Abstract
Isolated activation of metabolically sensitive skeletal muscle afferents (muscle metaboreflex) using post-exercise ischaemia (PEI) following handgrip partially maintains exercise-induced increases in arterial blood pressure (BP) and muscle sympathetic nerve activity (SNA), while heart rate (HR) declines towards resting values. Although masking of metaboreflex-mediated increases in cardiac SNA by parasympathetic reactivation during PEI has been suggested, this has not been directly tested in humans. In nine male subjects (23 ± 5 years) the muscle metaboreflex was activated by PEI following moderate (PEI-M) and high (PEI-H) intensity isometric handgrip performed at 25% and 40% maximum voluntary contraction, under control (no drug), parasympathetic blockade (glycopyrrolate) and β-adrenergic blockade (metoprolol or propranalol) conditions, while beat-to-beat HR and BP were continuously measured. During control PEI-M, HR was slightly elevated from rest (+3 ± 2 beats min−1); however, this HR elevation was abolished with β-adrenergic blockade (P < 0.05 vs. control) but augmented with parasympathetic blockade (+8 ± 2 beats min−1, P < 0.05 vs. control and β-adrenergic blockade). The HR elevation during control PEI-H (+9 ± 3 beats min−1) was greater than with PEI-M (P < 0.05), and was also attenuated with β-adrenergic blockade (+4 ± 2 beats min−1, P < 0.05 vs. control), but was unchanged with parasympathetic blockade (+9 ± 2 beats min−1, P > 0.05 vs. control). BP was similarly increased from rest during PEI-M and further elevated during PEI-H (P < 0.05) in all conditions. Collectively, these findings suggest that the muscle metaboreflex increases cardiac SNA during PEI in humans; however, it requires a robust muscle metaboreflex activation to offset the influence of cardiac parasympathetic reactivation on heart rate.
Introduction
Heart rate (HR) and arterial blood pressure (BP) increase during exercise in an intensity-dependent manner due to the integration of central and peripheral neural mechanisms. Feed-forward signals from the brain, known as central command, arise in parallel with descending motor drive to the exercising muscles and converge on the cardiovascular areas of the brain stem (Krogh & Lindhard, 1913; Goodwin et al. 1972; Degtyarenko & Kaufman, 2006; Potts, 2006). In addition, group III and IV afferent nerves from the active skeletal muscles provide feedback to these cardiovascular areas in response to both mechanical and metabolic stimulation (Coote et al. 1971; McCloskey & Mitchell, 1972; Kaufman et al. 1983). Furthermore, the arterial baroreflex plays an important regulatory role in modulating the cardiovascular responses to exercise (Fadel et al. 2003; Joyner, 2006; Raven et al. 2006).
In humans, isolated activation of metabolically sensitive skeletal muscle afferents (muscle metaboreflex) using suprasystolic cuff occlusion following isometric handgrip (post-exercise ischaemia; PEI) partially maintains exercise-induced increases in BP and sympathetic nerve activity (SNA), while HR declines towards resting values (Alam & Smirk, 1937; Mark et al. 1985; Victor et al. 1987). Accordingly, the muscle metaboreflex is not considered to influence HR, while it raises BP primarily through an increase in sympathetically mediated peripheral vasoconstriction (Rowell & O’Leary, 1990). Alternatively, it has been proposed that the decrease in HR occurs during PEI as the result of an overwhelming effect of cardiac parasympathetic reactivation, due to baroreflex mechanisms and/or the loss of central command and mechanically sensitive muscle afferents, which masks metaboreflex-mediated increases in cardiac SNA (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999). Indeed, O’Leary (1993) found that during PEI following treadmill running in dogs, the administration of atropine to abolish cardiac parasympathetic tone unmasked a pronounced sympathetically mediated tachycardia. However, the extent to which these findings can be extrapolated to humans remains unclear.
The evidence from human studies to support the contention that HR remains near resting values during PEI due to an overwhelming effect of parasympathetic reactivation to obscure metaboreflex-mediated increases in cardiac SNA is indirect and equivocal. Nishiyasu et al. (1994) demonstrated that an index of cardiac parasympathetic tone derived from HR variability (standard deviation of R–R intervals), was elevated during modest muscle metaboreflex activation with PEI. However, high-frequency HR variability, also suggested to be an index of cardiac parasympathetic tone, was unaltered from rest during PEI (Iellamo et al. 1999). Iellamo et al. (1999) found that low-frequency HR variability, used to estimate cardiac SNA, was elevated during moderate activation of the muscle metaboreflex with PEI. However, the validity of HR variability measures as indices of either cardiac parasympathetic or sympathetic activity remains controversial (Taylor et al. 2001; Taylor & Studinger, 2006) and the influence of pharmacological blockade on HR during PEI, as used by O’Leary (1993), has not been evaluated in humans.
This study directly tested whether muscle metaboreflex-mediated increases in cardiac SNA during PEI are overwhelmed by elevated parasympathetic nerve activity in humans. The cardiovascular responses to two levels of muscle metaboreflex activation were compared during PEI under control conditions, and after parasympathetic and β-adrenergic blockade. We hypothesised that elimination of cardiac parasympathetic tone would allow the tachycardic effect of cardiac sympathetic activation to be manifest during isolated muscle metaboreflex activation in humans.
Methods
Nine healthy male subjects participated in the study with a mean (±s.d.) age, height and weight of 23 ± 5 years, 180 ± 7 cm and 77 ± 8 kg, respectively. All subjects were free of any known cardiovascular or pulmonary disorders and they were not using prescribed or over the counter medications. All experimental protocols and procedures conformed to the Declaration of Helsinki and were approved by the local ethics committee in Copenhagen (H-B-2009-024). After receiving detailed verbal and written explanation of the experimental procedures and protocols, each subject gave informed written consent prior to participation. Subjects were requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h prior to experimentation. All studies were performed at an ambient room temperature of 23–24°C with external stimuli minimised.
Experimental measurements
Arterial BP was measured by a catheter (1.1 mm ID, 20-gauge) placed in the left brachial artery and connected to a pressure transducer (Baxter, Uden, the Netherlands) positioned at the level of the right atrium. Study drugs were administered through a catheter (2.4 mm; REF 681698 BD Medical Systems, Singapore) inserted retrogradely into the right internal jugular vein, under local anaesthesia (lidocain, 2%) and guided by ultrasound. Internal jugular vein catheterisation was specifically performed for purposes unrelated to the present report (i.e. examination of the influence of handgrip exercise on cerebral metabolism). HR and R–R interval were monitored using a lead II ECG, and together with the BP signals obtained through a Dialogue 2000 monitor (IBC-Danica, Copenhagen, Denmark), interfaced with a personal computer equipped with customized data acquisition software. Respiratory movements were recorded using a strain-gauge pneumograph placed around the subjects’ abdomen (Pneumotrace; UFI, Morro Bay, CA, USA) to ensure that the subjects did not perform inadvertent Valsalva manoeuvres during handgrip and PEI. Ratings of perceived exertion were expressed using a 6–20 scale (Borg, 1982). All cardiovascular variables were sampled at 1000 Hz, and real time beat-to-beat values of HR, systolic BP, diastolic BP and mean BP were calculated and stored for off-line analysis (Chart v5.2 and Powerlab, AD Instruments, Bella Vista, NSW, Australia).
Experimental protocol
On experimental days the subjects arrived at the laboratory at least 2 h following a light meal. The subjects were instrumented for measurement of HR and respiration, and catheterized for BP recordings and the administration of study drugs. Following instrumentation the subjects recovered for ∼30 min to offset arousal and nociceptive stimuli associated with catheterization on resting cardiovascular variables (Seifert et al. 2009).
Thereafter, the subjects were seated in a semi-recumbent position on a hospital bed with a handgrip dynamometer held in the right hand with the limb supported. The handgrip force exerted was interfaced with a personal computer and displayed at eye level to provide visual feedback to the subjects (Chart v5.2 and Powerlab). Maximum voluntary contraction (MVC) was determined as the highest of three to five maximal efforts, each separated by 1 min. After a 10 min rest period, each subject performed 2 min of isometric handgrip at a moderate (25% MVC) or high (40% MVC) intensity followed by 3 min and 15 s of forearm ischaemia to isolate muscle metaboreflex activation (PEI). PEI was achieved by inflation of a blood pressure cuff around the right arm to suprasystolic pressure (240 mmHg) 5 s before the end of handgrip. The additional 15 s of PEI was incorporated so that the initial changes in HR and BP that occur when exercise is stopped could be eliminated from analyses predicated on steady-state conditions (e.g. HR variability). PEI following both 25% and 40% MVC handgrip was used to evoke a graded and robust activation of the muscle metaboreflex (PEI-M and PEI-H, respectively). Our rationale was that previous human studies have utilized either low or moderate intensity muscle metaboreflex activation (Nishiyasu et al. 1994; Iellamo et al. 1999) whereas the canine study demonstrating a metaboreflex control of HR employed robust metaboreflex activation (O’Leary, 1993). The order of the 25% and 40% MVC trials was randomized, and trials were separated by 20–30 min to ensure restoration of resting cardiovascular variables. Subjects were instructed to maintain a constant and comfortable respiratory rate throughout the rest, PEI and recovery periods, and to avoid straining manoeuvres during handgrip.
Trials of 25% and 40% MVC handgrip were performed under three conditions: (1) control conditions (no drug), (2) following parasympathetic blockade with the muscarinic receptor blocker glycopyrrolate, and (3) after β-adrenergic blockade with either the selective β-1 receptor blocker metoprolol (n= 5) or the β-1 and β-2 receptor blocker propranolol (n= 4). Glycopyrrolate was chosen, in contrast to atropine, because it does not penetrate the blood–brain barrier (Proakis & Harris, 1978), thereby avoiding any direct central nervous system effects. However, for β-adrenergic blockade this was not possible as there is evidence to suggest that both propranolol and metoprolol cross the blood–brain barrier (Neil-Dwyer et al. 1981). All subjects performed control handgrip trials on the first study day, followed by either the muscarinic cholinergic blockade or the β-adrenergic blockade trials according to a counterbalanced design. Three to seven days later the subjects returned to the laboratory and repeated the protocol with the remaining study drug. Multiple study days were considered necessary to eliminate the effect of the previous drug used and considering that the high intensities of handgrip being used could provoke fatigue. Indeed, pilot studies indicated that six bouts of handgrip and PEI in one study session were not feasible. Metoprolol and propranolol were administered in step-wise infusions of 1 mg. Full blockade of β-adrenoreceptors was identified when resting HR was unchanged to consecutive doses (group average dose of 0.17 ± 0.01 and 0.19 ± 0.01 mg kg−1, for propranolol and metoprolol, respectively). Similarly, step-wise infusions of 0.2 mg of glycopyrrolate were administered, and complete cardiac parasympathetic blockade was considered achieved when consecutive doses caused no further increases in resting HR (group average dose of 19.6 ± 1.7 μg kg−1). Between handgrip trials during the β-adrenergic and parasympathetic blockade conditions, if HR was changed from post-drug resting baseline values, additional doses of metoprolol (0.03 ± 0.006 mg kg−1), propranolol (0.02 ± 0.005 mg kg−1) or glycopyrrolate (5.5 ± 1.3 μg kg−1) were administered until no further change in HR occurred. This procedure maintained the initial post-drug baseline HR, identified as complete cholinergic or β-adrenergic blockade (Ogoh et al. 2005; Fisher et al. 2006).
Heart rate variability and cardiac baroreflex sensitivity
To further evaluate whether exercise-induced increases in HR are not maintained during PEI because of an overwhelming effect of cardiac parasympathetic reactivation due to baroreflex mechanisms and/or the loss of central command and mechanically sensitive muscle afferents, indices of cardiac parasympathetic control were derived using analysis of cardiovascular variability. Time domain HR variability was performed using the square root of the mean of the sum of successive differences in R–R interval (RMSSD) as recommended for the estimation of short-term high-frequency variability of HR that is primarily mediated by parasympathetic nerve activity (Task Force, 1996). In addition, spontaneous cardiac baroreflex sensitivity was assessed using the sequence technique (Fisher et al. 2009). In brief, the beat-to-beat time series of systolic BP and RR interval were analysed off-line using a customized computer algorithm (Spike 2, Cambridge Electronic Design, Cambridge, UK). Sequences of three or more consecutive beats where systolic BP and R–R interval change in the same direction were identified as arterial baroreflex sequences. A linear regression was applied to each individual sequence and only those sequences in which r2 was >0.85 were accepted. The slope of systolic BP–R–R interval was calculated as a measure of cardiac baroreflex sensitivity. HR variability and cardiac baroreflex sensitivity measures were calculated over 3 min periods at rest, during PEI and during recovery. These indices were not assessed during handgrip or the first 15 s of PEI due to the confounding influence of inherent non-stationarities in HR.
Statistical analysis
Comparisons of physiological variables were made using three-way analyses of variance (ANOVA) with repeated measures, in which condition (control, parasympathetic blockade, β-adrenergic blockade), phase (rest, handgrip, PEI, recovery), and trial (25% MVC, 40% MVC) were the main factors. Post hoc analysis was employed using Student–Newman–Kuels test to investigate main effects and interactions. Statistical significance was set at P < 0.05 and values are presented as means ±s.e.m. Analyses were conducted using Statistica (Statsoft, Tulsa, Oklahoma, USA) for Windows.
Results
Rest
As expected, resting HR was reduced with β-adrenergic blockade, and increased with parasympathetic blockade (P < 0.05; Table 1 and Fig. 1). Mean BP at rest was slightly reduced with β-adrenergic blockade and increased with parasympathetic blockade (P < 0.05 vs. control; Table 1 and Fig. 2). RMSSD and cardiac baroreflex sensitivity were reduced with parasympathetic blockade (P < 0.05 vs. control), but unchanged following β-adrenergic blockade (P > 0.05 vs. control, Fig. 3).
Table 1.
Heart rate (HR) and blood pressure during isometric handgrip and post exercise ischaemia (PEI), for the control, β-adrenergic blockade and parasympathetic blockade conditions
| 25% MVC |
40% MVC |
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Handgrip | PEI | Recovery | Rest | Handgrip | PEI | Recovery | Drug | Phase | Trial | Interaction | |
| HR (beats min−1) | ||||||||||||
| Control | 56 ± 2 | 79 ± 3* | 59 ± 3*† | 56 ± 3† | 55 ± 2 | 101 ± 3*$ | 64 ± 3*†§ | 54 ± 3† | <0.001 | <0.001 | <0.001 | <0.001 |
| β block | 51 ± 3‡ | 63 ± 3*‡ | 50 ± 3†‡ | 52 ± 3†‡ | 52 ± 3‡ | 78 ± 3*‡$ | 56 ± 2*†‡§ | 50 ± 3†‡ | ||||
| Parasympathetic | 112 ± | 127 ± | 120 ± | 111 ± | 115 ± | 138 ± | 125 ± | 110 ± | ||||
| block | 3‡# | 4*‡# | 3*†‡# | 4†‡# | 3‡#§ | 4*‡#$ | 2*†‡#§ | 3*†‡# | ||||
| systolic BP (mmHg) | ||||||||||||
| Control | 133 ± 3 | 151 ± 3 | 158 ± 3 | 141 ± 3 | 134 ± 3 | 167 ± 4 | 174 ± 3 | 148 ± 4 | 0.079 | <0.001 | <0.001 | 0.975 |
| β block | 124 ± 4 | 141 ± 3 | 146 ± 4 | 131 ± 4 | 126 ± 3 | 156 ± 3 | 162 ± 3 | 138 ± 4 | ||||
| Parasympathetic block diastolic BP (mmHg) | 129 ± 4 | 147 ± 4 | 150 ± 5 | 132 ± 4 | 127 ± 4 | 162 ± 3 | 162 ± 3 | 136 ± 3 | ||||
| Control | 61 ± 2 | 74 ± 2 | 77 ± 2 | 62 ± 1 | 60 ± 2 | 87 ± 3 | 84 ± 2 | 65 ± 1 | <0.001 | <0.001 | <0.001 | 0.546 |
| β block | 61 ± 3 | 78 ± 3 | 79 ± 4 | 63 ± 3 | 62 ± 2 | 92 ± 3 | 88 ± 3 | 67 ± 2 | ||||
| Parasympathetic block | 72 ± 2 | 88 ± 2 | 86 ± 2 | 73 ± 2 | 73 ± 3 | 101 ± 2 | 91 ± 4 | 73 ± 2 | ||||
| mean BP (mmHg) | ||||||||||||
| Control | 85 ± 1 | 100 ± 1 | 104 ± 2 | 88 ± 1 | 85 ± 2 | 113 ± 3 | 114 ± 2 | 93 ± 2 | 0.064 | <0.001 | <0.001 | 0.830 |
| β block | 82 ± 3 | 99 ± 2 | 102 ± 3 | 86 ± 3 | 83 ± 2 | 113 ± 2 | 113 ± 3 | 91 ± 3 | ||||
| Parasympathetic block | 91 ± 3 | 108 ± 3 | 107 ± 3 | 92 ± 2 | 91 ± 3 | 121 ± 4 | 115 ± 2 | 94 ± 2 | ||||
Values represent means (±s.e.m.) over each experimental phase. BP, blood pressure; MVC, maximum voluntary contraction. P values are derived from ANOVA examining main effects of drug, phase, trial and interaction (drug × phase × trial).
P < 0.05 vs. rest,
P < 0.05 vs. exercise,
P < 0.05 vs. control,
P < 0.05 vs.β-blockade,
P < 0.05 vs. 25% MVC.
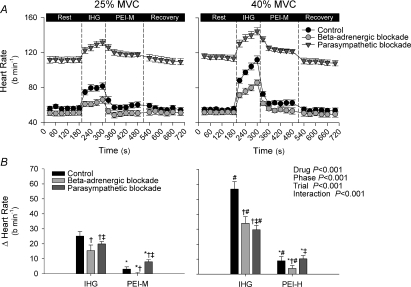
Figure 1.
Heart rate during isometric handgrip (IHG) and post-exercise ischaemia (PEI) under control (black symbols), β-adrenergic blockade (light grey symbols) and parasympathetic blockade (dark grey symbols) conditions A shows absolute heart rate during all experimental phases, while B shows the change (Δ) in heart rate from rest during IHG and PEI. PEI-M, PEI following 25% IHG; PEI-H, PEI following 40% IHG. *P < 0.05 vs. exercise, †P < 0.05 vs. control, ‡P < 0.05 vs.β blockade, #P < 0.05 vs. 25% MVC.
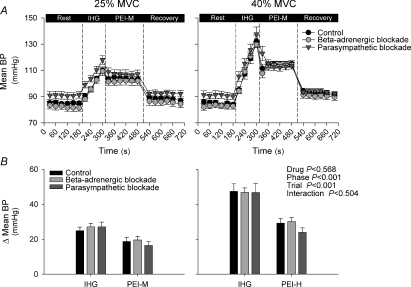
Figure 2.
Mean blood pressure (BP) during isometric handgrip (IHG) and post-exercise ischaemia (PEI) under control (black symbols), β-adrenergic blockade (light grey symbols) and parasympathetic blockade (dark grey symbols) conditions A shows absolute mean BP during all experimental phases, while B shows the change (Δ) in mean BP from rest during IHG and PEI. PEI-M, PEI following 25% IHG; PEI-H, PEI following 40% IHG.
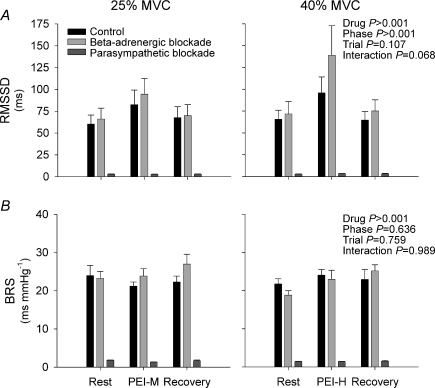
Figure 3.
Indices of heart rate variability (panel A) and cardiac spontaneous baroreflex sensitivity (panel B; BRS) at rest, post-exercise ischaemia (PEI) and recovery under control (black bars), β-adrenergic blockade (light grey bars) and parasympathetic blockade (dark grey bars) conditions Two subjects were omitted from cardiac baroreflex sensitivity analysis as no sequences were detected during PEI. PEI-M, PEI following 25% IHG; PEI-H, PEI following 40% IHG. RMSSD, square root of the mean of the sum of successive differences in R–R interval.
25% and 40% MVC isometric handgrip
The HR increased from rest during handgrip at 25% MVC under all conditions, and was additionally augmented during 40% MVC (Table 1 and Fig. 1). Under control conditions HR increased by 23 ± 3 beats min−1 and 57 ± 5 beats min−1 for the 25% MVC and 40% MVC conditions, respectively. However, compared with control, the magnitude of the increase in HR was attenuated by both parasympathetic and β-adrenergic blockade (P < 0.05). The exercise-induced increases in systolic BP, diastolic BP and mean BP were greater in the 40% MVC trial compared to the 25% MVC trial (P < 0.05; Table 1 and Fig. 2) with no significant differences between control, parasympathetic and β-adrenergic blockade conditions. Ratings of perceived exertion were higher for 40% MVC handgrip compared with 25% MVC handgrip (19 (16–20) vs. 14 (12–16) a.u.; median (range); P < 0.05), with no significant interaction observed between exercise intensity and condition (P > 0.05).
Graded muscle metaboreflex activation during PEI
During PEI, HR fell from end-exercise values under all conditions (P < 0.05 vs. handgrip; Table 1 and Fig. 1). During control PEI-M, HR remained slightly elevated from baseline (+3 ± 2 beats min−1), but this elevation was abolished with β-adrenergic blockade (Table 1 and Fig. 1). In contrast, during PEI-M with parasympathetic blockade, HR was increased from baseline (P < 0.05), with the magnitude of the elevation being more marked than under control conditions (+8 ± 2 beats min−1, P < 0.05 vs. control). During control PEI-H, HR also remained elevated from baseline (+9 ± 3 beats min−1). This elevation in HR was greater than during control PEI-M (Fig. 1). Notably, the elevation in HR during control PEI-H was attenuated with β-adrenergic blockade (P < 0.05 vs. control), but was not significantly different following parasympathetic blockade. The elevation in systolic BP, diastolic BP and mean BP during PEI-M and PEI-H was similar between conditions with the elevation being greater in PEI-H (P < 0.05 vs. PEI-M, Table 1 and Fig. 2).
Heart rate variability and cardiac baroreflex sensitivity
RMSSD was elevated from rest during PEI-M and PEI-H in the control and β-adrenergic blockade conditions (P < 0.05; Fig. 3). However, during PEI-H, RMSSD was higher with β-adrenergic blockade (P < 0.05 vs. control). In contrast, with parasympathetic blockade, RMSSD was unchanged from rest during PEI-M or PEI-H, and remained reduced compared to control and β-adrenergic blockade conditions (P < 0.05). Notably, cardiac baroreflex sensitivity was unchanged from rest during PEI-M and PEI-H in control, β-adrenergic blockade and parasympathetic blockade conditions, but remained significantly reduced with parasympathetic blockade (P < 0.05 vs. control and β-adrenergic blockade, Fig. 3). Furthermore, no significant differences in cardiac baroreflex sensitivity were observed between PEI-M and PEI-H.
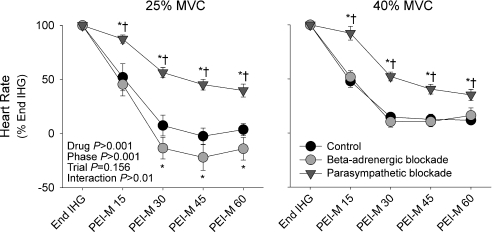
Cardiac recovery kinetics from end-exercise to PEI
Figure 4 shows the recovery of HR from end-exercise over the first minute of PEI-M and PEI-H. Notably, with parasympathetic blockade HR recovery was slower than in control and β-adrenergic blockade conditions. Indeed, during the first 15 s of PEI with parasympathetic blockade, HR remained at 87 ± 4% and 92 ± 6% of end-exercise values for PEI-M and PEI-H, respectively, whereas HR decreased by ∼50% under control and β-adrenergic blockade conditions.
Figure 4.
Heart rate during the first minute of post-exercise ischaemia expressed as a percentage of end isometric handgrip values (End IHG), under control (black symbols), β-adrenergic blockade (light grey symbols) and parasympathetic blockade (dark grey symbols) conditions PEI-M, PEI following 25% IHG; PEI-H, PEI following 40% IHG. Time-points represent 15s averages. *P < 0.05 vs. control, †P < 0.05 vs.β-adrenergic blockade.
Discussion
The present study examined autonomic control of HR by metabolically sensitive skeletal muscle afferents (muscle metaboreflex) using selective pharmacological elimination of cardiac parasympathetic or sympathetic tone in humans. We find evidence for muscle metaboreflex-mediated increases in HR via activation of cardiac SNA; however, this effect is quite modest and appears to require robust metaboreflex activation. Indeed, during moderate muscle metaboreflex activation (PEI-M), HR rapidly returned towards baseline under control and β-adrenergic blockade conditions, while the recovery of HR was slower and less complete with parasympathetic blockade. Accordingly, metaboreflex-mediated increases in cardiac SNA during PEI seem to be dampened by cardiac parasympathetic reactivation, probably due to the loss of central command and muscle mechanoreceptor inputs and/or baroreflex mechanisms, as proposed by others (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999). However, during robust metaboreflex activation, the elevation of HR from rest observed under control conditions (PEI-H) was unaltered with parasympathetic blockade but was attenuated with β-adrenergic blockade. Thus, with more robust muscle metaboreflex activation, increases in cardiac SNA begin to overcome cardiac parasympathetic reactivation. Collectively, these findings suggest that the muscle metaboreflex increases cardiac SNA during PEI in humans; however, for this to have an effect on HR, robust muscle metaboreflex activation is needed to offset cardiac parasympathetic reactivation.
In humans, increases in HR during isometric exercise are traditionally thought to be mediated by a withdrawal of parasympathetic tone primarily due to activation of central command (Mitchell et al. 1989). However, there may also be an important contribution from skeletal muscle mechanoreceptors (Gladwell et al. 2005). In contrast, the muscle metaboreflex is thought to play a less substantial role in cardiac regulation, although it does make an important contribution to increasing BP via augmented peripheral vasoconstriction (Rowell & O’Leary, 1990). Support for this dichotomy is based on the observation that isolated activation of the muscle metaboreflex during PEI, leads to a maintained elevation of exercise-induced increases in SNA, vascular resistance and BP, while HR returns towards baseline (Alam & Smirk, 1937; Mark et al. 1985; Seals, 1989). Although HR may remain near baseline during PEI because of an overwhelming effect of parasympathetic reactivation that obscures metaboreflex-mediated increases in cardiac SNA, the data supporting this notion in humans are indirect and somewhat conflicting. Thus, we undertook the approach of pharmacologically eliminating cardiac parasympathetic or sympathetic tone to examine muscle metaboreflex control of HR in humans, as when employed in a canine study this approach has revealed a muscle metaboreflex-mediated increase in HR via cardiac SNA (O’Leary, 1993).
The results of the present study demonstrate a greater elevation in HR during moderate muscle metaboreflex activation (PEI-M) with cardiac parasympathetic blockade, compared with control conditions or with β-adrenergic blockade. This observation is in line with work employing PEI with parasympathetic blockade following treadmill running in dogs (O’Leary, 1993), and suggests that the muscle metaboreflex does provide sympathetic drive to the heart in humans, but that this is ordinarily masked during PEI by an increase in cardiac parasympathetic tone. In this regard, RMSSD (an index of cardiac parasympathetic tone) was augmented during PEI under control conditions. This is probably explained by the loss of inputs from central command and mechanoreceptor inputs upon the cessation of exercise and/or baroreflex mechanisms (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999). These data suggest that at modest levels of metaboreflex activation with PEI, a sympathetically mediated tachycardia is masked by augmented cardiac parasympathetic tone in humans.
More compelling data demonstrating a role for the muscle metaboreflex in mediating HR responses were derived from the robust activation of the muscle metaboreflex. During PEI-H under control conditions, HR was elevated above resting values, as has occasionally been reported during PEI following high-intensity isometric exercise (Drew et al. 2008a,b;). We demonstrated that this HR elevation was sympathetically mediated, as it was attenuated with β-adrenergic blockade. Intriguingly, there was no significant difference observed in the magnitude of the HR elevation during PEI-H, between control and parasympathetic blockade conditions. This suggests that under control conditions, robust activation of the metaboreflex is able to increase cardiac SNA sufficiently to overcome the augmented cardiac parasympathetic tone elicited by the loss of central command and muscle mechanoreceptors and/or baroreflex mechanisms.
Despite a greater BP elevation during PEI-H compared with PEI-M (i.e. greater muscle metaboreflex activation), the elevation in HR with parasympathetic blockade was only modestly graded during PEI. This may suggest that there is a limit to the increase in HR that can be elicited by isolated metaboreflex activation in humans. Indeed, the elevation in HR during the actual handgrip exercise was more graded after parasympathetic blockade and different between 25% and 40% MVC at the end of exercise. These data suggest that in the absence of parasympathetic nerve activity, central command and/or muscle mechanoreceptors drive HR increases through cardiac SNA during handgrip. Moreover, these data are in contrast to O’Leary (1993) in which exercise-induced increases in HR were almost completely maintained during post-exercise occlusion of hind-limb circulation after parasympathetic blockade in dogs. The reasons for these differences are unclear but may be attributable to species differences and/or differences in the exercise modality employed. In this regard, disparities in baseline autonomic tone, central haemodynamics and skeletal muscle fibre type composition between humans and dogs has been reported (Rowell & O’Leary, 1990). In addition, when examining the muscle metaboreflex during or after exercise (i.e. PEI), the type of exercise (i.e. isometric vs. dynamic), the size of the exercising muscle mass, and the exercise intensity can be modulating factors (Mitchell, 1990; Fisher & White, 2004).
Following handgrip, the recovery of HR during PEI was slower with parasympathetic blockade compared with the control or β-adrenergic blockade conditions. This slower fall in HR during PEI is presumably due to gradual withdrawal of cardiac SNA activity (Warner & Cox, 1962) following the rapid loss of inputs from central command and/or muscle mechanoreceptors during sustained muscle metaboreflex activation. A delayed HR recovery from exercise is a powerful independent predictor of mortality in low risk patients (Cole et al. 1999) and has been attributed to rapid parasympathetic reactivation (Imai et al. 1994). The results of the present study broadly support this notion, and further suggest that in the absence of parasympathetic reactivation, increased cardiac SNA may also contribute to a delayed post-exercise recovery of HR. These data may be important given the known alterations in skeletal muscle afferent sensitivity in disease conditions associated with reduced parasympathetic tone (e.g. heart failure) (Smith et al. 2006).
The HR response to both 25% and 40% MVC isometric handgrip exercise was attenuated with β-adrenergic blockade, suggesting a sympathetic contribution to the HR response under control conditions. This observation is supported by the work of Maciel et al. (1987) and Martin et al. (1974); however, the precise mechanisms underlying this sympathetic HR response have remained obscure in humans (i.e. central command, metaboreflex, mechanoreflex). In the present study, despite a slower recovery of HR during PEI with parasympathetic blockade than under control and β-adrenergic blockade conditions, HR still fell markedly from the end of exercise. HR was increased by 30 ± 3 beats min−1 during 40% MVC handgrip with parasympathetic blockade, but was only elevated by 9 ± 2 beats min−1 during the subsequent PEI-H. Although these findings indicate that the muscle metaboreflex contribution to the sympathetically mediated tachycardia during handgrip was modest, it should be noted that the isolation of the muscle metaboreflex during PEI does not take into account interactions between mechanoreceptors and metaboreceptors, and the potential sensitization that can occur, particularly during high intensity handgrip (Kaufman & Rybicki, 1987). Nevertheless, these data suggest that central command and/or muscle mechanoreceptors may make significant contributions to exercise-induced increases in HR via elevated cardiac SNA.
Although there is evidence to suggest that muscle mechanoreceptor activation can modestly increase muscle SNA in humans (Cui et al. 2006) and cardiac SNA in animals (Tsuchimochi et al. 2009), evidence that mechanoreceptor activation can increase cardiac SNA in humans is presently lacking. It is also uncertain whether cardiac SNA is increased during exercise by the population of muscle mechanoreceptors that are sensitized by the presence of local metabolites (Kaufman & Rybicki, 1987; Fisher et al. 2005). Likewise, an effect of central command to increase cardiac SNA in humans has not been clearly demonstrated. Of note, Mitchell et al. (1989), using partial neuromuscular blockade (i.e. augmented central command), suggested that withdrawal of parasympathetic nerve activity by central command is the primary mechanism for the increase in HR during handgrip with little effect of central command on cardiac SNA. Although the reason previous studies have not found that central command and/or muscle mechanoreceptors cause a sympathetically mediated tachycardia is unclear, we suggest that the intensity of exercise may be a key factor and that the higher the intensity of exercise the more readily a cardiac SNA response can be discerned.
Robust activation of the muscle metaboreflex during PEI is associated with a rightward resetting of the carotid–cardiac baroreflex function curve to operate around the prevailing HR and BP, whilst maximal and operating point gain (i.e. sensitivity) are preserved (Fisher et al. 2008). In agreement, we found no evidence of muscle metaboreflex-mediated alterations in cardiac baroreflex sensitivity during either moderate (PEI-M) or robust (PEI-H) muscle metaboreflex activation. In contrast, spontaneous indices of cardiac baroreflex sensitivity have been reported to be reduced by muscle metaboreflex activation elicited by either PEI in humans (Iellamo et al. 2006), or partial terminal aortic occlusion in treadmill-exercising dogs (Sala-Mercado et al. 2007). Such reports of metaboreflex-mediated decreases in cardiac baroreflex sensitivity raise the possibility that increased cardiac SNA or reduced parasympathetic tone may potentially reduce cardiac baroreflex sensitivity. However, cardiac baroreflex sensitivity was not significantly influenced by moderate or robust muscle metaboreflex activation during PEI under control conditions, or with either pharmacological blockade of cardiac SNA or cardiac parasympathetic nerve activity.
The findings of the present study suggest that isolated muscle metaboreflex activation during PEI elicits a sympathetically mediated tachycardia that is ordinarily masked by cardiac parasympathetic reactivation. More importantly, with robust muscle metaboreflex activation, increases in cardiac SNA are able to overcome cardiac parasympathetic reactivation. Collectively, these findings suggest that the muscle metaboreflex increases cardiac SNA during PEI in humans, but to have an effect on HR, robust muscle metaboreflex activation is needed to offset cardiac parasympathetic reactivation.
Acknowledgments
The authors appreciate the time and effort expended by all the volunteer subjects. We thank Peter Nissen for his expert technical assistance and Dr David McIntyre for writing the Spike 2 script files. This research was supported by NIH Grant no. HL-093167 (P.J.F.), the Aase and Ejnar Danielsens Foundation (N.H.S), and a Wellcome Trust VIP Award (J.P.F.).
Glossary
Abbreviations
- BP
arterial blood pressure
- HR
heart rate
- MVC
maximal voluntary contraction
- PEI
post-exercise ischaemia
- PEI-H
post-exercise ischaemia following high intensity handgrip exercise
- PEI-M
post-exercise ischaemia following moderate intensity handgrip exercise
- RMSSD
square root of the mean of the sum of successive differences in R–R interval
- SNA
sympathetic nerve activity
Author contributions
J.P.F. contributed to study design, data acquisition, data analysis, data interpretation and wrote the first draft of the manuscript. T.S. contributed to data acquisition and critical review of the manuscript. D.H. contributed to data acquisition and data analysis. C.N.Y. contributed to data acquisition and critical review of the manuscript. N.H.S. provided clinical support and contributed to data acquisition and critical review of the manuscript. P.J.F. contributed to study design, data acquisition, data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Barosensory cells in the nucleus tractus solitarius receive convergent input from group III muscle afferents and central command. Neuroscience. 2006;140:1041–1050. doi: 10.1016/j.neuroscience.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Drew RC, Bell MP, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008a;104:716–723. doi: 10.1152/japplphysiol.00956.2007. [DOI] [PubMed] [Google Scholar]
- Drew RC, McIntyre DB, Ring C, White MJ. Local metabolite accumulation augments passive muscle stretchinduced modulation of carotid–cardiac but not carotid–vasomotor baroreflex sensitivity in man. Exp Physiol. 2008b;93:1044–1057. doi: 10.1113/expphysiol.2008.042234. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–781. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Dawson EA, Fadel PJ, Secher NH, Raven PB, White MJ. Cardiac and vasomotor components of the carotid baroreflex control of arterial blood pressure during isometric exercise in humans. J Physiol. 2006;572:869–880. doi: 10.1113/jphysiol.2005.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Junor C, Khaja A, Northrup M, Fadel PJ. Spontaneous baroreflex measures are unable to detect age-related impairments in cardiac baroreflex function during dynamic exercise in humans. Exp Physiol. 2009;94:447–458. doi: 10.1113/expphysiol.2008.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, White MJ. Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol. 2004;89:639–646. doi: 10.1113/expphysiol.2004.028639. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Effect of muscle metaboreflex activation on carotid-cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2296–H2304. doi: 10.1152/ajpheart.91497.2007. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Di Rienzo M, Lucini D, Legramante JM, Pizzinelli P, Castiglioni P, Pigozzi F, Pagani M, Parati G. Muscle metaboreflex contribution to cardiovascular regulation during dynamic exercise in microgravity: insights from mission STS-107 of the space shuttle Columbia. J Physiol. 2006;572:829–838. doi: 10.1113/jphysiol.2005.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–65. [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel BC, Gallo Junior L, Marin Neto JA, Martins LE. Autonomic nervous control of the heart rate during isometric exercise in normal man. Pflugers Arch. 1987;408:173–177. doi: 10.1007/BF00581348. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Martin CE, Shaver JA, Leon DF, Thompson ME, Reddy PS, Leonard JJ. Autonomic mechanisms in hemodynamic responses to isometric exercise. J Clin Invest. 1974;54:104–115. doi: 10.1172/JCI107731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol. 1989;413:433–445. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil-Dwyer G, Bartlett J, McAinsh J, Cruickshank JM. β-Adrenoceptor blockers and the blood-brian barrier. Br J Clin Pharmacol. 1981;11:549–553. doi: 10.1111/j.1365-2125.1981.tb01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol. 1994;77:2778–2783. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol. 2005;566:599–611. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol. 2006;91:59–72. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- Proakis AG, Harris GB. Comparative penetration of glycopyrrolate and atropine across the blood–brain and placental barriers in anesthetized dogs. Anesthesiology. 1978;48:339–344. doi: 10.1097/00000542-197805000-00007. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Sala-Mercado JA, Ichinose M, Hammond RL, Ichinose T, Pallante M, Stephenson LW, O’Leary DS, Iellamo F. Muscle metaboreflex attenuates spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol. 2007;292:H2867–H2873. doi: 10.1152/ajpheart.00043.2007. [DOI] [PubMed] [Google Scholar]
- Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Seifert TS, Brassard P, Jorgensen TB, Hamada AJ, Rasmussen P, Quistorff B, Secher NH, Nielsen HB. Cerebral non-oxidative carbohydrate consumption in humans driven by adrenaline. J Physiol. 2009;587:285–293. doi: 10.1113/jphysiol.2008.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2804–H2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Studinger P. Counterpoint: cardiovascular variability is not an index of autonomic control of the circulation. J Appl Physiol. 2006;101:678–681. doi: 10.1152/japplphysiol.00446.2006. discussion 681. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, Hayes SG, McCord JL, Kaufman MP. Both central command and exercise pressor reflex activate cardiac sympathetic nerve activity in decerebrate cats. Am J Physiol Heart Circ Physiol. 2009;296:H1157–H1163. doi: 10.1152/ajpheart.01219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest. 1987;79:508–516. doi: 10.1172/JCI112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner HR, Cox A. A mathematical model of heart rate control by sympathetic and vagus efferent information. J Appl Physiol. 1962;17:349–355. doi: 10.1152/jappl.1962.17.2.349. [DOI] [PubMed] [Google Scholar]