Abstract
Evidence showing the ability of glial cells to detect, respond to and modulate synaptic transmission and plasticity has contributed to the notion of glial cells as active synaptic partners. However, synaptically induced plasticity of glia themselves remains ill defined. Here we used the amphibian neuromuscular junction (NMJ) to study plasticity of perisynaptic Schwann cells (PSCs), glial cells at this synapse, following long-term in vivo modifications of synaptic activity. We used two models that altered synaptic activity in different manners. First, chronic blockade of postsynaptic nicotinic receptors using α-bungarotoxin (α-BTx) decreased facilitation, increased synaptic depression and decreased post-tetanic potentiation (PTP). Second, chronic nerve stimulation increased facilitation and resistance to synaptic depression, while leaving PTP unaltered. Our results indicate that there is no direct relationship between transmitter release and PSC calcium responses. Indeed, despite changes in transmitter release and plasticity in stimulated NMJs, nerve-evoked PSC calcium responses were similar to control. Similarly, PSC calcium responses in α-BTx treated NMJs were delayed and smaller in amplitude, even though basal level of transmitter release was increased. Also, when isolating purinergic and muscarinic components of PSC calcium responses, we found an increased sensitivity to ATP and a decreased sensitivity to muscarine in chronically stimulated NMJs. Conversely, in α-BTx treated NMJs, PSC sensitivity remained unaffected, but ATP- and muscarine-induced calcium responses were prolonged. Thus, our results reveal complex modifications of PSC properties, with differential modulation of signalling pathways that might underlie receptor regulation or changes in Ca2+ handling. Importantly, similar to neurons, perisynaptic glial cells undergo plastic changes induced by altered synaptic activity.
Introduction
There is now a growing body of evidence indicating that glial cells play an active role in synaptic communication throughout the nervous system. They are indeed involved in the regulation of synaptic transmission and plasticity (reviewed by Auld & Robitaille, 2003; Halassa et al. 2007), believed to be the functional basis of learning and memory. Hence, glial cells are full participants at synapses and contribute actively to neuronal processing and integration. Complementary to their active role in regulating synaptic plasticity, glial cells themselves exhibit activity-dependent changes in their properties during development (Pasti et al. 1997). However, there is no direct evidence that glial cell modifications are specific and adapted to the level of synaptic activity and that they can occur in mature systems. Such adaptation, concomitant with pre- and postsynaptic plasticity, would be essential to maintain appropriate communication between all components of the tripartite synapse. Hence, the goal of this work was to test whether the properties and activation of perisynaptic glial cells are differentially regulated in response to opposite changes in synaptic activity.
To this end we used the neuromuscular junction (NMJ), a useful simple model in the study of synapse–glia interactions. Perisynaptic Schwann cells (PSCs), glial cells at this synapse, are known to modulate synaptic transmission and plasticity by increasing or decreasing transmitter release (Robitaille, 1998; Castonguay & Robitaille, 2001). These cells and their interactions with the synaptic elements share a large number of similarities with astrocytes, their counterparts in the CNS (Auld & Robitaille, 2003).
Pre- and postsynaptic properties are dependant on the level of synaptic activity and can be modified by either a decrease or an increase in activity. For instance, lowering nerve–muscle communication increased quantal content following long-term presynaptic blockade of transmitter release (Snider & Harris, 1979; Tsujimoto et al. 1990) or chronic blockade of postsynaptic nicotinic receptors (nAChRs) with α-bungarotoxin (α-BTx, Plomp et al. 1992). Also, sprouting of nerve terminals and PSC processes has been observed when synaptic activity was lowered (Snider & Harris, 1979; Wernig et al. 1980; Wines & Letinsky, 1988; Diaz et al. 1989; Diaz & Pecot-Dechavassine, 1989; Tsujimoto et al. 1990). In contrast, phasic nerve terminals adapt to higher patterns of neuronal activity by decreasing their synaptic efficacy (releasing less neurotransmitter) and increasing their resistance to synaptic depression (Lnenicka & Atwood, 1985; Hinz & Wernig, 1988; Lnenicka & Zhao, 1991; Mercier et al. 1992; Wernig et al. 1996; Somasekhar et al. 1996; Cooper et al. 1998; Reid et al. 2003; Belair et al. 2005). Changes in protein expression and morphological transformations have also been reported (Lnenicka et al. 1986; Nguyen & Atwood, 1990; Somasekhar et al. 1996; Cooper et al. 1998). Thus, both presynaptic and postsynaptic as well as some morphological changes of PSCs were observed following alterations of synaptic activity.
Here we tested whether PSCs regulate their excitability and activity based on calcium signalling following long-term in vivo changes in synaptic activity. We used chronic blockade of nACHRs with α-BTx to decrease synaptic activity postsynaptically, with consequent presynaptic adjustments, and prolonged nerve stimulation to increase synaptic activity both pre- and postsynaptically. Our data reveal that PSCs undergo bidirectional plasticity involving a fine tuning of calcium response properties within a specific subset of PSCs. These results suggest that PSCs may adapt to the synapse they are associated with and may adjust their feedback accordingly.
Methods
All experimental procedures and protocols were approved by the Comité de déontologie de l’expérimentation sur les animaux of the Université de Montréal in accordance with the Canadian Council on Animal Care policy.
Animals
Experiments were performed on Rana pipiens frogs (body length 6–9 cm; Connecticut Valley Biological Supply). During chronic treatments, frogs were housed individually in an aqua-terrarium supplied with fresh running water, and fed with meal worms every other day.
Chronic in vivo nerve stimulation
Animals were prepared as previously described (Belair et al. 2005). Briefly, frogs were anaesthetised with tricaine methanesulfonate (MS-222; Sigma, Oakville, ON, Canada) and harnessed with two electrodes made with Teflon-coated wires (90% platinum–10% iridium; Medwire Corp., Mount Vernon, NY, USA) and polyethylene tubing (Becton Dickinson, Franklin Lakes, NJ, USA). Electrodes were fixed over the skin, ventrally and dorsally, parallel to each other, one at the shoulder level, the other at the caudal–lateral region of the cutaneus pectoris (CP) muscle. Stimulation of the nerve supplying the left CP was performed transcutaneously through a small uncoated portion of the wires, placed on either side of the nerve. Specific contractions of the left CP could be visually assessed during stimulation. The right unstimulated CP served as control. Stimulation protocol consisted of alternated 15 min trains of stimuli (0.5 ms duration, 0.4–0.9 V amplitude) delivered at 10 Hz with 5 min rest periods, during 7 days. Animals were anaesthetized using tricaine methanesulfonate (MS-222) and killed by double pithing, at the end of the conditioning period. No effect of MS-222 on synaptic function at the NMJ has been reported.
Chronic in vivoα-bungarotoxin blockade of nACHRs
Animals were anaesthetized with MS-222 (0.28 mg g−1 of body weight; Sigma) injected subcutaneously. Skin incision was made, approximately 1 cm long, perpendicular to the medial line, 2 cm below the sternum. Two pieces of chromatography paper (0.5 cm × 0.5 cm), soaked with 40 μl of α-BTx solution (260 nm; Invitrogen, Burlington, ON, Canada) or normal Ringer solution (sham animals), were inserted between the skin and muscles and were left over the two CP muscles for 30 min, allowing a complete blockade of muscle contractions. Papers were then removed and skin stitched up. A second blockade procedure was performed on day 3 or 4. On day 6 or 7, animals were anaesthetized using MS-222 (Sigma) and killed by double pithing.
Electrophysiology
Experiments were performed on NMJs of CP nerve–muscle preparations, pinned down on a Sylgard-coated recording chamber equipped with a suction electrode that delivered supra-threshold stimuli to the motor nerve. Except for chronically blocked preparations, muscles were incubated with α-BTx solution (65 nm; Invitrogen) until contractions were abolished. Nerve–muscle preparations were then perfused with normal physiological solution (mm: 120 NaCl; 2.0 KCl; 1.0 NaHCO3; 1.8 CaCl2; 5.0 Hepes). Intracellular recordings of endplate potentials (EPPs) were performed using sharp glass microelectrodes (10–20 MΩ, filled with KCl, 2 m) and a Neuroprobe amplifier (A-M systems, Carlsborg, WA, USA). Signals were further amplified a 1000 times with a Warner Instruments DC amplifier and filtered at 2 kHz. EPPs were recorded with WinWCP software (John Dempster, University of Strathclyde).
The effects of chronic treatments on high frequency-induced plasticity were studied by stimulating the motor nerve at 40 Hz during 30 s, between two periods of 0.2 Hz stimulation (square pulses of 0.1 ms duration). High frequency facilitation, high frequency depression and post-tetanic potentiation (PTP) were calculated relative to the mean EPP amplitude during the 0.2 Hz control period. High frequency facilitation refers to the largest EPP amplitude recorded at the beginning of the 40 Hz train; high frequency depression refers to the mean amplitude of the last five EPPs of the train; and PTP refers to the mean amplitude of five EPPs surrounding the peak EPP, after the 40 Hz train.
Calcium imaging of PSCs
CP muscles with their pectoralis proprius motor nerve were removed and pinned down on a Sylgard-coated recording chamber. Fluo-4 AM (10 μm; Invitrogen), a membrane-permeant form of calcium indicator, was dissolved in normal Ringer solution containing 0.02% pluronic acid, and 1% dimethyl sulfoxide (Sigma). The preparation was incubated in this solution, in the dark, for 90 min, at room temperature. Muscle contractions were blocked with normal Ringer solution containing α-BTx (15 μg ml−1; Invitrogen). After complete blockade, preparations were perfused with normal Ringer solution containing N,N,N′,N′-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN; 20 μm; Invitrogen) with 0.01% ethanol, 20 min prior to calcium imaging and during the whole experiment TPEN chelate heavy metals that can affect fluo-4 responses.
PSCs were activated either by transmitter release following motor nerve stimulation or by local application of agonists. Motor nerve stimulation was performed using a suction electrode that delivered supra-threshold stimulus (square pulses of 0.1 ms duration, 40 Hz, 30 s). For local application of agonists (ATP, 10 or 20 μm; muscarine, 10 or 75 μm; Sigma), a glass pipette with an internal diameter of less than 1 μm was placed in close vicinity to the PSC cell body, and pulses of pressure (69 to 138 kPa, 100 ms; Picospritzer II, Parker Instrumentation, Cleveland, OH, USA) were applied to deliver the drug. The agonist solutions were prepared with the same solution used for bath perfusion.
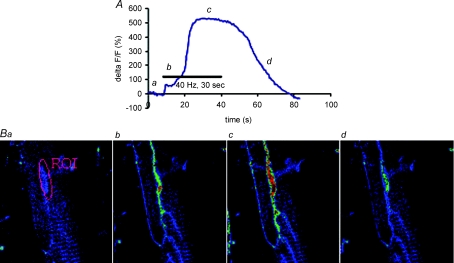
Ca2+ imaging of PSCs was performed using two confocal microscopes, a Zeiss LSM 510 and a Bio-Rad MRC600. The same system was used throughout for a given treatment and its related controls. The use of two different systems explains the difference in the size of Ca2+ responses observed in different conditions reported in this work (Table 1). Fluo-4 was excited at 488 nm with an argon ion laser and its intensity attenuated with neutral density filters. The emitted light was detected through a band-pass filter (505–543 nm) in the case of the LSM510 and through a long pass filter (LP515) with the Bio-Rad MRC600. Confocal images of PSCs were taken at the same focal plane, at rest, during and after motor nerve stimulation or local application of neurotransmitters (at 800 ms interval with the Bio-Rad and at 500 ms interval with the Zeiss). Resting fluorescence (Frest) was averaged from 20 images and cells with mean pixel intensity 25 ± 5 were selected. The fluorescence intensity (F) was averaged over the PSC cell body (Fig. 1) and relative changes in fluorescence intensities (%ΔF/F) were expressed as:
Table 1.
Effects of treatments on nerve evoked PSC calcium responses
| Treatments |
||||
|---|---|---|---|---|
| Chronic in vivoα-bungarotoxin blockade of nACHRs |
Chronic in vivo nerve stimulation |
|||
| PSC calcium response | Sham | Bungarotoxin | Control | Stimulated |
| Amplitude (%) | 913.8 ± 148.1 | 440.3 ± 95.7 | 400.3 ± 111.1 | 305.2 ± 56.4 |
| P= 0.008* | P= 0.438 | |||
| Duration (s) | 82.9 ± 7.3 | 76.0 ± 6.8 | 57.1 ± 3.7 | 55.8 ± 3.0 |
| P= 0.498 | P= 0.795 | |||
| Duration/amplitude (s/%) | 0.18 ± 0.04 | 0.5 ± 0.1 | 0.51 ± 0.09 | 0.41 ± 0.06 |
| P= 0.044* | P= 0.340 | |||
| Area under the curve (%/s2) | 36153.7 ± 8586.0 | 8789.1 ± 1705.7 | 7929.4 ± 2224.8 | 5459.3 ± 1297.9 |
| P < 0.001* | P= 0.329 | |||
| Half-width (s) | 34.5 ± 4.3 | 21.0 ± 1.9 | 18.9 ± 1.2 | 18.2 ± 1.2 |
| P= 0.003* | P= 0.674 | |||
| Delay (s) | 5.8 ± 1.1 | 13.8 ± 3.3 | 5.7 ± 0.8 | 7.3 ± 1.8 |
| P= 0.046* | P= 0.425 | |||
| Time-to-peak (s) | 17.5 ± 2.2 | 15.9 ± 2.4 | 14.0 ± 1.3 | 11.9 ± 0.7 |
| P= 0.631 | P= 0.156 | |||
| Rise slope (10–90%) (%/s) | 176.1 ± 39.3 | 64.9 ± 21.0 | 74.4 ± 27.6 | 46.7 ± 10.6 |
| P= 0.011* | P= 0.329 | |||
| Rise time (10–90%) (s) | 8.8 ± 1.7 | 14.8 ± 3.8 | 8.7 ± 1.0 | 6.8 ± 0.6 |
| P= 0.221 | P= 0.109 | |||
| Decay slope (10–90%) (%/s) | −25.1 ± 4.3 | −21.3 ± 6.1 | −20.7 ± 6.1 | −18.9 ± 5.4 |
| P= 0.631 | P= 0.830 | |||
| Decay time (10–90%) (s) | 31.1 ± 3.5 | 28.9 ± 6.1 | 20.1 ± 1.6 | 21.0 ± 1.9 |
| P= 0.771 | P= 0.704 | |||
Mean values and standard error for different PSC calcium response parameters measured in sham, α-BTx-treated, control and chronically stimulated NMJs. *Significant P values.
Figure 1.
Measurements of PSC Ca2+ responses at the amphibian NMJ A, typical Ca2+ response evoked in a PSC by sustained motor nerve stimulation is shown (top) as a relative change in fluorescence. Letters along the graph represent the time at which the figures shown below have been taken. B, false colour images obtained with a Bio-Rad MRC600 confocal microscope, where blue represents low level of Ca2+ and red high level. One can see the Z bands (regular striations on the muscle surface) as well as the elongated structure of the NMJ that extends along the main axis of the muscle fibre. The region of interest (ROI) illustrated in image 1 depicts the area that was used to measure the changes in pixel intensity over the PSC soma.
Statistical analysis
Results are expressed as the mean ± standard error of the mean. Data obtained from NMJs of treated muscles and their respective control were compared using Student's t test, with α= 0.05. Distributions of PSC sensitivities to neurotransmitters were compared using a chi-square test. Due to the use of different experimental conditions (set-ups and drug concentrations) for the two treatments, comparisons were only made between treated muscles and their respective control.
Results
We monitored intracellular Ca2+ dynamics in PSCs and used changes in calcium response properties as an indicator of PSC plasticity following changes in synaptic activity.
In vivo conditionings
Frogs were not agitated, and they behaved and fed normally during chronic in vivo stimulation and following postsynaptic receptor blockade. The procedures specifically affected the CP muscle such that chronic nerve stimulation allowed the visualization of its contractions throughout the conditioning period (Belair et al. 2005) while α-BTx blockade of nAChRs prevented its contractions specifically, leaving surrounding muscles unaffected. Importantly, after 7 days of either treatment, the wound was healing well, the skin was clean of any infections and the muscle integrity was preserved, showing no obvious signs of necrosis, infections or other damage. Muscles presenting any signs of damage were discarded.
Effects of treatments on short-term plasticity
Considering the known relationship between the level of transmitter release and PSC activation, we first characterized neurotransmission in chronically treated NMJs by recording EPPs before, during and after a 40 Hz, 30 s stimulation (α-BTx: n= 8 NMJs, N= 6 frogs; sham: n= 9 NMJs, N= 6 frogs; stimulated: n= 10 NMJs, control: n= 7 NMJs, N= 10 frogs). Such sustained stimulation results in a number of plasticity events occurring with different time courses (Zucker & Regehr, 2002). Facilitation is generally observed first and is followed by a period of synaptic depression that recovers at the end of the tetanus and even potentiates after longer trains, producing post-tetanic potentiation (PTP; Kamiya & Zucker, 1994). The extent of these changes in transmitter release is normally correlated with the basal quantal content of the NMJ (Nudell & Grinnell, 1982; Pawson and Grinnell, 1990).
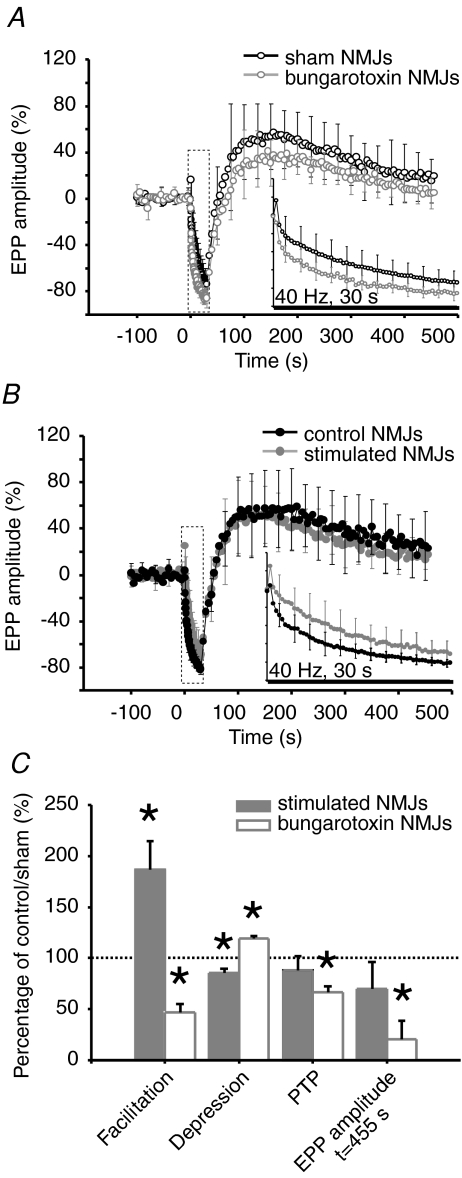
It was possible to record EPPs in chronically blocked preparations, even though muscle contractions were completely blocked, which is likely due to a small number of unblocked nAChRs leaving remnants of a low level of synaptic transmission. Consistent with an increase in transmitter release, high frequency facilitation was decreased in α-BTx treated NMJs compared to sham (α-BTx: 26.9 ± 5.0%; sham: 57.9 ± 12.3%, P= 0.042), while synaptic depression was more pronounced (α-BTx: 85.2 ± 1.8%; sham: 71.5 ± 3.8%, P= 0.008). Conversely, PTP was reduced (α-BTx: 43.5 ± 3.6%; sham: 65.3 ± 9.1%, P= 0.049), and EPP amplitude returned to basal level within a few minutes while sham NMJs were still potentiated at this time (t= 455 s) (α-BTx: 5.0 ± 4.4%; sham: 24.6 ± 5.8%, P= 0.019; Fig. 2A and C).
Figure 2.
Effects of treatments on short-term synaptic plasticity: high frequency facilitation, depression and PTP A and B, mean EPP amplitude before, during and after high frequency stimulation (40 Hz, 30 s). A, sham (black, open circles, N= 6 frogs; sham: n= 9 NMJs) and α-BTx-treated NMJs (grey, open circles, N= 6 frogs, n= 8 NMJs). B, control (black, filled circles, N= 7 frogs, control: n= 7 NMJs) and stimulated NMJs (grey, filled circles, N= 10 frogs, n= 10 NMJs). Mean amplitude is normalized to the mean EPP amplitude during the initial 0.2 Hz control period. Inset: enlarged view of the 40 Hz, 30 s stimulation period. C, bar graph depicting changes in short-term synaptic plasticity for α-BTx-treated (white) and stimulated NMJs (grey) relative to sham and control NMJs (100%, dotted line), respectively. Asterisks show statistically significant changes in EPP amplitude from EPP amplitude at rest (i.e. prior to high frequency stimulation). On average, α-BTx-treated NMJs presented weaker facilitation at the beginning of the stimulation train, more pronounced depression at the end and smaller PTP that did not last as long as sham NMJs. Stimulated NMJs presented greater facilitation at the beginning of the stimulation train and less depression at the end while PTP remained unchanged compared to control.
Conversely, and consistent with decreased level of transmitter release, high frequency facilitation was increased in stimulated NMJs compared to control (stimulated: 52.3 ± 7.8%; control: 28.0 ± 3.7%, P= 0.028) and high frequency depression was less pronounced (stimulated: 68.9 ± 3.8%; control: 80.9 ± 1.8%, P= 0.026). PTP was not affected by chronic nerve stimulation either in amplitude (stimulated: 55.0 ± 8.6%; control: 62.4 ± 12.9%, P= 0.626) or in duration. EPP amplitude remained elevated within the time course of the experiment (t= 455 s) in stimulated (15.8 ± 5.9%) and control preparations (22.7 ± 8.8%) (P= 0.511; Fig. 2B and C).
As a whole, this analysis confirms that the two in vivo models resulted in opposite alterations in short-term plasticity and supports parallel changes in synaptic efficacy. We then focused our attention on PSCs and whether they, too, have undergone plasticity changes.
Effects of treatments on nerve evoked PSC calcium responses
Considering the opposite regulation of synaptic plasticity and efficacy by chronic blockade of nAChRs and chronic nerve stimulation (Plomp et al. 1992), we also predicted opposite changes in nerve-evoked PSC activation for the two treatments.
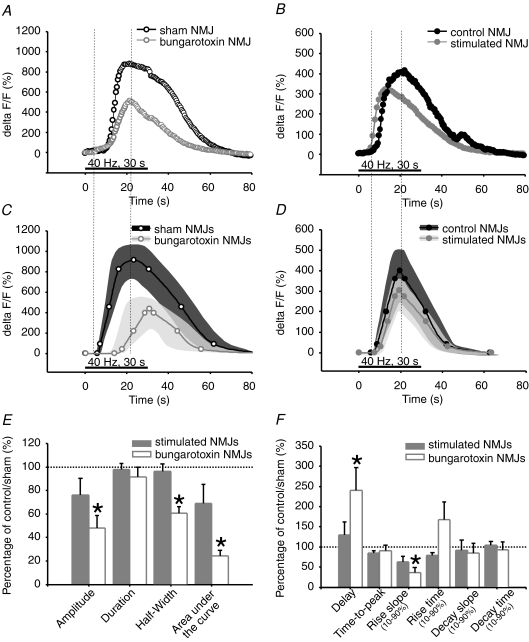
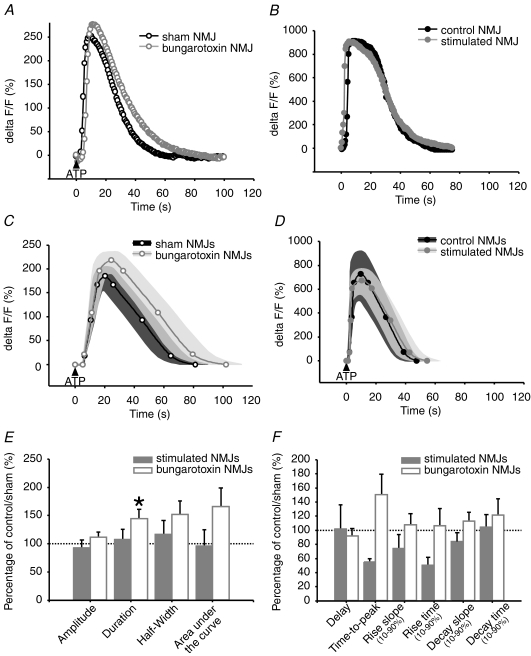
As shown in Fig. 3A, C, E and F and Table 1, properties of PSC calcium responses, evoked with 40 Hz, 30 s stimulation, were altered by chronic blockade of nAChRs with α-BTx (α-BTx: n= 24 PSCs; N= 8 frogs; sham: n= 18 PSCs; N= 8 frogs). PSC calcium responses of α-BTx-treated NMJs were significantly delayed by 140% compared to sham. The slope of rise was significantly reduced by 63%. This suggests that calcium release from PSC internal stores, the known mechanisms regulating the activity of PSCs (Jahromi et al. 1992; Robitaille, 1995; Robitaille et al. 1997), were slower in α-BTx-treated NMJs or that Ca2+ handling by endogenous chelators was altered. Moreover, the significant reduction of calcium response amplitude and area under the curve, by 51% and 75%, respectively, suggests that a smaller amount of calcium is released from PSC internal stores. Besides, duration of PSC calcium responses in α-BTx-treated NMJs was not different from sham; however, duration expressed per unit of amplitude was significantly increased by 173% in these NMJs, revealing a prolonged action of calcium for a given response amplitude. Taken together, these results suggest that the neuronal message perceived by PSCs is altered following chronic blockade of nAChRs and high frequency stimulation.
Figure 3.
Effects of treatments on nerve evoked PSC calcium responses A and B, characteristic calcium responses evoked by 40 Hz, 30 s stimulation in PSCs. A, α-BTx-treated (grey, open circles) and sham NMJs (black, open circles). B, chronically stimulated (grey, filled circles) and control NMJs (black, filled circles). C and D, mean calcium responses evoked by 40 Hz, 30 s stimulation in PSCs. C, α-BTx-treated (grey, open circles, n= 24 PSCs, N= 8 frogs) and sham NMJs (black, open circles, n= 18 PSCs, N= 8 frogs). D, stimulated (grey, filled circles, n= 40 PSCs, N= 19 frogs) and control NMJs (black, filled circles, n= 37 PSCs, N= 19 frogs). Variability for control and treated NMJs is represented as dark grey and light grey filled regions surrounding the mean. E and F, bar graphs depicting changes in nerve-evoked PSC calcium responses of α-BTx-treated (white) and stimulated NMJs (grey) relative to sham and control NMJs (100%, dotted line), respectively. Asterisks show statistically significant changes. Only α-BTx-treated NMJs show altered nerve-evoked PSC calcium responses, in terms of amplitude, half-width, area under the curve, delay and rise slope, compared to sham.
Interestingly, the properties of synaptically evoked PSC calcium responses were not affected by chronic nerve stimulation. Indeed, all parameters measured in stimulated NMJs were not significantly different from control NMJs (stimulated: n= 40 PSCs; control: n= 37 PSCs; N= 19 frogs) (Table 1, Fig. 3B, D, E and F). These results suggest that the neuronal message perceived by PSCs, during high frequency stimulation, might not be altered by chronic nerve stimulation.
Changes in transmitter release cannot account for changes in PSC responses
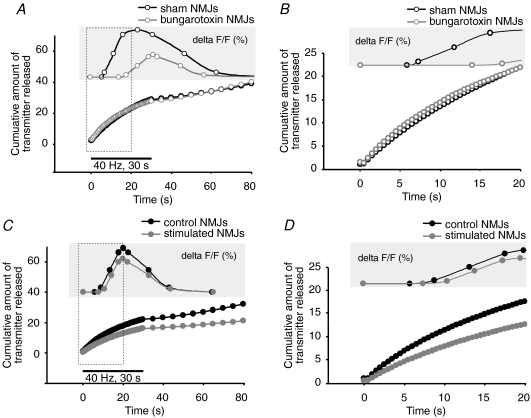
Because our chronic treatments are believed to modify quantal release concomitantly with synaptic plasticity, we wondered if the total amount of transmitter released, during the time course of PSC calcium responses, could correlate with their properties. To evaluate this, we modelled changes in transmitter release during the stimulation protocol. We attributed relative values to initial quantal release and calculated the cumulative amount of transmitter released as a function of the relative changes in EPP amplitude during the 40 Hz, 30 s stimulation. Initial transmitter release was set to 1 for sham NMJs and to 1.5 for α-BTx treated NMJs, according to observations by others showing a 50% increase in quantal release with such treatment (Plomp et al. 1992) and consistent with our observations of decreased facilitation and increased depression. Similarly, we set initial transmitter release to 1 for control NMJs and to 0.5 for stimulated NMJs, since we previously showed a 50% reduction in quantal release following chronic nerve stimulation (Belair et al. 2005).
As shown in Fig. 4A and B, α-BTx treated NMJs would have released equal or even slightly greater amount of neurotransmitter than sham NMJs, at least within the first 6 s of stimulation, which correspond to the mean time of PSC calcium response onset in control conditions (Fig. 4A and B). Hence, if PSC activation was proportional to the amount of transmitter released, one would have expected calcium responses with earlier onset and similar or greater amplitude in α-BTx-treated NMJs. However, as indicated above, PSC calcium responses in these conditions were markedly delayed and smaller in amplitude. Conversely, stimulated NMJs would have released a smaller amount of transmitters than control NMJs, which would result in delayed or smaller calcium responses, according to a proportional stimulus–response relationship. However, PSC calcium responses displayed similar properties in stimulated and control NMJs (Fig. 4C and D). These observations strongly argue against a linear stimulus–response relationship between PSC activation and transmitter release and support more complex modifications of PSC properties that depend on the level of synaptic activity imposed.
Figure 4.
Model of transmitter release during 40 Hz, 30 s stimulation A, mean PSC calcium response (upper trace) and estimated amount of transmitter released by α-BTx-treated NMJs (grey open circles), relative to sham NMJs (black, open circles). B, enlarged view of A showing the first 20 s of stimulation. Note the delay of PSC calcium response onset in α-BTx-treated NMJs, despite putative larger amount of transmitter released. C, mean PSC calcium response (upper trace) and estimated amount of transmitter released by stimulated NMJs (grey, filled circles) relative to control NMJs (black, filled circles) during the time course of PSC calcium responses. D, enlarged view of C showing the first 20 s of stimulation. Note the similarities between PSC calcium responses in stimulated and control NMJs, despite putative smaller amount of transmitter released in stimulated NMJs.
Changes in ATP-evoked PSC calcium responses
Because PSC activation, in treated preparations, cannot be explained directly by changes in the level of transmitter released, we next investigated whether the properties of PSCs themselves were altered. At the frog NMJ, PSCs are primarily activated by ACh and ATP, two neurotransmitters that are co-released by the presynaptic terminal. While ACh activates muscarinic receptors of an unknown type (Robitaille et al. 1997), ATP triggers Ca2+ rises in PSCs mainly via P2Y and, to a lesser extent, via P2X receptors (Robitaille, 1995). To characterize the effect of chronic treatments on these two components of PSC calcium responses, we performed calcium imaging of PSCs following local application of ATP or muscarine to their soma, using a puff micropipette.
ATP-induced calcium responses in PSCs are concentration dependant, with amplitude, duration and percentage of responding cells increasing with concentration (data not shown). Using a maximal effective concentration (20 μm) that evoked calcium rises in 100% of sham PSCs, we found that calcium responses were comparable for almost all parameters tested (α-BTx: n= 36 PSCs; N= 4 frogs; sham: n= 31 PSCs; N= 3 frogs). Duration was, however, significantly increased by 44% in PSCs of α-BTx treated NMJs, suggesting a prolonged action of calcium in these cells (Table 2, Fig. 5A, C, E and F). On the other hand, the ratio of duration over amplitude was not significantly altered. Therefore, duration of calcium responses for a given increase in amplitude was unchanged.
Table 2.
Effects of treatments on PSC calcium responses evoked with maximal effective ATP concentration
| Treatments |
||||
|---|---|---|---|---|
| Chronic in vivoα-bungarotoxin blockade of nACHRs |
Chronic in vivo nerve stimulation |
|||
| PSC calcium response | Sham | Bungarotoxin | Control | Stimulated |
| Amplitude (%) | 189.1 ± 16.9 | 211.3 ± 17.7 | 726.4 ± 194.6 | 672.4 ± 100.1 |
| P= 0.370 | P= 0.823 | |||
| Duration (s) | 71.2 ± 7.2 | 102.7 ± 12.0 | 44.2 ± 6.3 | 47.6 ± 8.0 |
| P= 0.033* | P= 0.739 | |||
| Duration/amplitude (s/%) | 0.42 ± 0.04 | 0.6 ± 0.1 | 0.11 ± 0.02 | 0.08 ± 0.01 |
| P= 0.091 | P= 0.231 | |||
| Area under the curve (%/s2) | 8336.7 ± 1608.4 | 13866.6 ± 2734.4 | 23471.7 ± 10155.1 | 22531.3 ± 6716.1 |
| P= 0.096 | P= 0.943 | |||
| Half-width (s) | 34.7 ± 5.1 | 52.9 ± 8.1 | 23.0 ± 3.7 | 26.8 ± 5.6 |
| P= 0.071 | P= 0.555 | |||
| Delay (s) | 3.7 ± 0.3 | 3.4 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| P= 0.599 | P= 0.969 | |||
| Time-to-peak (s) | 12.3 ± 2.1 | 18.5 ± 3.6 | 6.7 ± 1.4 | 3.7 ± 0.3 |
| P= 0.631 | P= 0.082 | |||
| Rise slope (10–90%) (%/s) | 53.8 ± 9.6 | 57.9 ± 8.6 | 480.5 ± 168.0 | 356.5 ± 94.4 |
| P= 0.753 | P= 0.582 | |||
| Rise time (10–90%) (s) | 6.1 ± 1.2 | 6.5 ± 1.5 | 3.1 ± 1.2 | 1.5 ± 0.3 |
| P= 0.818 | P= 0.313 | |||
| Decay slope (10–90%) (%/s) | −4.7 ± 0.6 | −5.3 ± 0.6 | −29.5 ± 6.4 | −24.9 ± 3.6 |
| P= 0.464 | P= 0.551 | |||
| Decay time (10–90%) (s) | 41.5 ± 4.3 | 50.6 ± 9.5 | 24.2 ± 3.5 | 25.3 ± 4.3 |
| P= 0.381 | P= 0.847 | |||
Mean values and standard error for different PSC calcium response parameters measured in sham, α-BTx-treated, control and chronically stimulated NMJs. *Significant P values.
Figure 5.
Effects of treatments on PSC calcium responses evoked with maximal effective ATP concentration A and B, characteristic PSC calcium responses evoked with maximal effective ATP concentration (giving 100% cell responsiveness). A, α-BTx-treated (grey, open circles) and sham NMJs (black, open circles). B, chronically stimulated (grey, filled circles) and control NMJs (black, filled circles). C and D, mean calcium responses evoked with maximal effective ATP concentration. C, α-BTx-treated (grey, open circles, n= 36 PSCs, N= 4 frogs) and sham NMJs (black, open circles, n= 31 PSCs, N= 3 frogs) D, chronically stimulated (grey, filled circles, n= 13 PSCs, N= 5 frogs) and control NMJs (black, filled circles, n= 17 PSCs, N= 5 frogs). Variability for control and treated NMJs is represented as dark grey and light grey filled regions surrounding the mean. E and F, bar graphs depicting changes in ATP evoked PSC calcium responses of α-BTx-treated (white) and stimulated NMJs (grey) relative to sham and control NMJs (100%, dotted line), respectively. Asterisks show statistically significant changes. Only α-BTx-treated NMJs show altered PSC calcium responses, in terms of duration, compared to sham at this maximal effective concentration.
Conversely, maximal effective concentration of ATP (20 μm) induced calcium responses in PSCs, from stimulated and control NMJs, that were not statistically different for all parameters tested (Table 2, Fig. 5B, D, E and F) (stimulated: n= 13 PSCs; control: n= 17 PSCs; N= 5 frogs). These results suggest that PSC properties can be differentially regulated by opposite changes in synaptic activity.
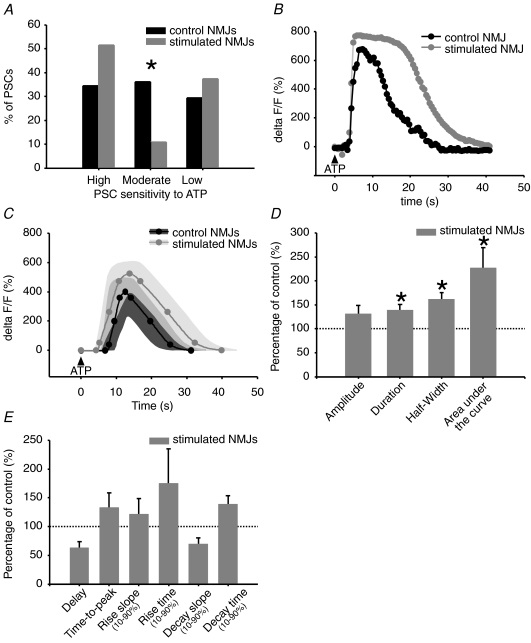
We next tested whether chronic stimulation changed the excitability of PSCs that could be masked by a supra-maximal concentration of ATP. This was achieved by using a concentration of ATP with submaximal effectiveness (10 μm), activating only a third of the cells in control NMJs. We found that PSCs could be grouped into three categories: high, moderate or low sensitivity, according to the number of ATP applications required for inducing a calcium response (1; 2 to 3; more than 3). With this grouping method, we found a shift in the distribution of PSC responsiveness in stimulated NMJs (Fig. 6A). Indeed, chronic nerve stimulation increased by 50% the proportion of cells belonging to the highly sensitive group (stimulated: 51.6%, n= 64 PSCs; control: 34.5%, n= 58 PSCs; N= 13 frogs, P= 0.0039, chi-square test), suggesting an increased sensitivity of PSCs to ATP.
Figure 6.
Effects of chronic nerve stimulation on PSC calcium responses evoked with submaximal effective ATP concentration A, frequency bar graph depicting the distribution of PSC sensitivity to submaximal effective ATP concentration (allowing sensitivity categorization) in chronically stimulated (grey) and control (black) NMJs. The proportion of highly sensitive PSCs is increased in stimulated NMJs, likely at the expense of moderately sensitive cells. B, characteristic calcium responses evoked with submaximal effective ATP concentration in low sensitive PSCs of stimulated (grey, filled circles) and control NMJs (black, filled circles). C, mean calcium response evoked with submaximal effective ATP concentration in low sensitive PSC of stimulated (grey, filled circles, n= 64 PSCs, N= 13 frogs) and control NMJs (black, filled circles, n= 58 PSCs, N= 13 frogs). Variability for control and stimulated NMJs is represented as dark grey and light grey filled regions surrounding the mean. D and E, bar graphs depicting changes in ATP evoked PSC calcium responses of stimulated NMJs (grey) relative to control NMJs (100%, dotted line). Asterisks show statistically significant changes. PSC calcium responses evoked with submaximal effective concentration of ATP are altered by chronic nerve stimulation, in terms of duration, half-width and area under the curve.
When compared, calcium rises evoked in highly and moderately sensitive PSCs were similar in control and stimulated NMJs. However, within the low sensitivity group, PSCs of stimulated NMJs presented calcium responses with significant 38% and 125% increases in duration and area under the curve, respectively (Table 3, Fig. 6B–E). This increase in duration indicates a prolonged action of calcium. However, the ratio of duration over amplitude remained unchanged, meaning that for a given amplitude, the duration of calcium response is the same. Group specific modulation of PSC calcium responses indicates that PSC properties are finely regulated by synaptic activity.
Table 3.
Effects of chronic nerve stimulation on PSC calcium responses evoked with submaximal effective ATP concentration
| Chronic in vivo nerve stimulation – ATP (10 μm) |
||||||
|---|---|---|---|---|---|---|
| Highly sensitive PSCs |
Moderately sensitive PSCs |
Weakly sensitive PSCs |
||||
| PSC calcium response | Control | Stimulated | Control | Stimulated | Control | Stimulated |
| Amplitude (%) | 457.7 ± 68.1 | 364.5 ± 57.1 | 289.2 ± 52.7 | 305.8 ± 77.7 | 400.4 ± 80.9 | 522.5 ± 72.8 |
| P= 0.308 | P= 0.872 | P= 0.274 | ||||
| Duration (s) | 25.2 ± 2.9 | 28.1 ± 3.5 | 22.1 ± 2.1 | 29.6 ± 4.2 | 23.3 ± 2.2 | 32.2 ± 3.0 |
| P= 0.571 | P= 0.102 | P= 0.034* | ||||
| Duration/Amplitude (s/%) | 0.09 ± 0.02 | 0.14 ± 0.02 | 0.15 ± 0.03 | 0.13 ± 0.03 | 0.15 ± 0.06 | 0.12 ± 0.04 |
| P= 0.068 | P= 0.734 | P= 0.684 | ||||
| Area under the curve (%/s2) | 7373.1 ± 1743.2 | 7293.1 ± 1754.3 | 3166.2 ± 720.8 | 5022.8 ± 1898.3 | 4148.5 ± 791.5 | 9349.9 ± 1809.5 |
| P= 0.976 | P= 0.269 | P= 0.027* | ||||
| Half-width (s) | 14.4 ± 2.0 | 15.3 ± 2.1 | 10.4 ± 1.4 | 13.0 ± 2.2 | 10.3 ± 0.9 | 16.5 ± 1.6 |
| P= 0.751 | P= 0.336 | P= 0.003* | ||||
| Delay (s) | 1.5 ± 0.3 | 1.6 ± 0.3 | 3.1 ± 0.9 | 2.7 ± 0.8 | 6.9 ± 1.9 | 4.3 ± 0.8 |
| P= 0.837 | P= 0.783 | P= 0.175 | ||||
| Time-to-peak (s) | 4.8 ± 0.9 | 5.9 ± 0.7 | 6.3 ± 0.8 | 7.7 ± 1.7 | 4.6 ± 0.5 | 6.1 ± 1.2 |
| P= 0.358 | P= 0.431 | P= 0.295 | ||||
| Rise slope (10–90%) (%/s) | 227.5 ± 57.3 | 159.6 ± 52.9 | 96.7 ± 37.5 | 47.6 ± 15.0 | 179.1 ± 55.5 | 215.5 ± 51.5 |
| P= 0..397 | P= 0.537 | P= 0.660 | ||||
| Rise time (10–90%) (s) | 3.6 ± 1.3 | 2.7 ± 2.2 | 4.8 ± 0.9 | 7.1 ± 1.8 | 3.1 ± 0.9 | 5.4 ± 1.9 |
| P= 0.763 | P= 0.235 | P= 0.403 | ||||
| Decay slope (10–90%) (%/s) | −36.3 ± 6.5 | −27.8 ± 5.3 | −27.8 ± 5.7 | −17.6 ± 3.3 | −46.7 ± 15.8 | −32.1 ± 5.4 |
| P= 0.331 | P= 0.286 | P= 0.330 | ||||
| Decay time (10–90%) (s) | 15.2 ± 2.1 | 18.2 ± 3.1 | 10.3 ± 1.1 | 13.4 ± 1.6 | 11.2 ± 1.5 | 15.5 ± 1.7 |
| P= 0.504 | P= 0.127 | P= 0.085 | ||||
Mean values and standard error for different PSC calcium response parameters measured in control and chronically stimulated NMJs. *Significant P values.
Changes in muscarinic-dependent PSC calcium responses
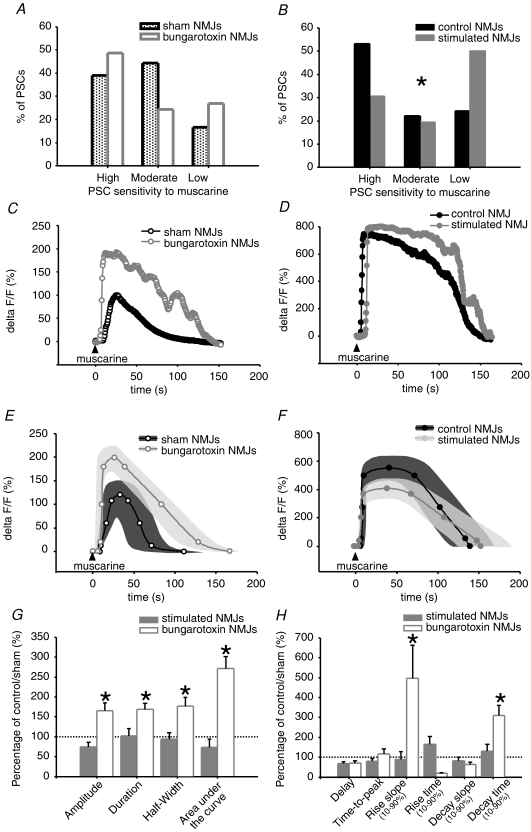
PSC calcium responses to muscarine are also concentration dependant, with the proportion of responding cells increasing with concentration, while the amplitude remained constant regardless of the concentration (Robitaille et al. 1997). Because of these all-or-none types of responses, we used a submaximal effective concentration of muscarine (10 μm) that decreased the proportion of responding PSCs below 100% to unmask an actual difference in sensitivity distribution. We classified PSCs of sham and α-BTx treated NMJs into highly, moderately and weakly sensitive PSCs, according to the number of muscarine applications required for inducing a calcium response (Fig. 7A). We found no difference in the distribution of PSCs of α-BTx treated NMJ in comparison to sham (α-BTx: n= 37 PSCs; N= 8 frogs; sham: n= 36 PSCs; N= 6 frogs; P= 0.1784, chi-square test). However, when comparing calcium responses within each group, we found that highly sensitive PSCs of α-BTx treated NMJs presented a significant increase in amplitude and area under the curve of 66% and 171%, respectively, suggesting that a greater amount of calcium is released from internal stores in these cells. Moreover, duration of PSC calcium responses was significantly increased by 69% following chronic blockade of nAChRs, indicating a prolonged action of calcium in these cells which might be accounted for by the significant prolongation of decay time by 210% (Fig. 7C, E, G and H). However, when expressing duration of PSC calcium responses in function of amplitude, significance was lost, indicating that the prolonged action of calcium might be the direct consequence of the greater amount of calcium released. Moreover, the slope of rise of these PSC calcium responses was almost 4 times steeper than in sham NMJ PSCs, suggestive of a faster calcium release from PSC internal stores and/or binding to fluo-4 dye (Table 4). Opposite to the highly sensitive group of PSCs, calcium responses evoked in moderately and weakly sensitive PSCs were not affected by chronic blockade of nAChRs indicating that a modulation occurred in a subpopulation of PSCs. These results also suggest that muscarinic signalling in PSCs of α-BTx treated NMJs might be altered mainly in conditions of low transmitter release, where only highly sensitive PSCs respond with a calcium elevation.
Figure 7.
Effect of treatments on muscarine-evoked PSC calcium responses A and B, bar graph depicting the distribution of PSC sensitivity to submaximal effective muscarine concentration (allowing sensitivity categorization). A, in α-BTx-treated (light grey) and sham NMJs (white). B, chronically stimulated (grey) and control NMJs (black). The distribution of PSCs in the different sensitivity groups is only altered for stimulated NMJs. The proportion of weakly sensitive PSCs is increased in chronically stimulated NMJs, which is likely to be at the expense of highly sensitive cells. C and D, characteristic calcium responses evoked with submaximal effective muscarine concentration. C, weakly sensitive PSCs of α-BTx-treated (grey, open circles) and sham NMJs (black, open circles). D, chronically stimulated (grey, filled circles) and control NMJs (black, filled circles). E and F, mean calcium responses evoked with submaximal effective concentration. E, weakly sensitive PSCs of α-BTx-treated (grey, open circles, n= 37 PSCs, N= 8 frogs) and shams NMJs (black, open circles, n= 36, PSCs, N= 6 frogs). F, chronically stimulated (grey, filled circles, n= 36 PSCs, N= 15 frogs) and control NMJs (black, filled circles, n= 45 PSCs, N= 15 frogs). Variability for control and treated NMJs is represented as dark grey and light grey filled regions surrounding the mean. G and H, bar graphs depicting changes in calcium responses evoked by muscarine in weakly sensitive PSCs of α-BTx-treated (white) and stimulated NMJs (grey) relative to sham and control NMJs (100%, dotted line), respectively. Asterisks show statistically significant changes. Only α-BTx-treated NMJs show altered PSC calcium responses compared to sham at this submaximal effective concentration, in terms of amplitude, duration, half-width, area under the curve, rise slope and decay time.
Table 4.
Effects of treatments on calcium responses evoked, in highly sensitive PSCs, with submaximal effective muscarine concentration
| Treatments |
||||
|---|---|---|---|---|
| Chronic in vivoα-bungarotoxin blockade of nACHRs |
Chronic in vivo nerve stimulation |
|||
| PSC calcium response | Sham | Bungarotoxin | Control | Stimulated |
| Amplitude (%) | 119.9 ± 28.2 | 199.1 ± 23.3 | 552.2 ± 79.5 | 409.6 ± 65.2 |
| P= 0.037* | P= 0.230 | |||
| Duration (s) | 95.0 ± 18.7 | 160.9 ± 14.2 | 110.4 ± 13.9 | 113.3 ± 20.1 |
| P= 0.008* | P= 0.904 | |||
| Duration/amplitude (s/%) | 1.8 ± 0.6 | 1.0 ± 0.1 | 0.27 ± 0.04 | 0.29 ± 0.04 |
| P= 0.140 | P= 0.769 | |||
| Area under the curve (%/s2) | 7217.5 ± 2188.4 | 19624.4 ± 2188.4 | 44758.8 ± 10562.9 | 32704.7 ± 9541.9 |
| P= 0.038* | P= 0.450 | |||
| Half-width (s) | 41.0 ± 12.7 | 72.8 ± 9.0 | 61.9 ± 12.2 | 58.4 ± 9.7 |
| P= 0.046* | P= 0.844 | |||
| Delay (s) | 8.8 ± 2.1 | 6.1 ± 1.1 | 7.4 ± 1.4 | 5.0 ± 0.8 |
| P= 0.255 | P= 0.232 | |||
| Time-to-peak (s) | 17.1 ± 4.5 | 20.1 ± 4.2 | 15.7 ± 3.1 | 12.4 ± 2.3 |
| P= 0.633 | P= 0.461 | |||
| Rise slope (10–90%) (%/s) | 11.4 ± 8.5 | 56.7 ± 18.8 | 217.3 ± 50.8 | 194.0 ± 86.6 |
| P= 0.048* | P= 0.806 | |||
| Rise time (10–90%) (s) | 21.2 ± 8.3 | 4.2 ± 0.6 | 3.1 ± 0.6 | 5.1 ± 1.2 |
| P= 0.079 | P= 0.114 | |||
| Decay slope (10–90%) (%/s) | −2.8 ± 1.0 | −1.8 ± 0.3 | −7.1 ± 1.6 | −5.9 ± 1.3 |
| P= 0.245 | P= 0.633 | |||
| Decay time (10–90%) (s) | 28.8 ± 8.1 | 89.4 ± 14.5 | 61.4 ± 12.1 | 80.2 ± 21.7 |
| P= 0.003* | P= 0.420 | |||
Mean values and standard error for different PSC calcium response parameters measured in sham, α-BTx-treated, control and chronically stimulated NMJs. *Significant P values.
When comparing the sensitivity of PSCs from stimulated and control NMJs, we observed a greater proportion of weakly sensitive cells in the treated group (stimulated: 50%, n= 36 PSCs; control: 24.4%, n= 45 PSCs; N= 15 frogs; P= 0.0468), suggesting that chronic nerve stimulation decreases PSC sensitivity to muscarine (Fig. 7B). Thus, in conditions of low transmitter release, less PSCs might be able to respond to neurotransmission with a calcium elevation. However, PSC calcium responses evoked in each group were statistically similar in stimulated and control NMJs (Fig. 7D, F, G and H).
Discussion
The active regulation of synaptic efficacy and plasticity by PSCs (Robitaille, 1998; Castonguay & Robitaille, 2001) implies that, in turn, PSCs should also adapt to their associated synapses and the changes in synaptic properties that occur. Therefore, one would predict that synaptic plasticity must underlie PSC plasticity. The results presented here confirm this hypothesis by showing activity-dependent PSC modifications that overlap with adaptations of synaptic transmission.
Mechanisms of PSC modifications
One of the main results of this work is that changes in properties of nerve-evoked PSC calcium responses cannot be accounted for only by the changes in basal transmitter release and the build up of transmitter release during sustained motor nerve stimulation following chronic treatments. The correlation between transmitter release and PSC activation is, indeed, more complex than a linear relationship and may reflect the specific cholinergic and purinergic alterations in conditions of modified transmitter release. For instance, changes in sensitivity are likely to be related to the availability of functional receptors and/or the efficiency of their coupling to intracellular mechanisms of calcium release. A decrease in muscarinic sensitivity and functional mAChRs is supported by studies showing a strict regulation of these receptors by agonists (van Koppen & Kaiser, 2003). Moreover, repeated activation of PSC mAChRs was shown to result in successively smaller Ca2+ signals until no signal could be triggered (Jahromi et al. 1992; Georgiou et al. 1999). On the other hand, ATP-induced calcium responses in frog PSCs are highly reproducible and show little or no fatigue (Georgiou et al. 1999). Besides, recent evidence obtained from cultured astrocytes supports an agonist-dependent upregulation of the metabotropic ATP receptor P2Y2, which is associated with an increased UTP-mediated intracellular calcium response (D’Alimonte et al. 2007). Therefore, tonic release of ACh and ATP during chronic nerve stimulation could alter the relative number of functional mAChR and P2Y receptors by similar mechanisms. In addition, there may be a number of parallel and complimentary mechanisms that take place to favour and facilitate a better activation of PSCs by ATP. For instance, intracellular coupling between receptors and Ca2+ internal stores may compensate for a weaker sensitivity of these cells while the receptor density and/or sensitivity would promote ATP activation of PSCs more sensitive to ATP.
Another possible mechanism involved in the changes in calcium response properties may be the regulation of InsP3 receptors (InsP3R) and mitochondria distribution. Indeed, in cultured astrocytes, distribution of mitochondria and regions of high density of InsP3R are correlated with sites of calcium response amplification (Sheppard et al. 1997; Simpson et al. 1997, 1998). Also, intracellular calcium oscillations in these cells result in similar mitochondrial calcium oscillations and inhibition of mitochondrial activity decreases the amplitude of InsP3-induced calcium responses (Simpson et al. 1998). Interestingly, this is consistent with the observation that PSC Ca2+ responses are relatively slow, suggesting that they are likely to be influenced by Ca2+ clearance mechanisms. Thus, such mechanisms could modulate purinergic and muscarinic calcium signalling in PSCs subjected to chronic changes in synaptic activity.
Physiological relevance of PSC modifications
The relationship between transmitter release and PSC activation is indicative of more complex interactions that go beyond the specific cholinergic and purinergic alterations in conditions of elevated transmitter release. Indeed, the striking differences we found in delay and amplitude of nerve-evoked PSC calcium responses in α-BTx-treated NMJs is suggestive of an extensive modification of neuron–glia communication parameters. In fact, the glial output may result from the integration of many interacting signals adjusted to the efficiency of synapse–glia interactions.
For instance, during presynaptic activity, nerve terminals release a variety of peptides and neurotrophins that, in turn, act as synaptic regulators (Matteoli et al. 1988, 1990; Bourque & Robitaille, 1998; Zhan et al. 2003). Moreover, PSCs possess receptors for these neuromodulators and some of them where shown to elicit and modulate calcium responses in PSCs (Bourque & Robitaille, 1998; Todd et al. 2008). Thus, the activity-dependent release of neuromodulators could be implicated in the fine tuning of nerve-evoked PSC Ca2+ responses and may explain differences observed between Ca2+ responses elicited by local neurotransmitter application and endogenous, nerve-evoked ones. Another possible explanation for the striking effect of α-BTx treatment on nerve-evoked PSC calcium responses would be a redistribution of their purinergic and muscarinic receptors farther away from release sites, leading to a weaker activation of PSC receptors. Importantly, it appears that the adjustments may be influenced by the extent of pre- and postsynaptic activation during the in vivo plasticity period.
Roles of calcium responses in PSCs
The modulation of PSC calcium responses implies that PSC calcium-dependant functions might also be altered. In particular, the regulation of transmitter release, since PSC calcium elevations were associated with delayed and transient increase of transmitter release (Castonguay & Robitaille, 2001). Such a role could be coherent with physiological properties of α-BTx-treated NMJs, which present stronger high-frequency depression and weaker PTP and are associated with smaller PSC calcium responses.
Interestingly, specific roles for purinergic and muscarinic signalling can also be envisaged, as different pathways are activated by different receptors. For instance, purinergic receptors were found to regulate the synthesis of prostaglandin (Gebicke-Haerter et al. 1988) and thromboxane A2 (Pearce et al. 1989) in cultured astrocytes. These molecules can also be produced by Schwann cells in vitro (Constable et al. 1994) while a potentiating effect was shown for prostaglandin in frog preparations (Madden & van der Kloot, 1982), supporting its role in the modulation of high-frequency induced synaptic depression.
On the other hand, muscarinic signalling in PSCs was shown to regulate glial fibrillary acidic protein (GFAP) expression (Georgiou et al. 1994). Increasing the duration of muscarine-induced calcium responses could prevent the upregulation of GFAP and prevent the sprouting of PSC processes and successively, of nerve terminals. Besides, the absence of major sprouting events during the first 8 days of α-BTx blockade (Wines & Letinsky, 1991) suggests that GFAP expression is maintained at low levels in these preparations, within this time window.
Nature of PSC modifications: local vs. global regulation
The main conclusion of this work is that PSCs undergo synaptic plasticity as they may adapt to changes in synaptic activity by adjusting their sensitivity to neurotransmitters. This leads to complex modifications that are specific to their initial state and to the level of synaptic activity, suggesting they adjust their activity or excitability to that level. Hence, activity-dependent plasticity of PSCs might be part of their active role at synapses.
The variability and changes in PSC excitability following in vivo long-term plasticity are likely to influence the nature of synapse–glia interactions. Indeed, changes in PSC calcium response properties are likely to alter calcium-dependant processes within a single cell while their differential activation might alter the number of PSCs activated by a given stimulus or the number of stimuli required for their activation. This would directly impact on the ability of PSCs to intervene in the plasticity process at the NMJ. As a whole, these properties could lead to either local or global synapse–glia interactions. A local regulation of synaptic function will occur if PSCs work independently from each other and if their range of action is limited to a few active zones. In that case, any changes in calcium response properties of a single PSC would affect the active zones it covers. Indeed, individual PSCs are known to influence a small region of an NMJ as indicated by alterations of endplate currents by specific injections of GTP analogues or BAPTA into a single PSC (Robitaille, 1998; Castonguay & Robitaille, 2001). This is consistent with the observation that not all regions of an NMJ have the same efficacy of transmitter release (Robitaille & Tremblay, 1991; Wu & Betz, 1999; Wyatt & Balice-Gordon, 2008). Local synapse–glia interactions are also consistent with our findings of the heterogeneity in PSC sensitivity along the same NMJ. Thus, PSC properties might vary in function of the strength of the NMJ or the active zones they are associated with and their calcium responses might adapt to their specific needs.
Conversely, a global regulation of whole NMJs is likely to occur if PSCs work as a functional unit of four to five cells per NMJ. In the latter case, calcium responses of all PSCs at an NMJ will act as a population code. The extent of glial regulation would be related to the level of transmitter release, whereby conditions of elevated transmitter release would activate PSCs of all sensitivity, thus providing a global regulation, whereas during low transmitter release, only PSCs with higher sensitivity would be activated, resulting in more local regulation. Interestingly, the global regulation by PSCs is supported by the observation that PSC number is correlated with endplate area, a relationship that is maintained even when endplate area changes (Lubischer & Bebinger, 1999).
Hence, PSCs are likely to work both individually and within a functional unit, so that the extent of their modifications may be local and global and regulated by the level of transmitter release.
Conclusion
This study revealed that PSCs are highly plastic cells that regulate their properties according to the level of synaptic activity. Their modifications are bidirectional and underlie different functional roles for purinergic and muscarinic signalling in the regulation of synaptic structure and function. Our work also raised the need for understanding the functioning mode of PSCs as a coordinated ensemble regulating whole NMJs or as independent cells adapted to the specific needs of specific active zones and their associated postsynaptic densities.
Acknowledgments
We thank Keith Todd for reading various versions of the manuscript and for helpful discussion. This work was supported by grants to R.R. from the Canadian Institutes for Health Research (CIHR), the National Sciences and Engineering Research Council (NSERC) of Canada and an infrastructure group from FRSQ. R.R. was a CIHR Investigator and now holds a FRSQ Chercheur National. E-L.B. first held a NSERC and then a FRSQ studentship.
Glossary
Abbreviations
- α-BTx
α-bungarotoxin
- CP
cutaneus pectoris muscle
- EPP
endplate potential
- InsP3R
InsP3 receptors
- NMJ
neuromuscular junction
- nAChR
nicotinic acetylcholine receptor
- PTP
post-tetanic potentiation
- PSC
perisynaptic Schwann cell
Author contributions
E-L.B. and J.V. participated in all steps of the experiments including the conception and design, the acquisition of the data as well as their analysis and interpretation. E-L.B. and J.V. also participated in the drafting of the manuscript and its revision up to the final approval of the version submitted. As the senior author, R.R. participated in every step of the experimental work besides data acquisition and data analysis.
References
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Belair EL, Vallee J, Robitaille R. Long-term in vivo modulation of synaptic efficacy at the neuromuscular junction of Rana pipiens frogs. J Physiol. 2005;569:163–178. doi: 10.1113/jphysiol.2005.094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Robitaille R. Endogenous peptidergic modulation of perisynaptic Schwann cells at the frog neuromuscular junction. J Physiol. 1998;512:197–209. doi: 10.1111/j.1469-7793.1998.197bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay A, Robitaille R. Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse. J Neurosci. 2001;21:1911–1922. doi: 10.1523/JNEUROSCI.21-06-01911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable AL, Armati PJ, Toyka KV, Hartung HP. Production of prostanoids by Lewis rat Schwann cells in vitro. Brain Res. 1994;635:75–80. doi: 10.1016/0006-8993(94)91425-7. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Warren WM, Ashby HE. Activity of phasic motor neurons partially transforms the neuronal and muscle phenotype to a tonic-like state. Muscle Nerve. 1998;21:921–931. doi: 10.1002/(sici)1097-4598(199807)21:7<921::aid-mus10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- D’Alimonte I, Ciccarelli R, Di Iorio P, Nargi E, Buccella S, Giuliani P, Rathbone MP, Jiang S, Caciagli F, Ballerini P. Activation of P2X7 receptors stimulates the expression of P2Y2 receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol. 2007;20:301–316. doi: 10.1177/039463200702000210. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pecot-Dechavassine M. Terminal nerve sprouting at the frog neuromuscular junction induced by prolonged tetrodotoxin blockade of nerve conduction. J Neurocytol. 1989;18:39–46. doi: 10.1007/BF01188422. [DOI] [PubMed] [Google Scholar]
- Diaz J, Molgo J, Pecot-Dechavassine M. Sprouting of frog motor nerve terminals after long-term paralysis by botulinum type A toxin. Neurosci Lett. 1989;96:127–132. doi: 10.1016/0304-3940(89)90045-1. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, Wurster S, Schobert A, Hertting G. P2-purinoceptor induced prostaglandin synthesis in primary rat astrocyte cultures. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:704–707. doi: 10.1007/BF00165638. [DOI] [PubMed] [Google Scholar]
- Georgiou J, Robitaille R, Charlton MP. Muscarinic control of cytoskeleton in perisynaptic glia. J Neurosci. 1999;19:3836–3846. doi: 10.1523/JNEUROSCI.19-10-03836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou J, Robitaille R, Trimble WS, Charlton MP. Synaptic regulation of glial protein expression in vivo. Neuron. 1994;12:443–455. doi: 10.1016/0896-6273(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hinz I, Wernig A. Prolonged nerve stimulation causes changes in transmitter release at the frog neuromuscular junction. J Physiol. 1988;401:557–565. doi: 10.1113/jphysiol.1988.sp017179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Atwood HL. Long-term facilitation and long-term adaptation at synapses of a crayfish phasic motoneuron. J Neurobiol. 1985;16:97–110. doi: 10.1002/neu.480160203. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Zhao YG. Seasonal differences in the physiology and morphology of crayfish motor terminals. J Neurobiol. 1991;22:561–569. doi: 10.1002/neu.480220602. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Atwood HL, Marin L. Morphological transformation of synaptic terminals of a phasic motoneuron by long-term tonic stimulation. J Neurosci. 1986;6:2252–2258. doi: 10.1523/JNEUROSCI.06-08-02252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubischer JL, Bebinger DM. Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci. 1999;19:RC46. doi: 10.1523/JNEUROSCI.19-24-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KS, Van Der Kloot W. At the frog neuromuscular junction prostaglandin synthase inhibitors depress and PGE partially restores quantal acetylcholine release. Brain Res. 1982;234:464–468. doi: 10.1016/0006-8993(82)90888-5. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Haimann C, De Camilli P. Substance P-like immunoreactivity at the frog neuromuscular junction. Neuroscience. 1990;37:271–275. doi: 10.1016/0306-4522(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Haimann C, Torri-Tarelli F, Polak JM, Ceccarelli B, De Camilli P. Differential effect of α-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci U S A. 1988;85:7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier AJ, Bradacs H, Atwood HL. Long-term adaptation of crayfish neurons depends on the frequency and number of impulses. Brain Res. 1992;598:221–224. doi: 10.1016/0006-8993(92)90186-d. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Atwood HL. Expression of long-term adaptation of synaptic transmission requires a critical period of protein synthesis. J Neurosci. 1990;10:1099–1109. doi: 10.1523/JNEUROSCI.10-04-01099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell BM, Grinnell AD. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibres of uniform input resistance. J Neurosci. 1982;2:216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson PA, Grinnell AD. Physiological differences between strong and weak frog neuromuscular junctions: a study involving tetanic and posttetanic potentiation. J Neurosci. 1990;10:1769–1778. doi: 10.1523/JNEUROSCI.10-06-01769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce B, Murphy S, Jeremy J, Morrow C, Dandona P. ATP-evoked Ca2+ mobilisation and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem. 1989;52:971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in α-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Martinov VN, Nja A, Lomo T, Bewick GS. Activity-dependent plasticity of transmitter release from nerve terminals in rat fast and slow muscles. J Neurosci. 2003;23:9340–9348. doi: 10.1523/JNEUROSCI.23-28-09340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Tremblay JP. Non-uniform response to Ca++ along the frog neuromuscular junction: effects on the efficacy of spontaneous and evoked transmitter release. Neuroscience. 1991;40:571–585. doi: 10.1016/0306-4522(91)90142-b. [DOI] [PubMed] [Google Scholar]
- Robitaille R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci. 1995;15:7121–7131. doi: 10.1523/JNEUROSCI.15-11-07121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Jahromi BS, Charlton MP. Muscarinic Ca2+ responses resistant to muscarinic antagonists at perisynaptic Schwann cells of the frog neuromuscular junction. J Physiol. 1997;504:337–347. doi: 10.1111/j.1469-7793.1997.337be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard CA, Simpson PB, Sharp AH, Nucifora FC, Ross CA, Lange GD, Russell JT. Comparison of type 2 inositol 1,4,5-trisphosphate receptor distribution and subcellular Ca2+ release sites that support Ca2+ waves in cultured astrocytes. J Neurochem. 1997;68:2317–2327. doi: 10.1046/j.1471-4159.1997.68062317.x. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Mehotra S, Lange GD, Russell JT. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J Biol Chem. 1997;272:22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Mehotra S, Langley D, Sheppard CA, Russell JT. Specialized distributions of mitochondria and endoplasmic reticulum proteins define Ca2+ wave amplification sites in cultured astrocytes. J Neurosci Res. 1998;52:672–683. doi: 10.1002/(SICI)1097-4547(19980615)52:6<672::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Snider WD, Harris GL. A physiological correlate of disuse-induced sprouting at the neuromuscular junction. Nature. 1979;281:69–71. doi: 10.1038/281069a0. [DOI] [PubMed] [Google Scholar]
- Somasekhar T, Nordlander RH, Reiser PJ. Alterations in neuromuscular junction morphology during fast-to-slow transformation of rabbit skeletal muscles. J Neurocytol. 1996;25:315–331. doi: 10.1007/BF02284805. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Auld DS, Robitaille R. Neurotrophins modulate neuron-glia interactions at a vertebrate synapse. Eur J Neurosci. 2008;25:1287–1296. doi: 10.1111/j.1460-9568.2007.05385.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Umemiya M, Kuno M. Terminal sprouting is not responsible for enhanced transmitter release at disused neuromuscular junctions of the rat. J Neurosci. 1990;10:2059–2065. doi: 10.1523/JNEUROSCI.10-07-02059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signalling. Pharmacol Ther. 2003;98:197–220. doi: 10.1016/s0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Wernig A, Pecot-Dechavassine M, Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980;9:278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]
- Wernig A, Dorlochter M, Palazis P. Differential sensitivity to Mg2+- and tubocurarine-block of frog neuromuscular junctions in summer and winter. Neurosci Lett. 1996;207:41–44. doi: 10.1016/0304-3940(96)12483-6. [DOI] [PubMed] [Google Scholar]
- Wines MM, Letinsky MS. Motor nerve terminal sprouting in formamide-treated inactive amphibian skeletal muscle. J Neurosci. 1988;8:3909–3919. doi: 10.1523/JNEUROSCI.08-10-03909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines MM, Letinsky MS. Inactivity-induced motor nerve terminal sprouting in amphibian skeletal muscles chronically blocked by α-bungarotoxin. Exp Neurol. 1991;111:115–122. doi: 10.1016/0014-4886(91)90057-j. [DOI] [PubMed] [Google Scholar]
- Wyatt RM, Balice-Gordon RJ. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin. J Neurosci. 2008;28:325–335. doi: 10.1523/JNEUROSCI.3544-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Spatial variability in release at the frog neuromuscular junction measured with FM1-43. Can J Physiol Pharmacol. 1999;77:672–678. [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Acta Physiol Sinica. 2003;55:617–624. [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]