scott J. D., Pawson T. (2000) Nat. Cell Biol. 2, 540–547 [DOI] [PubMed] [Google Scholar]
- 8.Joberty G., Petersen C., Gao L., Macara I. G. (2000) Nat. Cell Biol. 2, 531–539 [DOI] [PubMed] [Google Scholar]
- 9.Hirose T., Izumi Y., Nagashima Y., Tamai-Nagai Y., Kurihara H., Sakai T., Suzuki Y., Yamanaka T., Suzuki A., Mizuno K., Ohno S. (2002) J. Cell Sci. 115, 2485–2495 [DOI] [PubMed] [Google Scholar]
- 10.Hurd T. W., Gao L., Roh M. H., Macara I. G., Margolis B. (2003) Nat. Cell Biol. 5, 137–142 [DOI] [PubMed] [Google Scholar]
- 11.Mellman I., Nelson W. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balklava Z., Pant S., Fares H., Grant B. D. (2007) Nat. Cell Biol. 9, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Macara I. G. (2005) Nat. Cell Biol. 7, 262–269 [DOI] [PubMed] [Google Scholar]
- 14.Du Q., Stukenberg P. T., Macara I. G. (2001) Nat. Cell Biol. 3, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 15.Kisurina-Evgenieva O., Mack G., Du Q., Macara I., Khodjakov A., Compton D. A. (2004) J. Cell Sci. 117, 6391–6400 [DOI] [PubMed] [Google Scholar]
- 16.Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007) Cell 128, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fivaz M., Vilbois F., Thurnheer S., Pasquali C., Abrami L., Bickel P. E., Parton R. G., van der Goot F. G. (2002) EMBO J. 21, 3989–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabharanjak S., Sharma P., Parton R. G., Mayor S. (2002) Dev. Cell 2, 411–423 [DOI] [PubMed] [Google Scholar]
- 19.Lu H., Bilder D. (2005) Nat. Cell Biol. 7, 1232–1239 [DOI] [PubMed] [Google Scholar]
- 20.Harris K. P., Tepass U. (2008) J. Cell Biol. 183, 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Chen X. W., Margolis B. (2007) Mol. Biol. Cell 18, 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells C. D., Fawcett J. P., Traweger A., Yamanaka Y., Goudreault M., Elder K., Kulkarni S., Gish G., Virag C., Lim C., Colwill K., Starostine A., Metalnikov P., Pawson T. (2006) Cell 125, 535–548 [DOI] [PubMed] [Google Scholar]
- 23.Troyanovsky B., Levchenko T., Månsson G., Matvijenko O., Holmgren L. (2001) J. Cell Biol. 152, 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernkvist M., Luna Persson N., Audebert S., Lecine P., Sinha I., Liu M., Schlueter M., Horowitz A., Aase K., Weide T., Borg J. P., Majumdar A., Holmgren L. (2009) Blood 113, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aase K., Ernkvist M., Ebarasi L., Jakobsson L., Majumdar A., Yi C., Birot O., Ming Y., Kvanta A., Edholm D., Aspenström P., Kissil J., Claesson-Welsh L., Shimono A., Holmgren L. (2007) Genes Dev. 21, 2055–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugihara-Mizuno Y., Adachi M., Kobayashi Y., Hamazaki Y., Nishimura M., Imai T., Furuse M., Tsukita S. (2007) Genes Cells 12, 473–486 [DOI] [PubMed] [Google Scholar]
- 27.Ross J. W., Ashworth M. D., Stein D. R., Couture O. P., Tuggle C. K., Geisert R. D. (2009) Physiol. Genomics 36, 140–148 [DOI] [PubMed] [Google Scholar]
- 28.Shimono A., Behringer R. R. (2003) Curr. Biol. 13, 613–617 [DOI] [PubMed] [Google Scholar]
- 29.Ernkvist M., Birot O., Sinha I., Veitonmaki N., Nyström S., Aase K., Holmgren L. (2008) Biochim. Biophys. Acta 1783, 429–437 [DOI] [PubMed] [Google Scholar]
- 30.Caplan S., Naslavsky N., Hartnell L. M., Lodge R., Polishchuk R. S., Donaldson J. G., Bonifacino J. S. (2002) EMBO J. 21, 2557–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radhakrishna H., Donaldson J. G. (1997) J. Cell Biol. 139, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colwill K., Wells C. D., Elder K., Goudreault M., Hersi K., Kulkarni S., Hardy W. R., Pawson T., Morin G. B. (2006) BMC Biotechnol. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babbey C. M., Ahktar N., Wang E., Chen C. C., Grant B. D., Dunn K. W. (2006) Mol. Biol. Cell 17, 3156–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S. A., Eyeson R., Cheever M. L., Geng J., Verkhusha V. V., Burd C., Overduin M., Kutateladze T. G. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13052–13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bittova L., Stahelin R. V., Cho W. (2001) J. Biol. Chem. 276, 4218–4226 [DOI] [PubMed] [Google Scholar]
- 36.Stahelin R. V., Long F., Diraviyam K., Bruzik K. S., Murray D., Cho W. (2002) J. Biol. Chem. 277, 26379–26388 [DOI] [PubMed] [Google Scholar]
- 37.Letunic I., Doerks T., Bork P. (2009) Nucleic Acids Res. 37, D229–D232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finn R. D., Tate J., Mistry J., Coggill P. C., Sammut S. J., Hotz H. R., Ceric G., Forslund K., Eddy S. R., Sonnhammer E. L., Bateman A. (2008) Nucleic Acids Res. 36, D281–D288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahelin R. V., Rafter J. D., Das S., Cho W. (2003) J. Biol. Chem. 278, 12452–12460 [DOI] [PubMed] [Google Scholar]
- 40.Brockman H. (1999) Curr. Opin. Struct. Biol. 9, 438–443 [DOI] [PubMed] [Google Scholar]
- 41.Wolfe D. H., Brockman H. L. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4285–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. (1975) Biochim. Biophys. Acta 406, 97–107 [DOI] [PubMed] [Google Scholar]
- 43.Marsh D. (1996) Biochim. Biophys. Acta 1286, 183–223 [DOI] [PubMed] [Google Scholar]
- 44.Halet G. (2005) Biol. Cell 97, 501–518 [DOI] [PubMed] [Google Scholar]
- 45.Balla A., Tuymetova G., Tsiomenko A., Várnai P., Balla T. (2005) Mol. Biol. Cell 16, 1282–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson K., Mouritsen O. G., Anderson R. G. (2007) Nat. Cell Biol. 9, 7–14 [DOI] [PubMed] [Google Scholar]
- 47.Smart E. J., Ying Y. S., Mineo C., Anderson R. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orci L., Brown D. (1983) Lab. Invest. 48, 80–89 [PubMed] [Google Scholar]
- 49.Hölttä-Vuori M., Uronen R. L., Repakova J., Salonen E., Vattulainen I., Panula P., Li Z., Bittman R., Ikonen E. (2008) Traffic 9, 1839–1849 [DOI] [PubMed] [Google Scholar]
- 50.Anderson R. G. (1998) Annu. Rev. Biochem. 67, 199–225 [DOI] [PubMed] [Google Scholar]
- 51.Liu P., Anderson R. G. (1995) J. Biol. Chem. 270, 27179–27185 [DOI] [PubMed] [Google Scholar]
- 52.Yu C., Alterman M., Dobrowsky R. T. (2005) J. Lipid Res. 46, 1678–1691 [DOI] [PubMed] [Google Scholar]
- 53.Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. (2004) Science 303, 495–499 [DOI] [PubMed] [Google Scholar]
- 54.Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. (2002) Nature 419, 361–366 [DOI] [PubMed] [Google Scholar]
- 55.Stahelin R. V., Long F., Peter B. J., Murray D., De Camilli P., McMahon H. T., Cho W. (2003) J. Biol. Chem. 278, 28993–28999 [DOI] [PubMed] [Google Scholar]
- 56.Ullrich O., Reinsch S., Urbé S., Zerial M., Parton R. G. (1996) J. Cell Biol. 135, 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terai T., Nishimura N., Kanda I., Yasui N., Sasaki T. (2006) Mol. Biol. Cell 17, 2465–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. (1992) Cell 70, 715–728 [DOI] [PubMed] [Google Scholar]
- 59.Zerial M., McBride H. (2001) Nat. Rev. Mol. Cell Biol. 2, 107–117 [DOI] [PubMed] [Google Scholar]
- 60.Weigert R., Yeung A. C., Li J., Donaldson J. G. (2004) Mol. Biol. Cell 15, 3758–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmermann P., Zhang Z., Degeest G., Mortier E., Leenaerts I., Coomans C., Schulz J., N′Kuli F., Courtoy P. J., David G. (2005) Dev. Cell 9, 377–388 [DOI] [PubMed] [Google Scholar]
- 62.Naslavsky N., Weigert R., Donaldson J. G. (2003) Mol. Biol. Cell 14, 417–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown F. D., Rozelle A. L., Yin H. L., Balla T., Donaldson J. G. (2001) J. Cell Biol. 154, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnston J. A., Ward C. L., Kopito R. R. (1998) J. Cell Biol. 143, 1883–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habermann B., The B. A. (2004) EMBO Rep. 5, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallop J. L., Jao C. C., Kent H. M., Butler P. J., Evans P. R., Langen R., McMahon H. T. (2006) EMBO J. 25, 2898–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farsad K., Ringstad N., Takei K., Floyd S. R., Rose K., De Camilli P. (2001) J. Cell Biol. 155, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stahelin R. V., Digman M. A., Medkova M., Ananthanarayanan B., Rafter J. D., Melowic H. R., Cho W. (2004) J. Biol. Chem. 279, 29501–29512 [DOI] [PubMed] [Google Scholar]
- 69.Henne W. M., Kent H. M., Ford M. G., Hegde B. G., Daumke O., Butler P. J., Mittal R., Langen R., Evans P. R., McMahon H. T. (2007) Structure 15, 839–852 [DOI] [PubMed] [Google Scholar]
- 70.Yoon Y., Tong J., Lee P. J., Albanese A., Bhardwaj N., Källberg M., Digman M. A., Lu H., Gratton E., Shin Y. K., Cho W. (2010) J. Biol. Chem. 285, 531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin-Belmonte F., Mostov K. (2007) Cell Cycle 6, 1957–1961 [DOI] [PubMed] [Google Scholar]
- 72.Blatner N. R., Stahelin R. V., Diraviyam K., Hawkins P. T., Hong W., Murray D., Cho W. (2004) J. Biol. Chem. 279, 53818–53827 [DOI] [PubMed] [Google Scholar]
- 73.Jones S. M., Alb J. G., Jr., Phillips S. E., Bankaitis V. A., Howell K. E. (1998) J. Biol. Chem. 273, 10349–10354 [DOI] [PubMed] [Google Scholar]
- 74.Simon J. P., Morimoto T., Bankaitis V. A., Gottlieb T. A., Ivanov I. E., Adesnik M., Sabatini D. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Matteis M., Godi A., Corda D. (2002) Curr. Opin. Cell Biol. 14, 434–447 [DOI] [PubMed] [Google Scholar]
- 76.Simonsen A., Lippé R., Christoforidis S., Gaullier J. M., Brech A., Callaghan J., Toh B. H., Murphy C., Zerial M., Stenmark H. (1998) Nature 394, 494–498 [DOI] [PubMed] [Google Scholar]
- 77.Schuck S., Simons K. (2004) J. Cell Sci. 117, 5955–5964 [DOI] [PubMed] [Google Scholar]
- 78.Wang W., Yang L., Huang H. W. (2007) Biophys. J. 92, 2819–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mukherjee S., Zha X., Tabas I., Maxfield F. R. (1998) Biophys. J. 75, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., Geuze H. J. (2003) Traffic 4, 222–231 [DOI] [PubMed] [Google Scholar]
- 81.Hao M., Lin S. X., Karylowski O. J., Wüstner D., McGraw T. E., Maxfield F. R. (2002) J. Biol. Chem. 277, 609–617 [DOI] [PubMed] [Google Scholar]
- 82.Naslavsky N., Weigert R., Donaldson J. G. (2004) Mol. Biol. Cell 15, 3542–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naslavsky N., Rahajeng J., Rapaport D., Horowitz M., Caplan S. (2007) Biochem. Biophys. Res. Commun. 357, 792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters P. J., Hsu V. W., Ooi C. E., Finazzi D., Teal S. B., Oorschot V., Donaldson J. G., Klausner R. D. (1995) J. Cell Biol. 128, 1003–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schweitzer J. K., Pietrini S. D., D'Souza-Schorey C. (2009) PLoS One 4, e5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hölttä-Vuori M., Tanhuanpää K., Möbius W., Somerharju P., Ikonen E. (2002) Mol. Biol. Cell 13, 3107–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones M. C., Caswell P. T., Norman J. C. (2006) Curr. Opin. Cell Biol. 18, 549–557 [DOI] [PubMed] [Google Scholar]
- 88.Muralidharan-Chari V., Hoover H., Clancy J., Schweitzer J., Suckow M. A., Schroeder V., Castellino F. J., Schorey J. S., D'Souza-Schorey C. (2009) Cancer Res. 69, 2201–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palacios F., Price L., Schweitzer J., Collard J. G., D'Souza-Schorey C. (2001) EMBO J. 20, 4973–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang H., Lu F. I., Jia S., Meng S., Cao Y., Wang Y., Ma W., Yin K., Wen Z., Peng J., Thisse C., Thisse B., Meng A. (2007) Development 134, 979–988 [DOI] [PubMed] [Google Scholar]
- 91.Zheng Y., Vertuani S., Nystrom S., Audebert S., Meijer I., Tegnebratt T., Borg J. P., Uhlen P., Majumdar A., Holmgren L. (2009) Circ. Res. 105, 260–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.