Abstract
Axonally initiated action potentials back-propagate into spiny dendrites of central mammalian neurons and thereby regulate plasticity at excitatory synapses on individual spines as well as linear and supralinear integration of synaptic inputs along dendritic branches. Thus, the electrical behaviour of individual dendritic spines and terminal dendritic branches is critical for the integrative function of nerve cells. The actual dynamics of action potentials in spines and terminal branches, however, are not entirely clear, mostly because electrode recording from such small structures is not feasible. Additionally, the available membrane potential imaging techniques are limited in their sensitivity and require substantial signal averaging for the detection of electrical events at the spatial scale of individual spines. We made a critical improvement in the voltage-sensitive dye imaging technique to achieve multisite recordings of backpropagating action potentials from individual dendritic spines at a high frame rate. With this approach, we obtained direct evidence that in layer 5 pyramidal neurons from the visual cortex of juvenile mice, the rapid time course of somatic action potentials is preserved throughout all cellular compartments, including dendritic spines and terminal branches of basal and apical dendrites. The rapid time course of the action potential in spines may be a critical determinant for the precise regulation of spike timing-dependent synaptic plasticity within a narrow time window.
Introduction
In cortical layer 5 pyramidal neurons, action potentials are usually triggered in the axonal initial segment distal to the axon hillock (Palmer & Stuart, 2006). Following their initiation, action potentials propagate forward along the axon but also backwards into the dendrites (Stuart & Sakmann, 1994). Dendritic patch-clamp recordings indicate a distance-dependent broadening of back-propagating action potentials (bAPs), from 1–2 ms in the soma to approximately 10 ms at remote dendritic sites (for example see Fig. 8 in Larkum et al. 2001). One of the many roles of bAPs is their involvement in spike timing-dependent synaptic plasticity (STDP; reviewed in Caporale & Dan, 2008). A very narrow time window for the pairing of bAPs with afferent synaptic potentials, in the range of 10–20 ms, determines whether STDP results in long-term potentiation (LTP) or long-term depression (LTD). Therefore, the duration of the bAPs at the spines, the site of coincidence detection, may be a critical determinant for STDP. Yet, accurate information on the bAP waveform in dendritic spines and terminal dendritic branches has not been available. While important electrical roles of spines in postsynaptic signalling have been proposed for quite some time (e.g. Jack et al. 1975), direct experimental evidence is largely missing because dendritic spines are not accessible to electrode measurements.
Because of these technical limitations, there have recently been two alternative attempts involving optical imaging. First, Nuriya et al. (2006) reported in a pioneering study the first optical voltage recordings in dendritic spines using second harmonic generation (SHG) microscopy and provided evidence for the invasion of spines by bAPs. Second, Palmer & Stuart (2009) used confocal microscopy in the line scan mode to record bAPs and excitatory postsynaptic potentials in spines. However, the sensitivity of these methods is quite low because of the high fractional shot noise related to the small number of photons available for collection (Wu & Cohen, 1993; Kuhn et al. 2004; Dombeck et al. 2005). Thus, these first attempts to monitor electrical events from individual spines required extensive signal averaging and the signal-to-noise ratio of the recordings was not sufficient for an adequate analysis of AP dynamics (Nuriya et al. 2006; Palmer & Stuart, 2009). Only two-photon excitation of voltage-sensitive dye fluorescence has, thus far, exhibited the requisite sensitivity in a uniquely favourable preparation (tightly packed excitable membrane of nerve terminals in the neurohypophysis; Fisher et al. 2008), but this approach has not yet been tested in measurements of electrical activity from dendritic spines. It has, thus, been a challenge, both conceptually and technically, to carry out an accurate study of membrane potential (Vm) dynamics at the level of individual dendritic spines.
We have improved the sensitivity of multisite voltage-sensitive dye recording of membrane potential changes (Vm-imaging) and achieved single spine resolution by using monochromatic, laser light excitation in wide-field epifluorescence microscopy mode. With this approach, we obtained direct evidence that the rapid time course of somatic action potentials is accurately preserved (within the narrow limits set by 0.5 ms sampling interval) throughout all cellular compartments, including terminal apical dendritic branches as well as spines in basal and apical dendrites. The similar time course of bAP signals in all dendritic compartments and approximate calibration of optical signals suggested that bAPs invade dendritic spines without significant voltage loss.
Methods
Ethical approval
All surgical and experimental procedures were performed in accordance with ethical standards as outlined in Drummond (2009) as well as with institutional animal welfare guidelines approved by the state government of Bavaria, Germany.
Slices, patch-clamp recording and intracellular application of dyes
Experiments were carried out on visual cortex slices from 10- to 20-day-old (BALB/c) mice. All measurements were carried out at 33–35°C. The mice were quickly decapitated following CO2 anaesthesia and 300 μm thick slices were cut in ice-cold solution using a Leica VT1200S vibrating blade microtome (Leica Microsystems AG, Wetzlar, Germany). This microtome has a blade holder design that minimizes vertical deflection of the cutting tool to achieve sections of the highest quality that retain viable cells on the section surface (Bischofberger et al. 2006). Slices were incubated at 37°C for 30 min and then maintained at room temperature (23–25°C). The standard extracellular solution used during recording contained (in mm): 125 NaCl, 25 NaHCO3, 20 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2 and 1 MgCl2, pH 7.4 when bubbled with a gas mixture (95% O2, 5% CO2). Slicing was done in modified standard extracellular solution with decreased CaCl2 (0.5 mm) and increased MgCl2 (6 mm) concentration. Somatic whole-cell recordings were made with 5–8 MΩ patch pipettes using an EPC9 patch-clamp amplifier (HEKA, Lambrecht, Germany). The pipette solution contained (in mm): 140 potassium gluconate, 12 KCl, 4 NaCl, 4 Mg-ATP, 2 Na2-ATP, 0.4 Na-GTP, 10 K-Hepes (pH 7.3, adjusted with KOH) and 1 mm of the voltage-sensitive dye (JPW3028 synthesized and kindly provided by J. P. Wuskell and L. M. Loew, University of Connecticut, Farmington, CT, USA). Layer 5 pyramidal cells were visually identified using infrared DIC video-microscopy.
Glass pipettes were first filled from the tip with dye-free solution by applying negative pressure for about 15 s and then back-filled with the solution containing the indicator dye. Intracellular staining was accomplished by free diffusion of the dyes into the soma in 15–60 min, depending on the electrode resistance. After diffusion of the dye into the soma was completed, as determined by measuring the resting light intensity from the soma, the patch-electrode with the dyes was detached from the neuron by forming an outside-out patch and the preparation was typically incubated for an additional 1.5 h at room temperature to allow the voltage-sensitive dye to spread into distal processes. To obtain electrical recordings from the soma the cell body was repatched using an electrode filled with clear intracellular solution before making optical measurements.
Backpropagating APs were evoked by 30–60 ms long depolarizing current pulses delivered from the somatic patch electrode. These relatively long pulses, compared to 0.5–2 ms brief current pulses used in some of the previous studies, were chosen to mimic the effect of synaptic stimulation. The response pattern was similar to the one evoked by localized synaptic stimulation via an extracellular electrode positioned close to a particular dendritic branch (Supplemental Fig 1S; see also insets in Fig. 1b and c in Stuart et al. 1997). This method of stimulation was favoured over localized synaptic stimulation used in our previous study (Canepari et al. 2007) because it simplifies and standardizes experimental conditions between measurements from different neurons. Conversely, synaptic stimulation creates far more complex conditions. Relatively long-lasting (∼50 ms) EPSP-evoked depolarizing events in the dendrites spread along dendritic processes toward the soma–axon region so that the dendrites can be widely depolarized at the time of AP initiation and backpropagation. In the general case, neither the spatial nor the amplitude profile of this synaptically evoked depolarization in the dendritic tree can be experimentally determined. Also, the spatial and the amplitude profiles will be different in each experiment depending on dendritic geometry and the site of locally activated synapses. Finally, a typical response of the cortical pyramidal cell is a spike doublet; the analysis of the time course of the bAPs described here was carried out on the first evoked spike.
Figure 1. Advance in sensitivity of epifluorescence wide-field voltage imaging from subcellular neuronal compartments.
A representative improvement in S/N (by a factor of 15) realized by replacing a conventional excitation light source (arc lamp) providing a collection of excitation light wavelengths selected by an interference filter with monochromatic excitation light from a DPSS laser. Two neurons shown in A and B were loaded with a similar amount of dye. Recordings of evoked bAP signals from apical dendrites of two neurons were made on the same apparatus using an arc lamp in A and a laser in B. All recordings are single-trial, shot noise limited measurements at a frame rate of 2 kHz.
Optical recording
We used a stationary stage upright microscope (Model BX51WI, Olympus Inc., Japan) equipped with three camera ports. One camera port had a standard high spatial resolution CCD camera for infrared DIC video-microscopy. The second camera port had a fast data acquisition camera with relatively low spatial resolution (80 × 80 pixels) but outstanding dynamic range (14 bits) and exceptionally low read noise (NeuroCCD-SM, RedShirtImaging LLC, Decatur, GA, USA). The third camera port had a CCD camera with high spatial resolution (1392 × 1024 pixels) mounted on a spinning-disc confocal scanner used to collect z-stacks of confocal images for detailed morphological reconstruction of the stained cell. The final refinement of the anatomy of dendrites and spines was obtained by sharpening the confocal image using deconvolution software (Huygens, Scientific Volume Imaging, Hilversum, The Netherlands).
The acquisition, analysis and display of data were carried out using the NeuroPlex program (RedShirtImaging) written in IDL (ITT Visual Information Solutions, Boulder, CO, USA). A small chamber with a brain slice was placed on the stage of the microscope and the fluorescence image of the stained neuron projected by a water immersion objective (60×/1.0 NA, Nikon) onto the fast data acquisition CCD. This objective was selected as a compromise between imaging area, spatial resolution and the signal-to-noise ratio (S/N).
Optical recording of voltage-sensitive dye signals from dendritic branches and individual spines was carried out in the wide-field epifluorescence microscopy mode. A frequency-doubled 200 mW diode-pumped Nd:YVO4 continuous wave (CW) laser emitting at 532 nm (Excelsior 532 single mode; Newport-Spectra-Physics, Mountain View, CA, USA) was the source of excitation light. The laser beam was directed to a light guide coupled to the microscope via a single-port epifluorescence condenser (TILL Photonics GmbH, Gräfelfing, Germany) designed to overfill the back aperture of the objective. In this way, near uniform illumination of the object plane was attained. The fractional noise of low-noise solid-state lasers (<0.2%) is below typical fractional shot noise in fluorescence voltage-sensitive dye recordings (Zhou et al. 2007). In our experiments, the laser light was used in place of a conventional xenon arc lamp to maximize the sensitivity of Vm-imaging by: (1) using a monochromatic excitation light at the extreme red edge of the absorption spectrum to maximize Vm sensitivity of the dye (Loew, 1982; Kuhn et al. 2004) and (2) increasing the intensity of the excitation light beyond the level that can be achieved by an arc lamp. The excitation light was reflected to the preparation by a dichroic mirror with the central wavelength of 560 nm and the fluorescence light was passed through a 610 nm barrier filter (a Schott RG610). The combined effect of an increase in light intensity and the use of near optimal monochromatic excitation wavelengths was a dramatic improvement in the sensitivity of voltage imaging by a factor that varied within a range of 10–40 in different measurements. The proper way to compare the previously available sensitivity with the enhanced sensitivity described here is to determine the S/N in single-trial recording of a standard membrane potential transient (e.g. AP) normalized to the membrane surface area projected onto a single pixel (see Fig. 2 in Djurisic et al. 2004). Figure 1 illustrates such a comparison; the data show a representative (not an extreme) increase in the S/N of optical recording of dendritic bAPs. Figure 1A shows the available sensitivity in one of the best recordings of an AP signal from a CA1 pyramidal neuron using the maximum excitation light intensity that can be obtained from a 250 W xenon arc lamp together with an optimized interference filter (520/90 nm). Figure 1B is an analogous measurement from a different pyramidal neuron stained to a similar level, using the monochromatic excitation light at the full output of a 300 mW, 532 nm DPSS laser. It is noteworthy that the improvement in sensitivity shown in Fig. 1B is equivalent to averaging 225 recording trials shown in Fig. 1A.
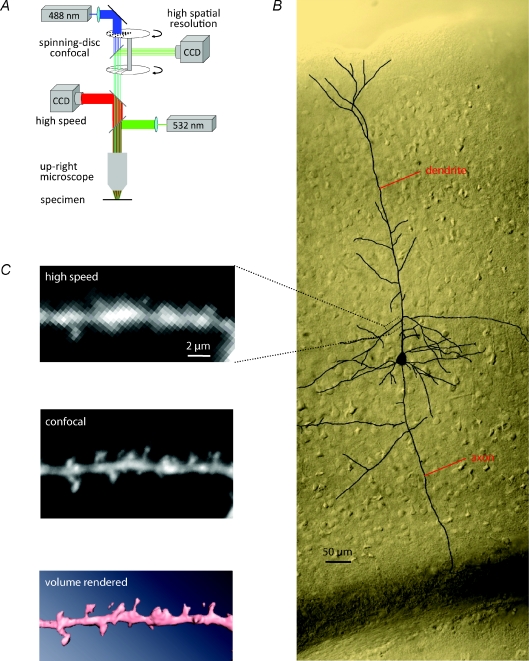
Figure 2. Experimental set-up.
A, the voltage-sensitive dye was excited using a 532 nm solid-state laser in wide-field illumination mode. Emission light was recorded with a high-speed CCD-camera. A spinning-disc confocal scanner was utilized for morphological reconstruction. B, camera lucida drawing of a stained neuron in a cortical slice imaged using differential interference contrast. C, high optical magnification images of a spiny dendritic branch obtained with the high speed CCD-camera (upper) or the confocal high resolution system (middle). Bottom image: a reconstruction from confocal data using volume rendering software.
Membrane potential signals were recorded at a frame rate of 2 kHz at two optical magnifications. For the relatively low optical magnification used in recordings from large areas of the dendritic tree, the image of a stained neuron was projected by a 60× objective onto a CCD chip via a 0.09–0.11× optical coupler (RedShirtImaging). At this magnification the full CCD frame (80 × 80 pixels) corresponded to a 300 × 300 μm area in the object plane with each individual pixel receiving light from an area of 3.8 × 3.8 μm. For the relatively high optical magnification used in recordings from individual dendritic spines, the image of a stained neuron was projected by a 60× objective directly onto a CCD chip positioned in the primary image plane. At this magnification, the full CCD frame (80 × 80 pixels) corresponded to a 30 × 30 μm area in the object plane with each individual pixel receiving light from an area of ∼ 0.4 × 0.4 μm.
Somatic AP signals in both our electrode and optical measurements had a half-width of ∼2 ms at 33–35°C. This value is higher than what was reported as an average AP duration in some previous studies using mature animals and patch-electrode recordings with optimized capacitive compensation (e.g. Stuart et al. 1997; Zhou et al. 2007). This was not caused by either the pharmacological effect of the dye or photodynamic damage because the AP shape remained unaltered after staining and after many recording trials (see below). The main reason for slower APs is almost certainly the young age of the animals, as clearly documented in McCormick & Prince (1987). In addition, we did not maximize the whole-cell capacitance compensation in recording AP electrically from the soma to avoid harmful ‘ringing’ of the amplifier. This will inevitably filter out the fastest frequency components of the AP signal in the electrical recordings. At the same time, spike signals recorded optically were acquired at a sampling rate of 2 kHz due to technical limitations to the CCD speed. This sampling rate is less than optimal for the precise reconstruction of the AP signal with a raising phase which is completed in less than 1 ms. For these reasons, the waveform of AP signals in this work, as recorded both electrically and optically should be regarded as an approximation, albeit characterized by a very small margin of error. In principle, the accuracy of our optical measurements of the AP half-width is better than 0.5 ms sampling interval because the measurements are made along a rapidly changing signal (Malmstadt et al. 1974; Ross & Krauthamer, 1984).
With the improved sensitivity of Vm-imaging under monochromatic excitation light, relatively good signal-to-noise ratios could be obtained in single-trial recordings from individual dendritic spines. Modest signal averaging (4–9 trials) was used to improve the signal-to-noise ratio further. Slow changes in light intensity due to bleaching of the dye were corrected by subtracting an appropriate exponential function derived from the recording trials with no stimulation. The residual slow changes in baseline after bleaching correction, if present, had no effect on bAP waveform because they were small and approximately 100 times slower than the rising phase of an action potential. The waveform of the bAP signal was reconstructed from a set of data points using Cubic Spline Interpolation, a piecewise continuous curve passing through each data point (Mathews & Fink, 2004).
The conclusions regarding the time course of the bAP signals derived from our measurements are based on the direct comparison of optical signals recorded from different dendritic locations. Such a comparison is valid only if the light intensity is linearly proportional to the membrane potential over the entire range of signal amplitudes. This has been demonstrated repeatedly in experiments where the same dye has been shown to track the full-size action potential exactly in several neuronal types (see Canepari et al. 2007 for references). This result implies a strictly linear relationship between the dye signal and the membrane potential in the entire physiological range.
Pharmacological effects and photodynamic damage
A number of previous studies have documented in detail that the voltage-sensitive dyes used for intracellular labelling (JPW 1114 or a close analogue, JPW 3028) have little or no pharmacological effect and cause little or no photodynamic damage when applied at functional concentrations to both invertebrate and vertebrate neurons (see Canepari et al. 2007 for references and quantitative data documenting the absence of significant pharmacological effects and photodynamic damage regarding both AP characteristics and long-term plasticity). It was important to confirm that the same conclusion is valid for layer 5 pyramidal neurons under laser based wide-field epi-illumination (see online Supplemental Material). First, action potential amplitude, voltage threshold and the width at half-height were routinely recorded in neurons at the beginning and end of the staining period lasting 15–60 min as well as after repatching the same neuron following equilibration of the dye inside the cell, which typically lasted ∼120 min. We consistently found that there was a characteristic and reversible change in the AP shape at the end of the staining period (data from a representative experiment shown in Supplemental Fig. 2Sa). This effect is not well understood and is most likely based on an additional membrane capacitive load introduced by a very high initial concentration of the charge shift probe inserted into the lipid bilayer of the somatic membrane and the axonal spike initiation site membrane near the tip of the loading electrode. During the ∼120 min recovery period, the dye diffuses from the somatic region into the dendritic and axonal branches, reducing the concentration of the probe in the soma significantly. This equilibration resulted in the AP response returning to control conditions. Typically, the AP amplitude, voltage threshold for excitation and the width at half-height are fully restored after the recovery period (Supplemental Fig. 2Sa–d). The full recovery of these parameters was a precondition for including the neuron in further analyses. Second, we established that laser illumination in our experimental protocol did not cause photodynamic damage to the preparation. An important step in minimizing possible damage was the restriction of the illumination to the region of interest by manipulating the field stop in the epi-illumination pathway so that the somatic region was never exposed to high intensity light. Additionally, recording periods were strictly limited to 50–100 ms. The results indicate that, under these conditions, AP signal shape and size remained unaltered after 20 recordings while a typical experiment in this study required no more than four to nine recording trials (Supplemental Fig. 2Se).
Results
The analysis of bAP signals in individual dendritic spines required a substantial improvement in the sensitivity of Vm-imaging as well as minimizing light scattering in wide-field epifluorescence microscopy recording mode from brain slices. We minimized light scattering effects by restricting the recordings to neurons located near the surface of acute brain slices (< 30 μm). This required optimizing the slicing procedure of the mouse visual cortex to obtain a high percentage of healthy neurons in the upper layer of the slice (Bischofberger et al. 2006). The heightened sensitivity was mandatory in order to compensate for the reduction in the S/N caused by the increase in optical magnification required to resolve individual spines.
The rules governing the sensitivity of light intensity measurements (expressed as the S/N) may be put in a nutshell with an expression S/N∝ (ΔF/F) (Wu & Cohen, 1993). The term ΔF/F is the fractional signal per unit change in Vm and Φ is the number of detected photons per unit time. Thus, one way to increase the S/N with a given voltage dye is to increase Φ by increasing the excitation light intensity. An additional possibility is to increase the fractional fluorescence change per unit change in Vm (sensitivity of the dye) by selecting an excitation wavelength with the best response. Following these rationales, we used the most sensitive voltage dye (in terms of S/N) for intracellular application (JPW3028; Canepari et al. 2007) and optimized both the resting florescence light intensity (Φ) and the relative fluorescence change in response to Vm change (ΔF/F) by utilizing a single wavelength laser excitation at 532 nm. A series of control experiments were carried out to test three critical methodological parameters: (1) improvement in the S/N; (2) the limits of spatial resolution as determined by light scattering; and (3) the extent of photodynamic damage at the required excitation light intensity.
(Wu & Cohen, 1993). The term ΔF/F is the fractional signal per unit change in Vm and Φ is the number of detected photons per unit time. Thus, one way to increase the S/N with a given voltage dye is to increase Φ by increasing the excitation light intensity. An additional possibility is to increase the fractional fluorescence change per unit change in Vm (sensitivity of the dye) by selecting an excitation wavelength with the best response. Following these rationales, we used the most sensitive voltage dye (in terms of S/N) for intracellular application (JPW3028; Canepari et al. 2007) and optimized both the resting florescence light intensity (Φ) and the relative fluorescence change in response to Vm change (ΔF/F) by utilizing a single wavelength laser excitation at 532 nm. A series of control experiments were carried out to test three critical methodological parameters: (1) improvement in the S/N; (2) the limits of spatial resolution as determined by light scattering; and (3) the extent of photodynamic damage at the required excitation light intensity.
Figure 2A depicts our experimental set-up. Imaging was performed with a high-speed CCD camera. A second, conventional CCD camera mounted on a spinning-disc confocal scanner was used for morphological analysis. Neurons with easily discernable dendrites were loaded from a patch-pipette with the voltage-sensitive dye JPW3028. Figure 2B shows a camera lucida drawing of a labelled pyramidal neuron. In dendrites located within the top 15–30 μm below the surface of the slice, long-neck spines were easily discerned with the high-speed camera. The morphology of the spiny dendrites was further verified using reconstructions from z-stacks of confocal images (Fig. 2C; Fig. 4A and B).
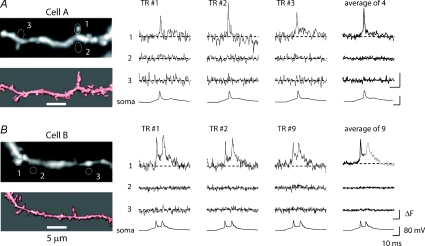
Figure 4. Backpropagated AP signals recorded optically from individual dendritic spines of two neurons.
A and B, left panels, upper micrographs, fluorescence images of dendritic spiny branches obtained with the CCD camera for voltage-imaging. Lower micrographs, anatomical reconstructions obtained from deconvoluted stacks of spinning-disk confocal images. Right panels, fluorescence intensity traces from locations 1–3 outlined on CCD images. Single-trial recordings and temporal averages of 4 (cell A) and 9 trials (cell B) are shown. Bottom traces, electrode recordings from the soma. The bAP signals, clearly recorded in spines, are absent from regions without spines (locations 2 and 3).
In a series of experiments, we monitored the extent of propagation and the time course of bAP signals at relatively low optical magnification (the full CCD frame (80 × 80 pixels) corresponded to a 300 × 300 μm area in the object plane). At this magnification the dendritic spines could not be resolved because their image was smaller than individual pixels. The dye-loaded pyramidal neurons were re-patched with a dye-free pipette (Fig. 3). Spikes were elicited by depolarizing current pulses delivered from a patch electrode in the soma and bAPs were recorded optically from various dendritic compartments covering the entire dendritic tree Fig. 3A–C. Recordings from different areas were obtained by sequentially repositioning the field of view manually under low light fluorescence. All dendritic sites, including the terminal apical tuft (Fig. 3A) as well as the oblique (Fig. 3C) and the basal dendrites (Fig. 3B), exhibited bAPs that could be monitored with excellent signal-to-noise ratio in single-trial measurements. The combined effect of an increase in light intensity and the use of near optimal excitation wavelengths resulted in a dramatic, 10- to 40-fold improvement in the sensitivity of voltage imaging.
Figure 3. Time course of dendritic bAPs as a function of distance from the soma.
A, the bAP signals from the apical terminal tuft 700 μm from the soma (spatial average from location 1 in B). Single-trial recordings and a temporal average of 4 trials illustrate the sensitivity of recording (S/N 15 and 30 respectively). B, a composite fluorescence image of a pyramidal neuron. The size of the full frame imaging region is outlined by dashed-line rectangle. Traces on the right are temporal averages of 4 trials and spatial averages from locations 1–5 indicated on the image. The peak of the first bAP used as a reference point for averaging. The second spike shown in grey did not average coherently because of temporal jitter. The electrode recording from the soma is on the left. C, a composite fluorescence image of a dendritic region from another neuron with several oblique dendrites in focus for voltage imaging. Single-trial recordings on the left are spatial averages from locations 1–4 indicated on the image. D, bAP waveforms from different dendritic locations from the 2 neurons shown in the figure. Superimposed optical signals are scaled to the same height. The somatic optical signal (bottom trace) is superimposed with the four sets of traces for reference. E, summary data from basal, apical and oblique dendrites from 7 pyramidal neurons showing the relationship between the width of the bAP and the distance of the dendritic site from the soma.
Charge-shift dyes have rapid kinetics in the microsecond range and exhibit a linear relationship between fluorescence changes and membrane potential (Salzberg et al. 1993). The optical signal, therefore, tracks the membrane potential without distortion and the time course of the signal is obtained directly from optical data. Somewhat surprisingly, the results indicated that the time course of bAP signals was independent of location in the apical, basal and oblique dendrites. In Fig. 3D, the waveforms of the first evoked bAP signals (used as a reference signal for temporal averaging) from several locations was compared on an expanded time scale and found to be nearly identical at all recording sites. The graph in Fig. 3E shows the summary results from seven experiments and indicates that bAP duration was independent of dendritic location along the whole length of the apical, oblique, and basal dendrites in layer 5 pyramidal neurons. As explained in Methods, the accuracy of this measurement (AP waveform) was better than 0.5 ms sampling interval.
The improved sensitivity of the above measurements allowed us to increase the optical magnification by a factor of 10 and record Vm-transients from individual dendritic spines. The spine bAP signals were clearly detected in single trial measurements, as illustrated in Fig. 4. The S/N was further improved by averaging a small number of trials (Fig. 4A and B). One concern in these measurements was the amount of scattered light from the parent dendrite that might contaminate the measurement from individual spines. To address this issue, we compared dendritic signals from individual spines with recordings from analogous regions without spines and found that the signals from regions without spines were smaller than the noise in the measurement (Fig. 4A and B, locations 2 and 3). Thus, the interference from scattered light was insignificant in the superficial layers of the slice. Another concern was the amount of photodynamic damage caused by the high intensity excitation light. In several experiments, we compared the very first single-trial optical recording of the bAP (control signal) with the last bAP signal at the end of the experiment. The results indicated that, in the range tested (4–25 recording trials) the first and the last bAP signal were identical indicating that the photodynamic damage was not significant.
From the type of data shown in Fig. 4, we compared the time course of the bAP signals in spines and the parent dendrites (Fig. 5). The waveform of the bAP signals was reconstructed from the data using cubic spline interpolation and compared on an expanded time scale. Figure 5E shows that both the upstroke and the downstroke of the AP in spines and dendrites closely overlapped. The summary result from 20 neurons (Fig. 5F) showed that signals from spines and dendrites did not differ significantly. Thus, our results provide confirmation that bAPs in spines have a similar rapid time course to the spikes recorded in the parent dendrite, as suggested by Palmer & Stuart (2009) on the basis of data obtained at lower temporal resolution. The rapid time course of the AP in the spines may be a critical determinant for the precise regulation of STDP within a very narrow time window (Caporale & Dan, 2008).
Figure 5. The comparison of the time course and amplitude of bAPs in individual dendritic spines and parent dendrites.
A, the fluorescence image of a section of a spiny dendrite in recording position. B, anatomical reconstruction from a stack of confocal images. C, spatial averages from locations 1 (spines) and 2 (dendrite) indicated on the image; temporal averages of four trials. Bottom trace, electrode recording from the soma. D, spatial average from exact same locations as in B related to 60 mV hyperpolarizing pulses; temporal averages of nine trials. E, comparison of the shape and size of the bAPs in spines and parent dendrites. F and G, summary results: individual results of waveform comparison from 20 neurons and amplitude comparison from 8 neurons are shown together with averaged values ±s.e.m.
Although the same time course of the bAP in dendritic branches and spines suggested that the two regions are likely to also be equipotential, the amplitude of the spike could, in principle, be different due to possible qualitative and/or quantitative differences in voltage-gated channel distribution. In our measurements the comparison of signal amplitudes from different sites required calibration of Vm-signals on an absolute scale (in terms of membrane potential) because the sensitivity of optical Vm-recording from multiple sites is not spatially uniform (Canepari et al. 2007). To provide this calibration we normalized bAP signals in every measurement to the optical signal related to a steady-state hyperpolarizing pulse obtained from identical locations. This calibration rests on an assumption that the long-lasting hyperpolarizing pulse will have approximately the same amplitude in the parent dendrite and in the spines. A typical measurement is shown in Fig. 5D. In this experiment, the amplitude difference of the bAP signals from spines and dendrites was small and comparable to the noise in measurement. The same result, without exceptions, was obtained in a total of eight neurons. The summary result (Fig. 5G) showed that the AP amplitude in spines and parent dendrites did not differ significantly.
Discussion
We improved the sensitivity of the Vm-imaging method to achieve optical detection of bAPs in the entire dendritic structure of individual layer 5 neurons in brain slices at the single spine resolution. A major finding of our study is that bAPs had a rapid time course throughout all cellular compartments, including the terminal apical tuft branches, a region that has never been probed for electrical signals (Fig. 3). This novel result, demonstrating clear invasion of the dendritic tuft of juvenile mice by bAPs, is at odds with previous work in cortical layer 5 neurons from more mature animals; numerous studies based on patch electrode recordings indicated that bAPs normally propagate with decrement and fail to invade terminal dendritic branches in the apical tuft (see Vetter et al. 2001 for references). It is possible that the main reason for the discrepancy between patch-electrode data versus our optical recording results is the young age of the animals used in our study. Juvenile animals are characterized by smaller and hence more electrically compact neurons which would be less likely to show location-dependent bAP failure. It is also well established that pyramidal neurons from juvenile animals are characterized by slower time course of the APs (McCormick & Prince, 1987), which will reduce the filtering effect of the dendritic cable. In addition, very small changes in the AP waveform could not be detected optically because the highest frequency components of the AP signal were filtered out by the sub-optimal sampling rate. Nevertheless, the accuracy of our measurements of the AP half-width was better than 0.5 ms sampling interval because the measurements are made along a rapidly changing signal (Malmstadt et al. 1974; Ross & Krauthamer, 1984). Finally, the results from layer 5 neurons from the visual cortex of immature mice cannot be directly compared with the recordings from layer 2/3 or layer 5 neurons from the somatosensory cortex from more mature rats; clearly, pyramidal cells from different brain areas, from different species and from different developmental stages are likely to have different characteristics.
At the same time, while considerable variation in the extent of AP back-propagation has been reported even between neurons of the same class (e.g. Larkum et al. 2001), it is noteworthy that the apparent disagreement between patch-electrode data and our optical recording results may be, at least in part, due to incomplete compensation for the RC filtering effect of high resistance electrodes as well as to the additional capacitance load introduced by the patch pipette. A similar disagreement between optical data (Zhou et al. 2008) and patch-electrode recordings (Kampa & Stuart, 2006; Nevian et al. 2007) has been described for bAP signals in basal dendrites of the rat pyramidal neurons. The possibility of signal distortion is a known problem that is particularly pronounced in distal recordings which require high resistance patch electrodes (Stuart et al. 1993). Voltage-sensitive dye recording of the fast voltage transients from small dendritic structures is free from these effects, has an advantage over glass electrode measurements in this respect, and can be used in the future to confirm timing information from more mature animals.
The second major result of our study is that the bAP time course is not altered as the signal propagates across thin dendritic spine necks into the spine head in layer 5 neurons of the visual cortex. Similar results, obtained at lower temporal resolution, were reported in two previous reports (Nuriya et al. 2006; Palmer & Stuart, 2009). Because no evidence was found that the electrical resistance of spine necks is high enough to cause filtering of the fastest dendritic Vm-signals, slower signals, like local dendritic spikes, would be expected to also invade spines unaltered. Furthermore, approximate calibration of optical signals in terms of membrane potential showed that the amplitude of the bAP signals from spines and dendrites did not differ significantly. In these measurements, the timing information derived from optical data is precise while the amplitude information is less reliable and should be considered as an approximation because of uncertainties in calibrating optical signals.
As mentioned above, there are only two prior attempts to analyse electrical events in individual spines by direct measurement. The first study (Nuriya et al. 2006) was based on monitoring voltage-sensitive SHG signals. This method required extensive averaging (approximately 300 trials) but still resulted in an insufficient S/N precluding analysis of the signal size and shape. Similarly, in the other study, the use of conventional confocal microscopy for voltage sensitive dye-based spine imaging was characterized by low sensitivity and, thus, necessitated extensive averaging and relatively low sampling rate (Palmer & Stuart, 2009). Nevertheless, the results obtained by Nuriya et al. (2006) and by Palmer & Stuart (2009) showed no evidence for significant voltage loss when bAPs invade dendritic spines; our data confirm these results at more appropriate amplitude resolution. Additionally, the similar waveform of the AP in spines and the immediately adjacent dendrite (Fig. 2D in Palmer & Stuart, 2009) is in full agreement with our results obtained at more appropriate temporal resolution.
The functional significance of our results is related to the prominent role of bAPs in dendritic signal integration in general and in synaptic plasticity in particular. Several studies indicated that the critical signal responsible for [Ca2+]i-transients that trigger STDP is the bAP (e.g. Watanabe et al. 2002). Other studies show that, under certain conditions, bAPs are neither sufficient nor necessary for LTP. According to many patch-electrode studies, the bAPs attenuate with distance from the soma and fail to invade the distal dendrites and synapses. In these regions, local regenerative responses were identified as a critical determinant of LTP (Golding et al. 2002) or LTD induction (Holthoff et al. 2004). Additionally, recent studies indicate that bAPs cause global inhibition of dendritic spikes (Remy et al. 2009) making plasticity rules even more complex. Thus, it is clear that the extent of bAP propagation into the dendritic tree is one of the key factors regulating dendritic integration and plasticity. Presently, the most efficient way to characterize AP backpropagation into the thin terminal dendritic branches is by using the spatially well resolved optical recording of the type described here. Our data argue that, in layer 5 pyramidal neurons in the visual cortex of juvenile mice, bAPs propagate throughout the dendritic structure, including spines, with invariant duration. Thus, in these neurons, the time course of the bAP in distal dendritic locations does not seem to participate in the mechanism that underlies non-uniform STDP in different dendritic regions (Froemke et al. 2005; Letzkus et al. 2006; Sjostrom & Hausser, 2006).
Acknowledgments
We are grateful to Larry Cohen for useful discussions and to Leslie M. Loew and Joseph P. Wuskell (Center for Cell Analysis and Modeling, UConn Health Center, Farmington, CT 06030, USA) for kindly providing dyes. This work was supported by a grant of the DFG to K.H. and A.K. and by NSF grant IOS-0817969, NIH grants NS068407 and M136043 and by the Kavli Institute for Neuroscience at Yale University to D.Z. A.K. is a Carl von Linde Senior Fellow of the Institute for Advanced Study of the TUM.
Glossary
Abbreviations
- AP
action potential
- bAP
backpropagating AP
- DPSS
Diode Pumped Solid State
- LTD
long-term depression
- LTP
long-term potentiation
- RC
resistor-capacitor circuit
- SHG
second harmonic generation
- STDP
spike-timing dependent plasticity
- Vm
membrane potential
- Vm-imaging
voltage-sensitive dye recording of membrane potential changes
Author contributions
K.H., D.Z. and A.K. designed the experiments, wrote the manuscript and approved the version to be published. K.H. and D.Z. carried out experiments and analysed data.
Author's present address
K. Holthoff: Hans-Berger-Klinik für Neurologie, Friedrich-Schiller-Universität Jena, D-07747 Jena, Germany.
Supplemental material
Figure 1S. The AP initiation in pyramidal neurons in responses to synaptic activation (left) and a depolarizing transmembrane current pulse delivered to the soma (right). Synaptic stimulation: presynaptic axons stimulated by a pair of brief (0.1 ms) current pulses delivered from an extracellular patch electrode. Somatic stimulation: a just suprathreshold transmembrane current pulse (30 ms in duration) delivered from a patch pipette in the whole-cell configuration.
Figure 2S. Pharmacological effects of staining and photodynamic damage were negligible. (a) Whole-cell patch electrode recordings from the soma during dye loading and recovery. (b) Full recovery after staining: electrode recording of AP signal at the start of recording superimposed with the AP signal after ∼120 min recovery period following staining (expanded time scale). (c) A fluorescence image of an apical dendrite of a stained cell in recording position. (d) Two APs evoked by soma stimulation. Single trial optical signal is a spatial average from location 1 indicated in panel c. (e) The 1st and the 20th AP signal recordings (first spike) from a representative experiment. Superimposed signals are from 100 ms optical recording trials from location 1 in panel c (expanded time scale).
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patch-clamp recording from mossy fibre terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Canepari M, Djurisic M, Zecevic D. Dendritic signals from rat hippocampal CA1 pyramidal neurons during coincident pre- and post-synaptic activity: a combined voltage- and calcium-imaging study. J Physiol. 2007;580:463–484. doi: 10.1113/jphysiol.2006.125005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Djurisic M, Antic S, Chen WR, Zecevic D. Voltage imaging from dendrites of mitral cells: EPSP attenuation and spike trigger zones. J Neurosci. 2004;24:6703–6714. doi: 10.1523/JNEUROSCI.0307-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Sacconi L, Blanchard-Desce M, Webb WW. Optical recording of fast neuronal membrane potential transients in acute mammalian brain slices by second-harmonic generation microscopy. J Neurophysiol. 2005;94:3628–3636. doi: 10.1152/jn.00416.2005. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JA, Barchi JR, Welle CG, Kim GH, Kosterin P, Obaid AL, Yodh AG, Contreras D, Salzberg BM. Two-photon excitation of potentiometric probes enables optical recording of action potentials from mammalian nerve terminals in situ. J Neurophysiol. 2008;99:1545–1553. doi: 10.1152/jn.00929.2007. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, Yuste R, Konnerth A. Single-shock LTD by local dendritic spikes in pyramidal neurons of mouse visual cortex. J Physiol. 2004;560:27–36. doi: 10.1113/jphysiol.2004.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electric Current Flow in Excitable Cells. Oxford: Oxford University Press; 1975. [Google Scholar]
- Kampa BM, Stuart GJ. Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts. J Neurosci. 2006;26:7424–7432. doi: 10.1523/JNEUROSCI.3062-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B, Fromherz P, Denk W. High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys J. 2004;87:631–639. doi: 10.1529/biophysj.104.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann BJ. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol. 2001;533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew LM. Design and characterization of electrochromic membrane probes. J Biochem Biophys Methods. 1982;6:243–260. doi: 10.1016/0165-022x(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Malmstadt H, Enke CG, Grouch SR. Electronic Measurements for Scientists. W. A. Benjamin Inc. Publishers; 1974. [Google Scholar]
- Mathews JH, Fink KD. Numerical Methods Using Mathlab. Upper Saddle River, NJ, USA: Pearson Prentice Hall; 2004. [Google Scholar]
- McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurons. J Physiol. 1987;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- Nuriya M, Jiang J, Nemet B, Eisenthal KB, Yuste R. Imaging membrane potential in dendritic spines. Proc Natl Acad Sci U S A. 2006;103:786–790. doi: 10.1073/pnas.0510092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Membrane potential changes in dendritic spines during action potentials and synaptic input. J Neurosci. 2009;29:6897–6903. doi: 10.1523/JNEUROSCI.5847-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Csicsvari J, Beck H. Activity-dependent control of neuronal output by local and global dendritic spike attenuation. Neuron. 2009;61:906–916. doi: 10.1016/j.neuron.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Ross WN, Krauthamer V. Optical measurements of potential changes in axons and processes of neurons of a barnacle ganglion. J Neurosci. 1984;4:659–672. doi: 10.1523/JNEUROSCI.04-03-00659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg BM, Obaid AL, Bezanilla F. Microsecond response of a voltage-sensitive merocyanine dye: fast voltage-clamp measurements on squid giant axon. Japn J Physiol. 1993;43(Suppl. 1):S37–S41. [PubMed] [Google Scholar]
- Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;42:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K-channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Wu JY, Cohen LB. Fast multisite optical measurement of membrane potential. In: Mason WT, editor. Biological Techniques: Fluorescent and Luminescent Probes for Biological Activity. New York: Academic Press; 1993. [Google Scholar]
- Zhou W-L, Yan P, Wuskell JP, Loew LM, Antic SD. Intracellular long-wavelength voltage-sensitive dyes for studying the dynamics of action potentials in axons and thin dendrites. J Neurosci Methods. 2007;164:225–239. doi: 10.1016/j.jneumeth.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Dynamics of action potential backpropagation in basal dendrites of prefrontal cortical pyramidal neurons. Eur J Neurosci. 2008;27:923–936. doi: 10.1111/j.1460-9568.2008.06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.